Novel Elastic Threads for Intestinal Anastomoses: Feasibility and Mechanical Evaluation in a Porcine and Rabbit Model
Abstract
1. Introduction
2. Results
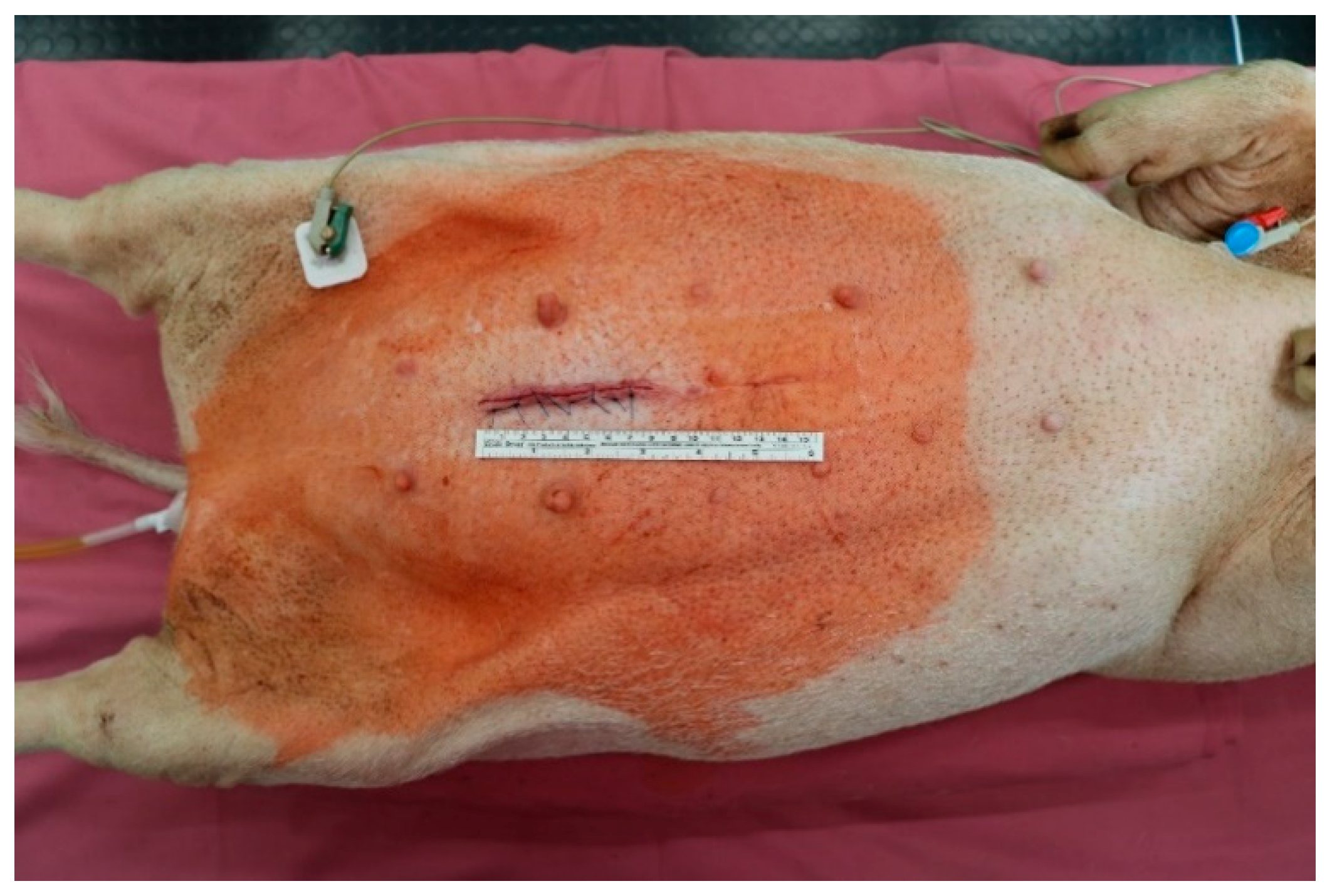
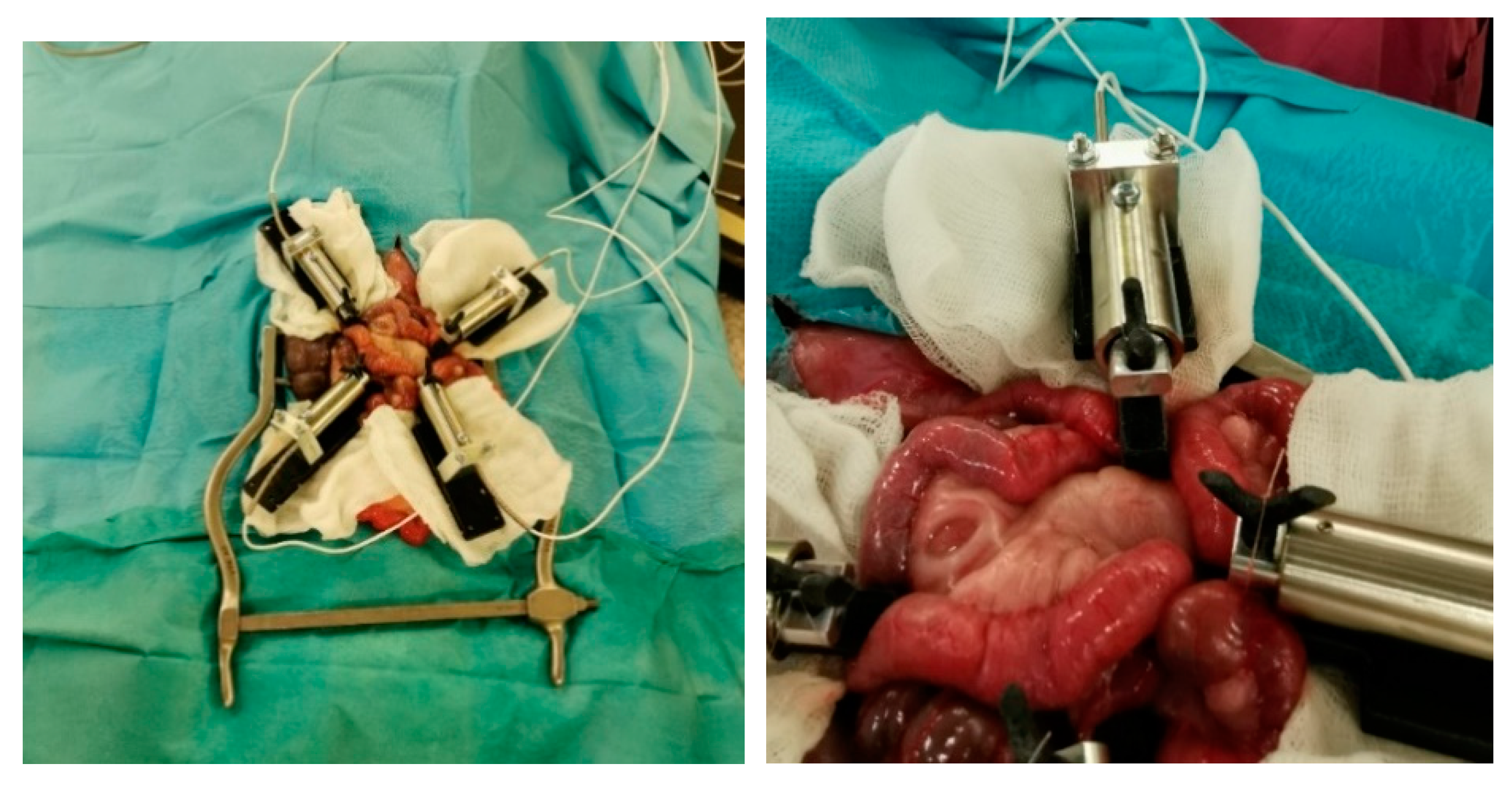
2.1. Minipig Surgery
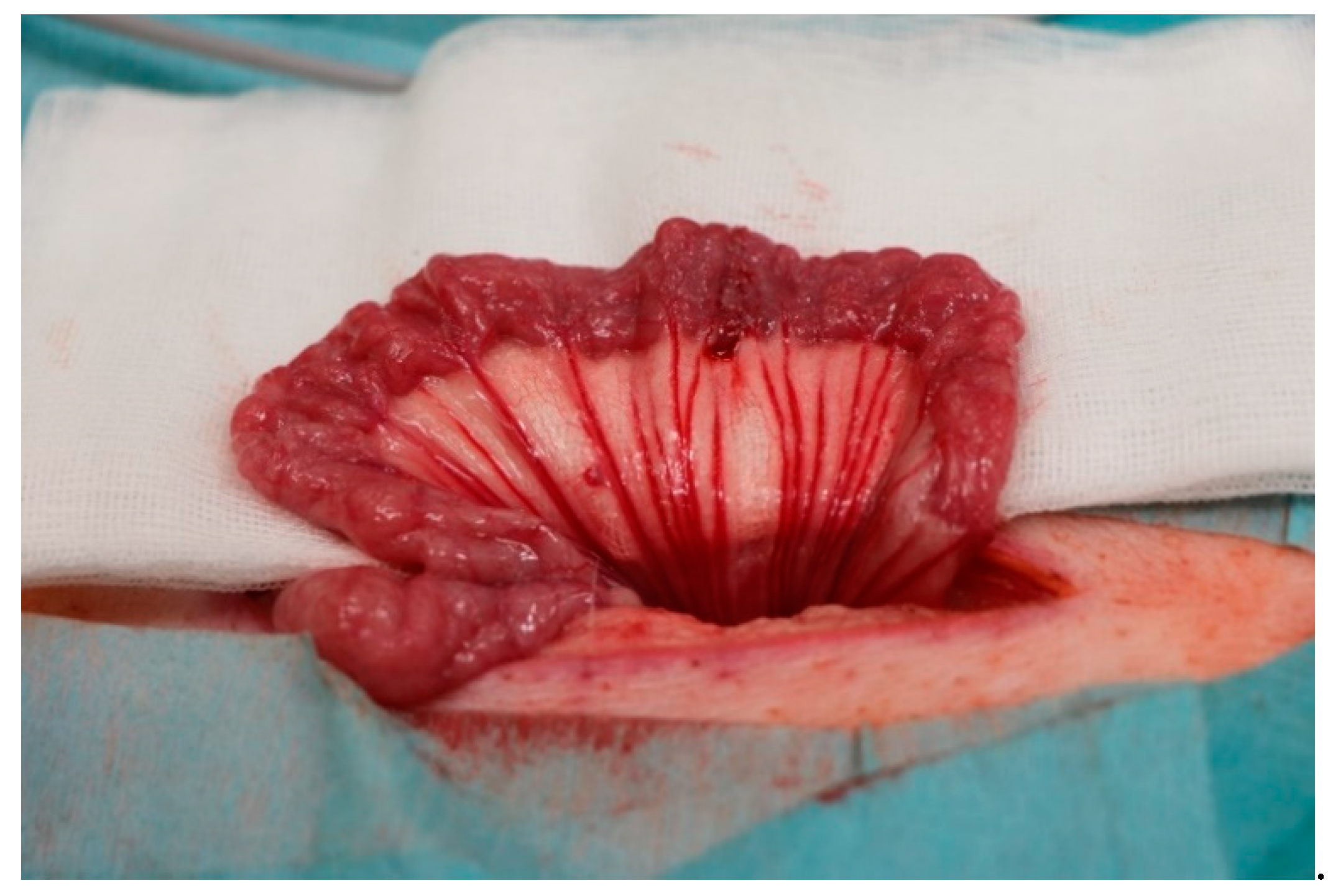
2.2. Macroscopic Evaluation
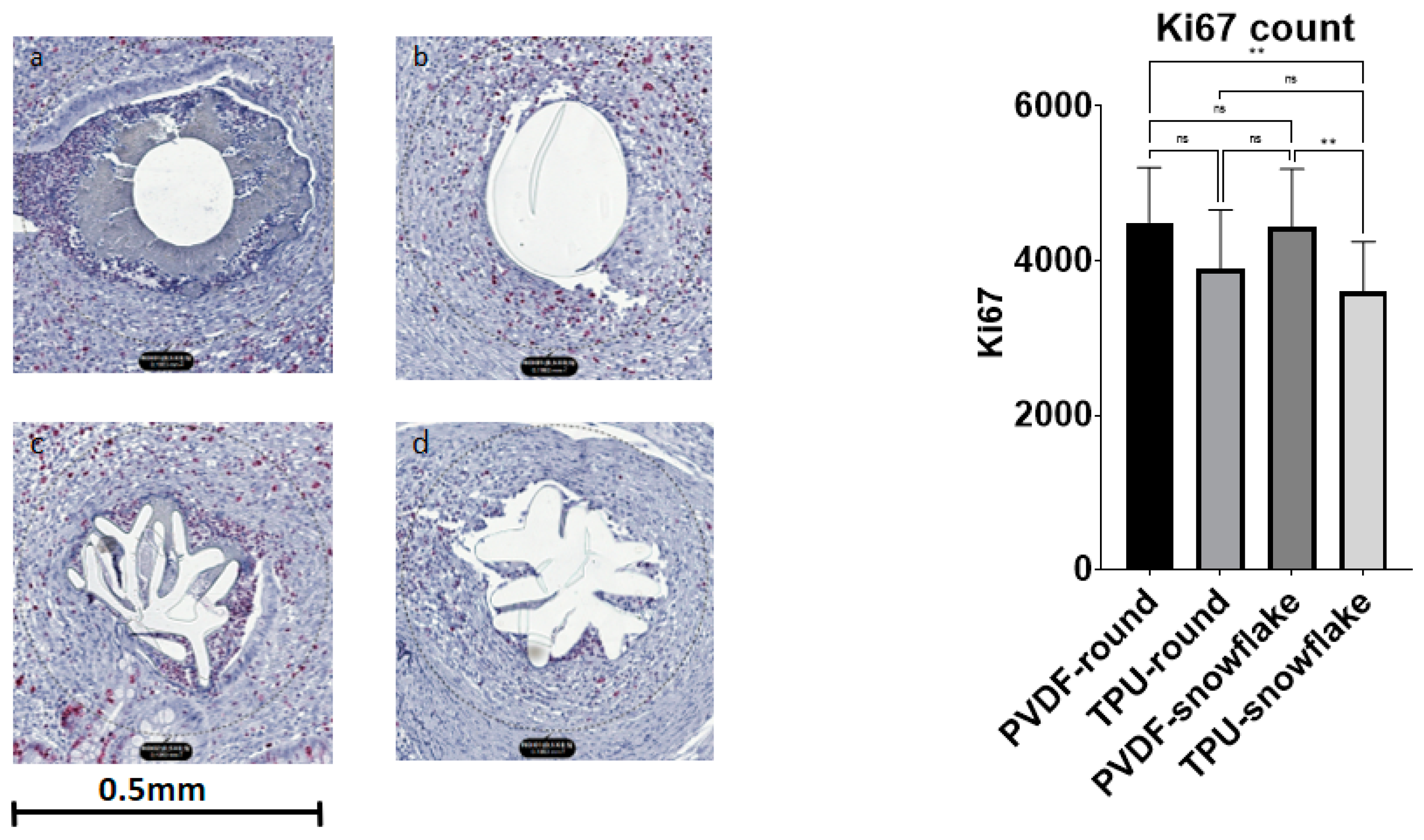
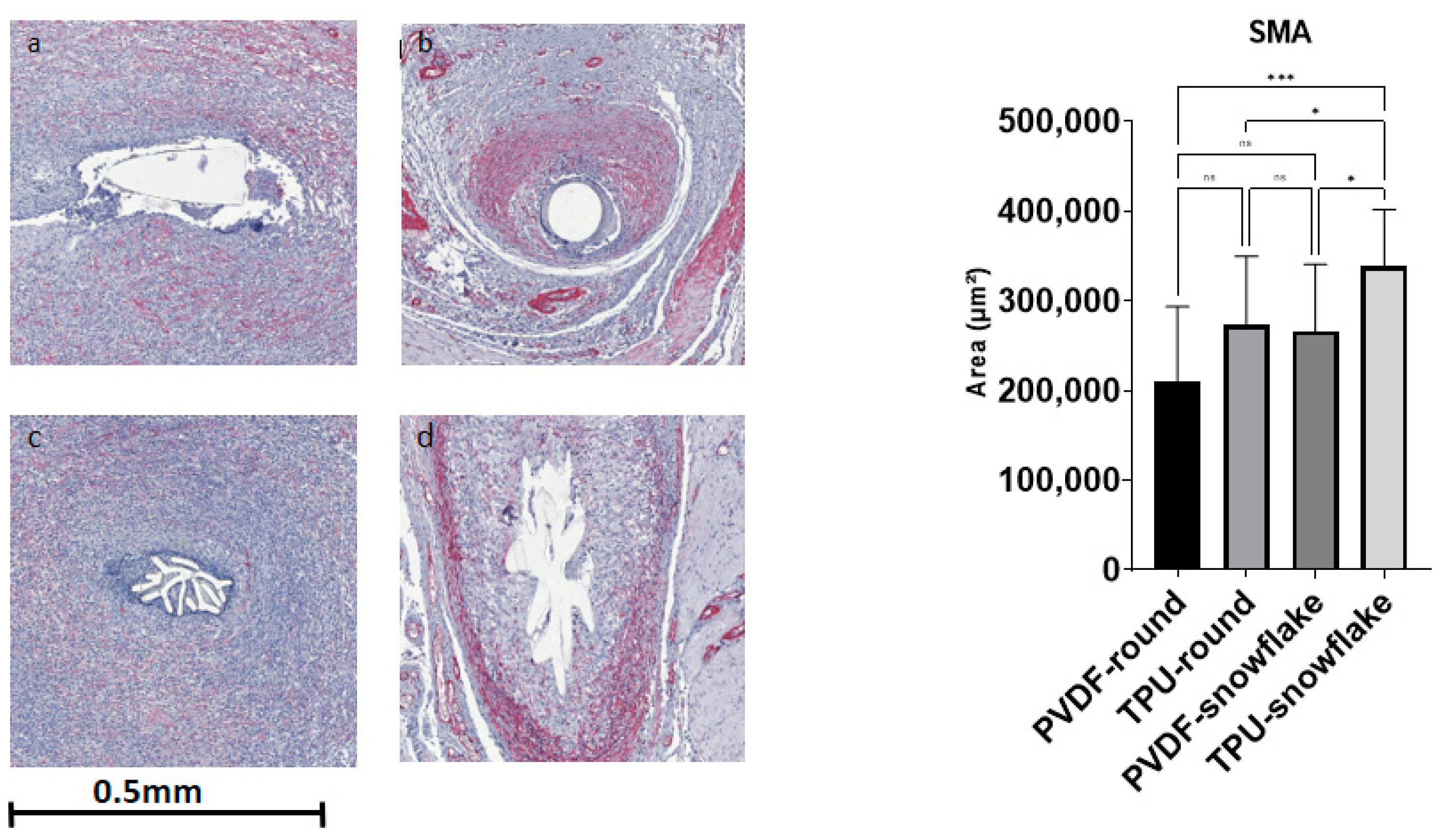
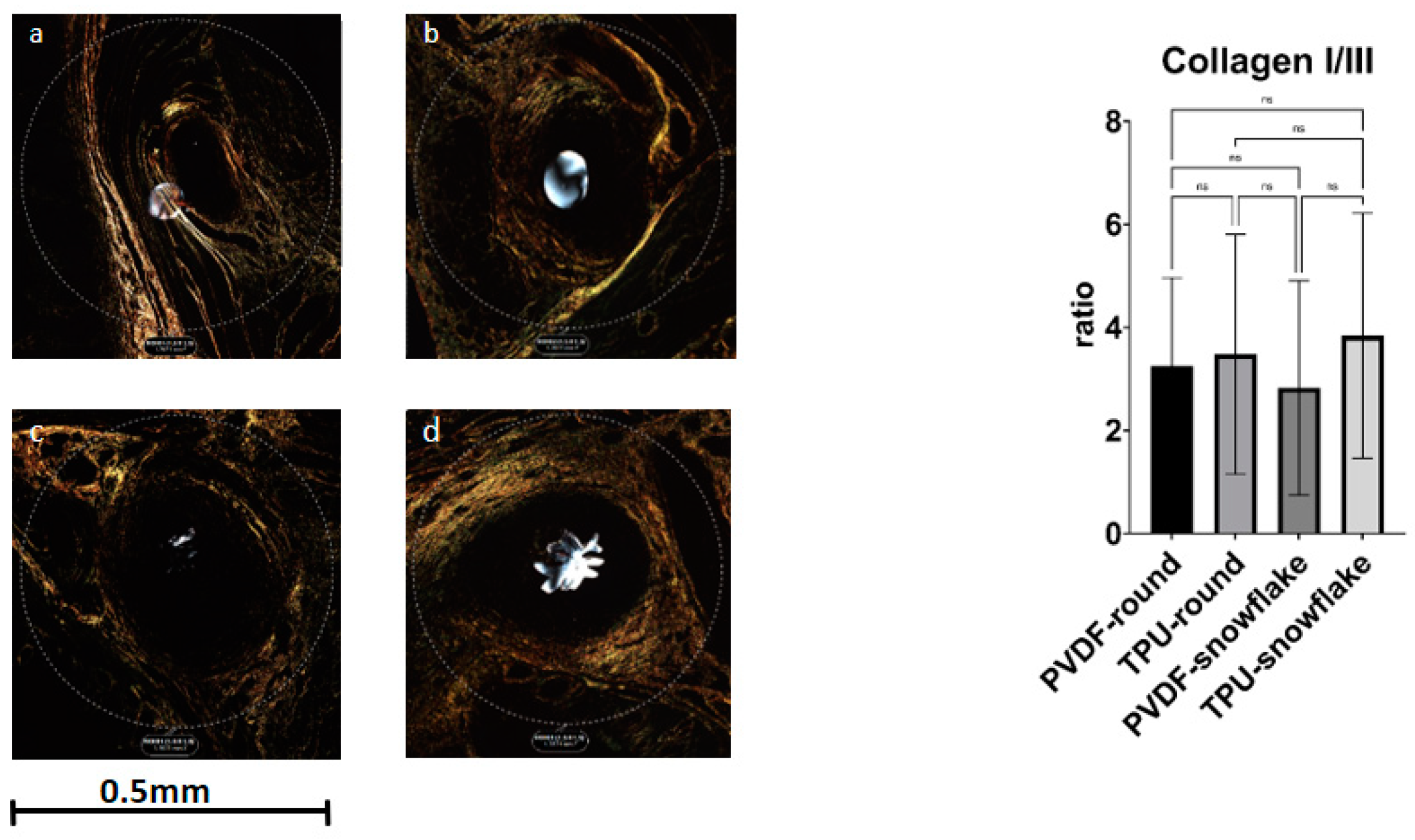
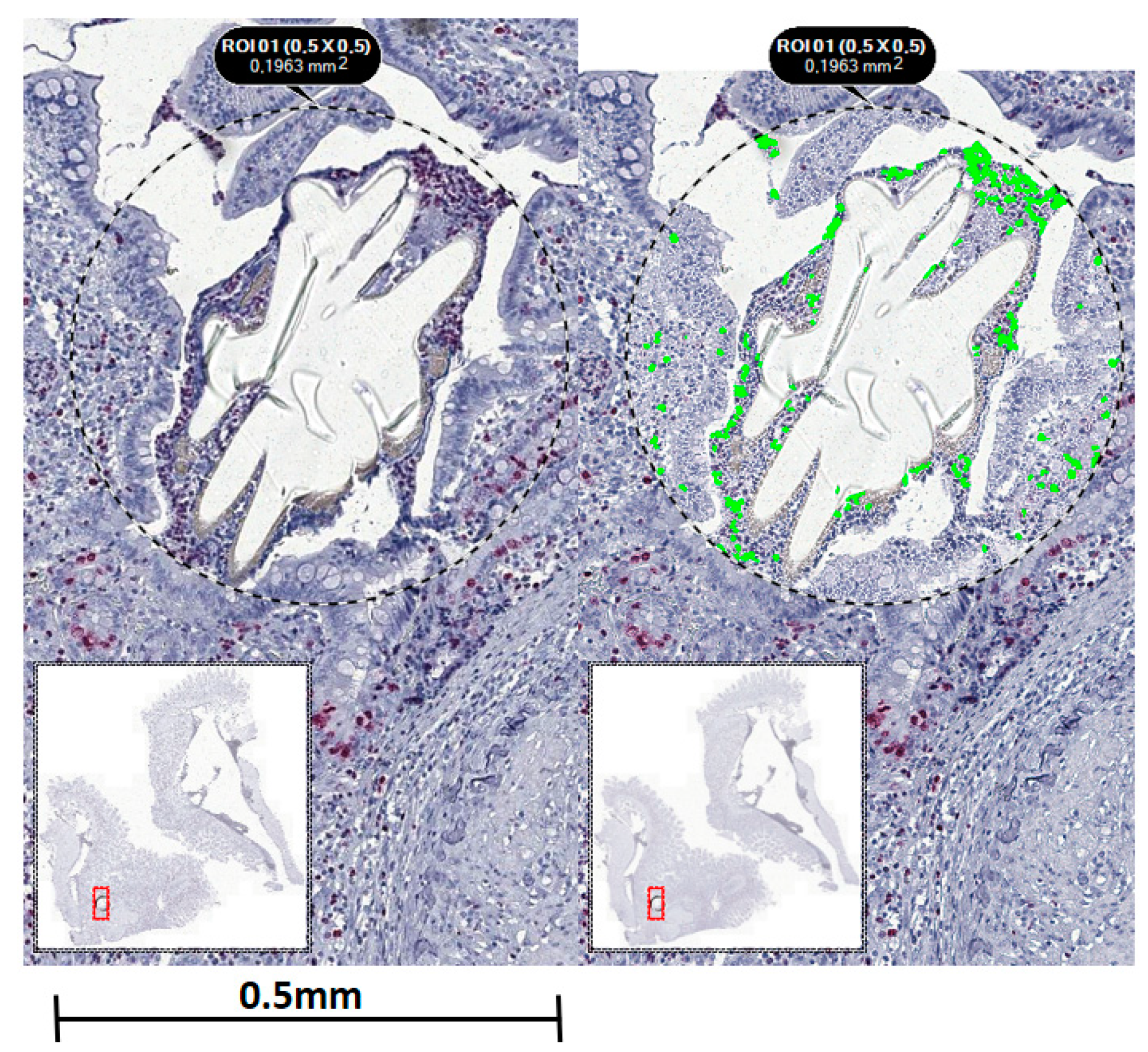
2.3. Microscopic Evaluation including Immunohistochemistry
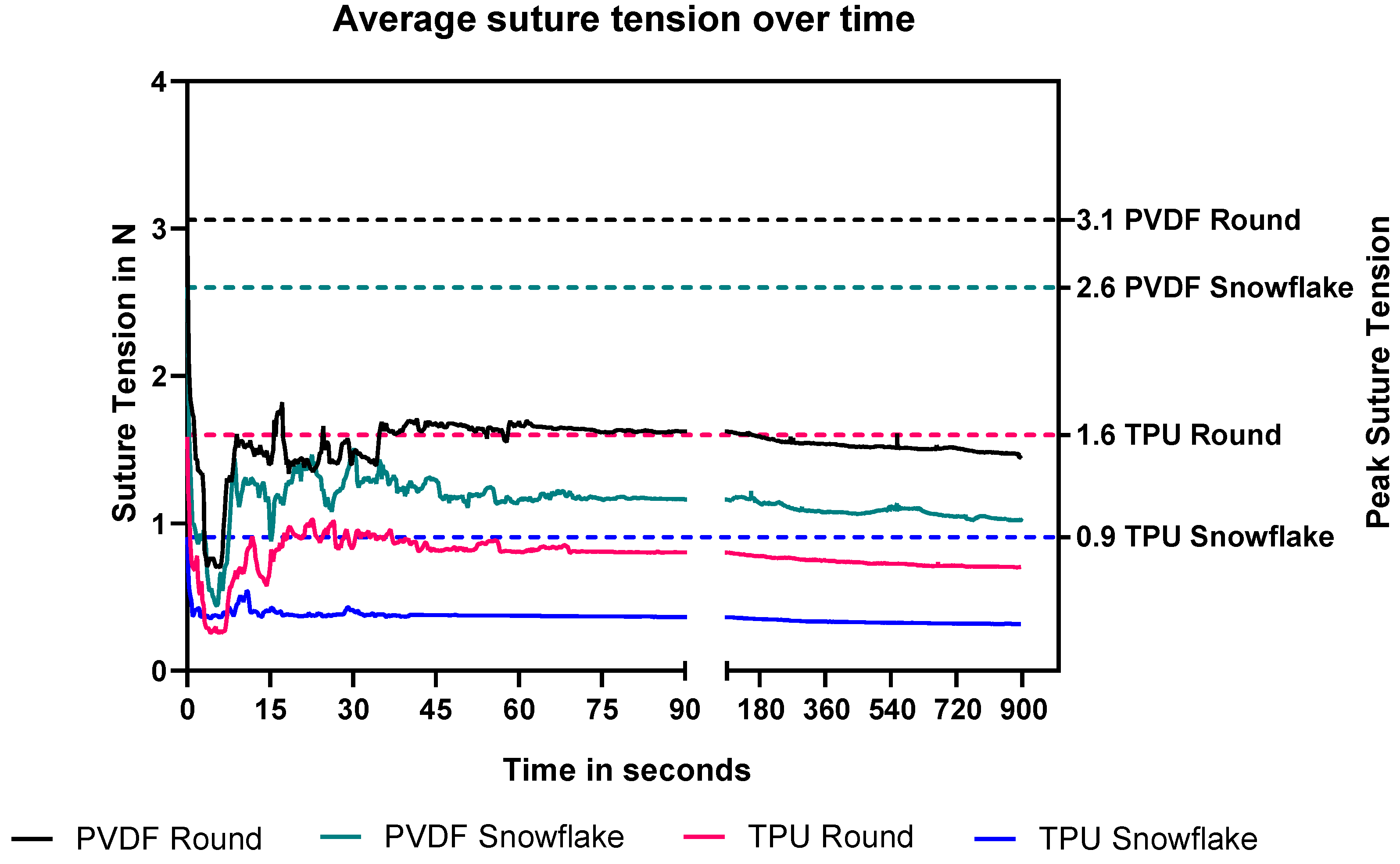
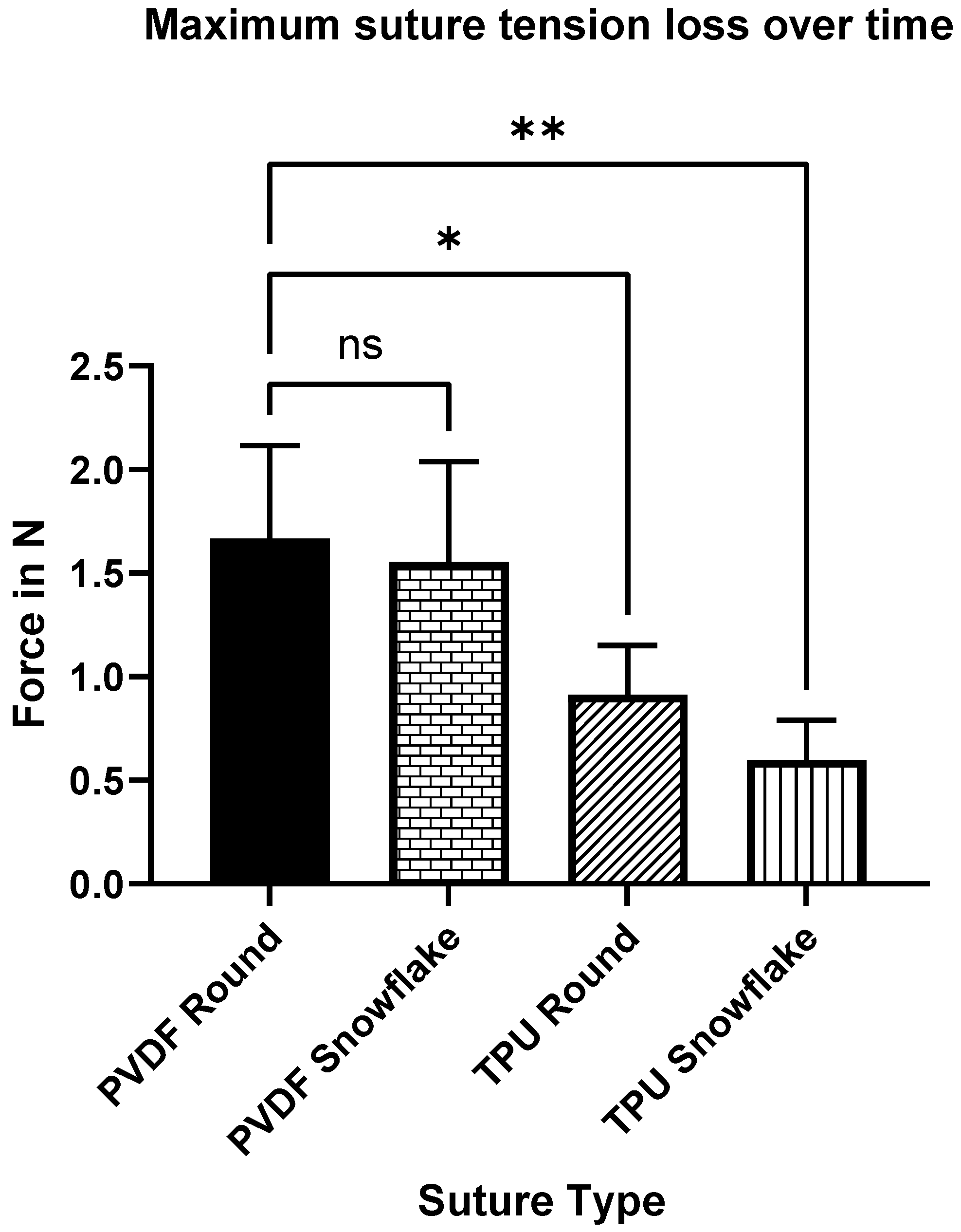
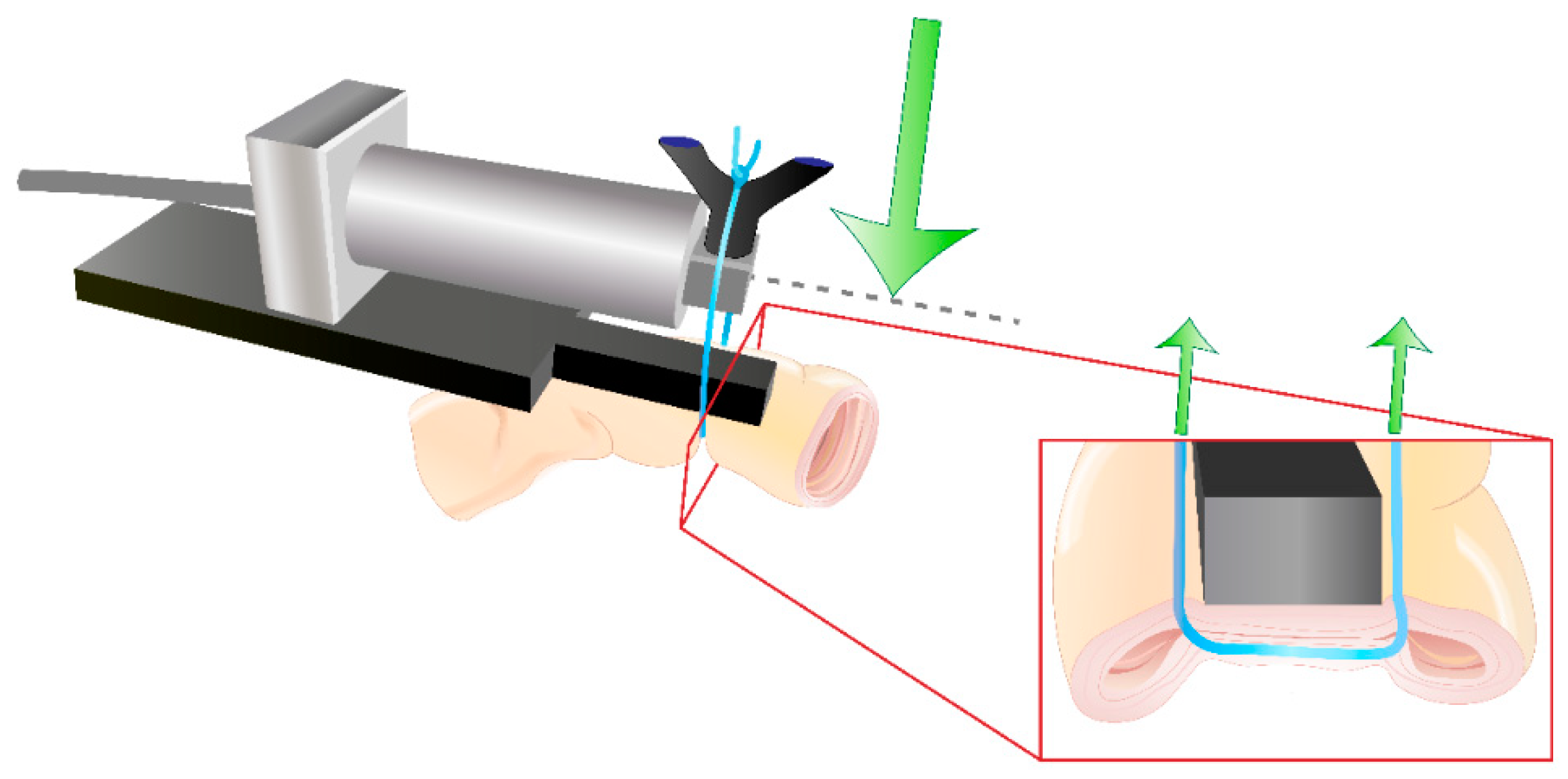
2.4. Rabbit Suture Tension Measurements
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedures
4.2.1. Minipig Small Bowel Anastomoses
4.2.2. Histological Assessment
4.2.3. Rabbit Small Bowel Suture Tension Measurements
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertelsen, C.A.; Andreasen, A.H.; Jorgensen, T.; Harling, H.; Danish Colorectal Cancer Group. Anastomotic leakage after curative anterior resection for rectal cancer: Short and long-term outcome. Colorectal Dis. 2010, 12, e76–e81. [Google Scholar] [CrossRef] [PubMed]
