Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice
Abstract
:1. Introduction
2. Methods
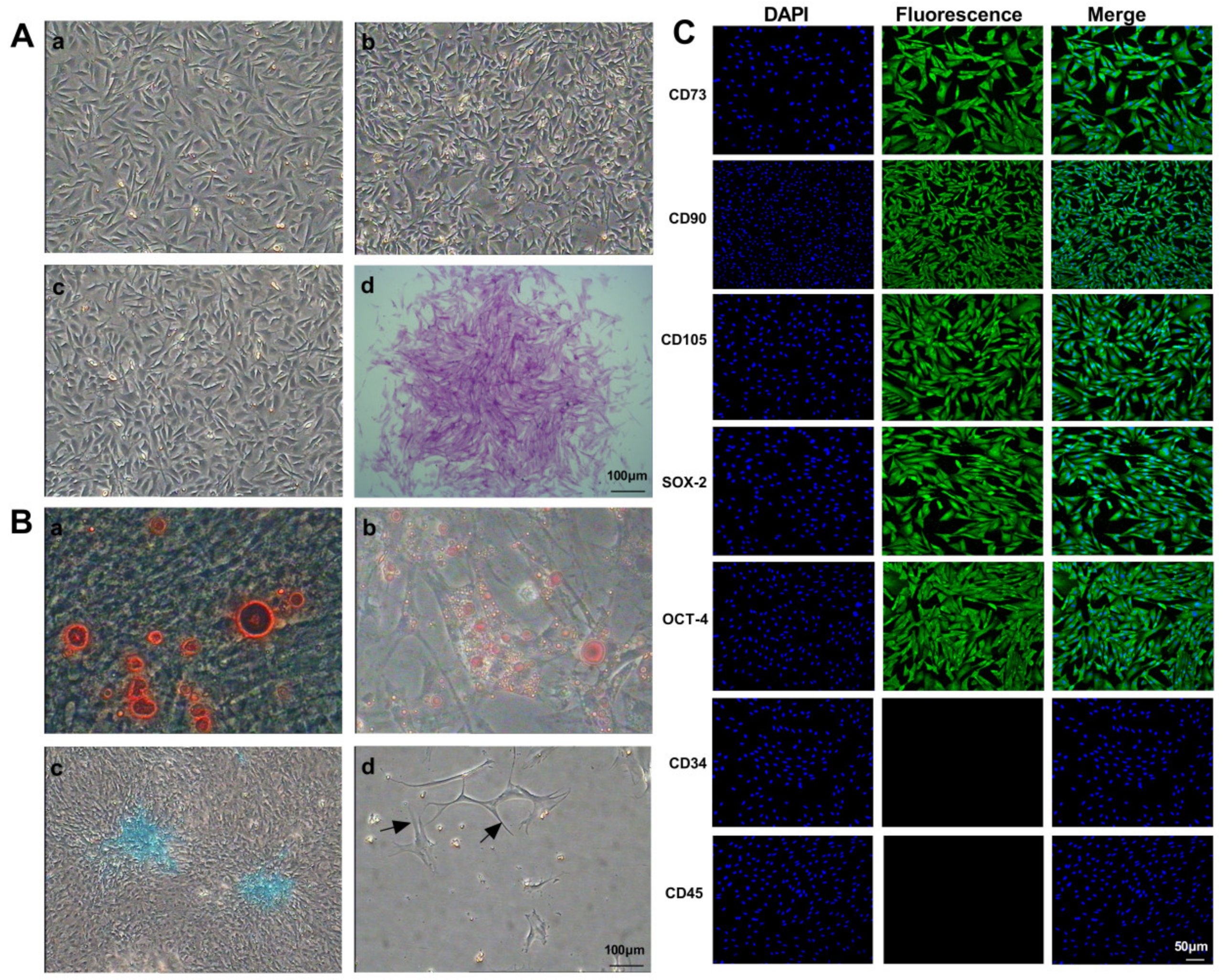
2.1. Isolation and Culture of GMSCs
2.2. Immunofluorescence Staining
2.3. Multi-Differentiation Potential of GMSCs
2.3.1. Osteogenic Differentiation of GMSCs