- Branagan, G.; Finnis, D.; Wessex Colorectal Cancer Audit Working Group. Prognosis after anastomotic leakage in colorectal surgery. Dis. Colon Rectum 2005, 48, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.; Junge, K.; Rosch, R.; Krones, C.; Klinge, U.; Schumpelick, V. Suture-free small bowel anastomoses using collagen fleece covered with fibrin glue in pigs. J. Investig. Surg. 2009, 22, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, R.M.; Bolle, T.; Kossel, K.; Heise, D.; Kroh, A.; Lambertz, A.; Blaeser, A.; Gries, T.; Jockenhoevel, S.; Neumann, U.P.; et al. Improved biocompatibility of profiled sutures through lower macrophages adhesion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Helmedag, M.; Heise, D.; Eickhoff, R.; Kossel, K.M.; Gries, T.; Jockenhoevel, S.; Neumann, U.P.; Klink, C.D.; Lambertz, A. Cross-section modified and highly elastic sutures reduce tissue incision and show comparable biocompatibility: In-vitro and in-vivo evaluation of novel thermoplastic urethane surgical threads. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, A.; Schroder, K.M.; Schob, D.S.; Binnebosel, M.; Anurov, M.; Klinge, U.; Neumann, U.P.; Klink, C.D. Polyvinylidene Fluoride as a Suture Material: Evaluation of Comet Tail-Like Infiltrate and Foreign Body Granuloma. Eur. Surg. Res. 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Klink, C.D.; Binnebosel, M.; Alizai, H.P.; Lambertz, A.; Vontrotha, K.T.; Junker, E.; Disselhorst-Klug, C.; Neumann, U.P.; Klinge, U. Tension of knotted surgical sutures shows tissue specific rapid loss in a rodent model. BMC Surg. 2011, 11, 36. [Google Scholar] [CrossRef]
- Schuster, P.; Kossel, K.-M.; Lambertz, A.; Vogels, R.R.M.; Klink, C.D.; Klinge, U.; Gries, T.; Jockenhoevel, S. Elastic filaments from thermoplastic polyurethanes for application in highly elastic mesh implants. BioNanoMaterials 2014, 15, 21–24. [Google Scholar] [CrossRef][Green Version]
- Lambertz, A.; Vogels, R.R.; Busch, D.; Schuster, P.; Jockenhovel, S.; Neumann, U.P.; Klinge, U.; Klink, C.D. Laparotomy closure using an elastic suture: A promising approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 417–423. [Google Scholar] [CrossRef]
- France, L.A.; Fancey, K.S. Viscoelastically active sutures—A stitch in time? Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111695. [Google Scholar] [CrossRef]
- Vogels, R.R.; Lambertz, A.; Schuster, P.; Jockenhoevel, S.; Bouvy, N.D.; Disselhorst-Klug, C.; Neumann, U.P.; Klinge, U.; Klink, C.D. Biocompatibility and biomechanical analysis of elastic TPU threads as new suture material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, H.; Seyidova, N.; Schreiber, C.; Strobl, M.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Frohlich, S.; Marchetti-Deschmann, M.; et al. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater. 2015, 11, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zdrahala, R.J.; Zdrahala, I.J. Biomedical applications of polyurethanes: A review of past promises, present realities, and a vibrant future. J. Biomater. Appl. 1999, 14, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Heise, D.; Eickhoff, R.; Kroh, A.; Binnebösel, M.; Klinge, U.; Klink, C.D.; Neumann, U.P.; Lambertz, A. Elastic TPU Mesh as Abdominal Wall Inlay Significantly Reduces Defect Size in a Minipig Model. J. Investig. Surg. 2018, 32, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, A.; Vogels, R.R.; Binnebosel, M.; Schob, D.S.; Kossel, K.; Klinge, U.; Neumann, U.P.; Klink, C.D. Elastic mesh with thermoplastic polyurethane filaments preserves effective porosity of textile implants. J. Biomed. Mater. Res. Part A 2015, 103, 2654–2660. [Google Scholar] [CrossRef]
- Enayati, M.; Eilenberg, M.; Grasl, C.; Riedl, P.; Kaun, C.; Messner, B.; Walter, I.; Liska, R.; Schima, H.; Wojta, J.; et al. Biocompatibility Assessment of a New Biodegradable Vascular Graft via In Vitro Co-culture Approaches and In Vivo Model. Ann. Biomed. Eng. 2016, 44, 3319–3334. [Google Scholar] [CrossRef]
- Kang, C.Y.; Halabi, W.J.; Chaudhry, O.O.; Nguyen, V.; Pigazzi, A.; Carmichael, J.C.; Mills, S.; Stamos, M.J. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013, 148, 65–71. [Google Scholar] [CrossRef]
- Matthiessen, P.; Hallbook, O.; Andersson, M.; Rutegard, J.; Sjodahl, R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004, 6, 462–469. [Google Scholar] [CrossRef]
- Hyman, N.; Manchester, T.L.; Osler, T.; Burns, B.; Cataldo, P.A. Anastomotic leaks after intestinal anastomosis: It’s later than you think. Ann. Surg. 2007, 245, 254–258. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Andreasen, A.H.; Jorgensen, T.; Harling, H.; Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: Risk factors. Colorectal Dis. 2010, 12, 37–43. [Google Scholar] [CrossRef]
- Yauw, S.T.; Wever, K.E.; Hoesseini, A.; Ritskes-Hoitinga, M.; van Goor, H. Systematic review of experimental studies on intestinal anastomosis. Br. J. Surg. 2015, 102, 726–734. [Google Scholar] [CrossRef] [PubMed]
- von Trotha, K.T.; Grommes, J.; Butz, N.; Lambertz, A.; Klink, C.D.; Neumann, U.P.; Jacobs, M.; Binnebosel, M. Surgical sutures: Coincidence or experience? Hernia 2017, 21, 505–508. [Google Scholar] [CrossRef]
- Simon-Allue, R.; Perez-Lopez, P.; Sotomayor, S.; Pena, E.; Pascual, G.; Bellon, J.M.; Calvo, B. Short- and long-term biomechanical and morphological study of new suture types in abdominal wall closure. J. Mech. Behav. Biomed. Mater. 2014, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bellon, J.M.; Perez-Lopez, P.; Simon-Allue, R.; Sotomayor, S.; Perez-Kohler, B.; Pena, E.; Pascual, G.; Calvo, B. New suture materials for midline laparotomy closure: An experimental study. BMC Surg. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Klink, C.D.; Binnebosel, M.; Kaemmer, D.; Schachtrupp, A.; Fiebeler, A.; Anurov, M.; Schumpelick, V.; Klinge, U. Comet-tail-like inflammatory infiltrate to polymer filaments develops in tension-free conditions. Eur. Surg. Res. 2011, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Cossermelli, W.; Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch. Histol. Jpn. 1978, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]

| PVDF-Round | TPU-Round | PVDF-Snowflake | TPU-Snowflake | p-Value | |
|---|---|---|---|---|---|
| Inner Granuloma | 30.8 (15.1) | 40.6 (25) | 29.5 (5.6) | 33.4 (14.2) | 0.3711 |
| Outer Granuloma | 97.1 (45.6) | 118.4 (71.8) | 100.3 (34.4) | 113.5 (41.9) | 0.7141 |
| Comet-Tail Infiltrate | 395.8 (123.9) | 357.7 (160.1) | 340.2 (98.6) | 400.9 (117.5) | 0.6187 |
| PVDF-Round | TPU-Round | PVDF-Snowflake | TPU-Snowflake | p-Value | |
|---|---|---|---|---|---|
| Ki67 [nuclei count] | 4497 (711) | 3903 (756) | 4438 (749) | 3610 (637) | 0.0013 |
| SMA [area, µm2] | 210,784 (82,404) | 272,770 (76,473) | 265,719 (74,756) | 337,952 (63,691) | 0.0005 |
| Collagen I/III ratio | 3.3 (1.7) | 3.5 (2.4) | 2.8 (0.2) | 3.8 (2.4) | 0.6863 |
| CD68 [nuclei count] | 3531 (1243) | 3346(886) | 2978 (760) | 2833.12 (531) | 0.0558 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz, S.M.; Helmedag, M.J.; Kossel, K.-M.; Eickhoff, R.M.; Heise, D.; Kroh, A.; Mechelinck, M.; Gries, T.; Jockenhoevel, S.; Neumann, U.P.; et al. Novel Elastic Threads for Intestinal Anastomoses: Feasibility and Mechanical Evaluation in a Porcine and Rabbit Model. Int. J. Mol. Sci. 2022, 23, 5389. https://doi.org/10.3390/ijms23105389
Schmitz SM, Helmedag MJ, Kossel K-M, Eickhoff RM, Heise D, Kroh A, Mechelinck M, Gries T, Jockenhoevel S, Neumann UP, et al. Novel Elastic Threads for Intestinal Anastomoses: Feasibility and Mechanical Evaluation in a Porcine and Rabbit Model. International Journal of Molecular Sciences. 2022; 23(10):5389. https://doi.org/10.3390/ijms23105389
Chicago/Turabian StyleSchmitz, Sophia M., Marius J. Helmedag, Klas-Moritz Kossel, Roman M. Eickhoff, Daniel Heise, Andreas Kroh, Mare Mechelinck, Thomas Gries, Stefan Jockenhoevel, Ulf P. Neumann, and et al. 2022. "Novel Elastic Threads for Intestinal Anastomoses: Feasibility and Mechanical Evaluation in a Porcine and Rabbit Model" International Journal of Molecular Sciences 23, no. 10: 5389. https://doi.org/10.3390/ijms23105389
APA StyleSchmitz, S. M., Helmedag, M. J., Kossel, K.-M., Eickhoff, R. M., Heise, D., Kroh, A., Mechelinck, M., Gries, T., Jockenhoevel, S., Neumann, U. P., & Lambertz, A. (2022). Novel Elastic Threads for Intestinal Anastomoses: Feasibility and Mechanical Evaluation in a Porcine and Rabbit Model. International Journal of Molecular Sciences, 23(10), 5389. https://doi.org/10.3390/ijms23105389