2.3.2. Adipogenic Differentiation of GMSCs
2.3.3. Chondrogenic Differentiation of GMSCs
2.3.4. Neuroblastic Differentiation of GMSCs
2.4. Clone Formation Assay of GMSCs
2.5. Experimental Design
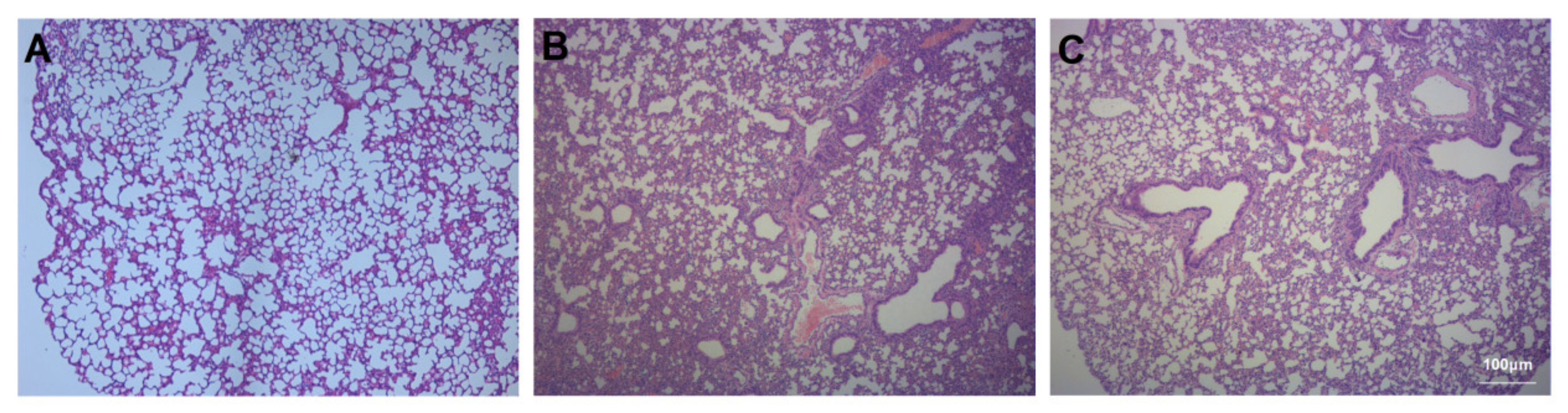
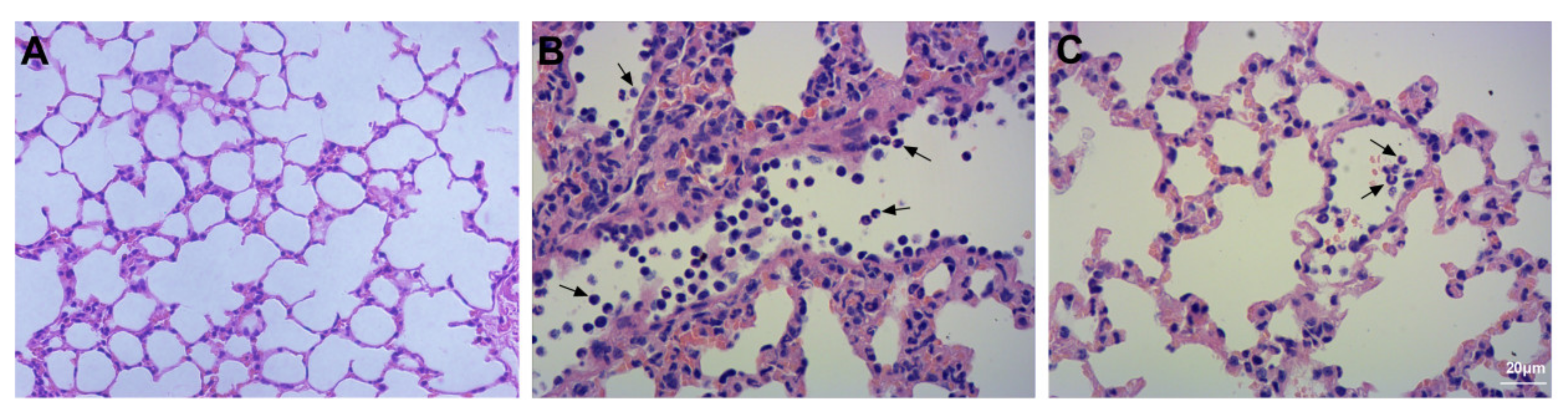
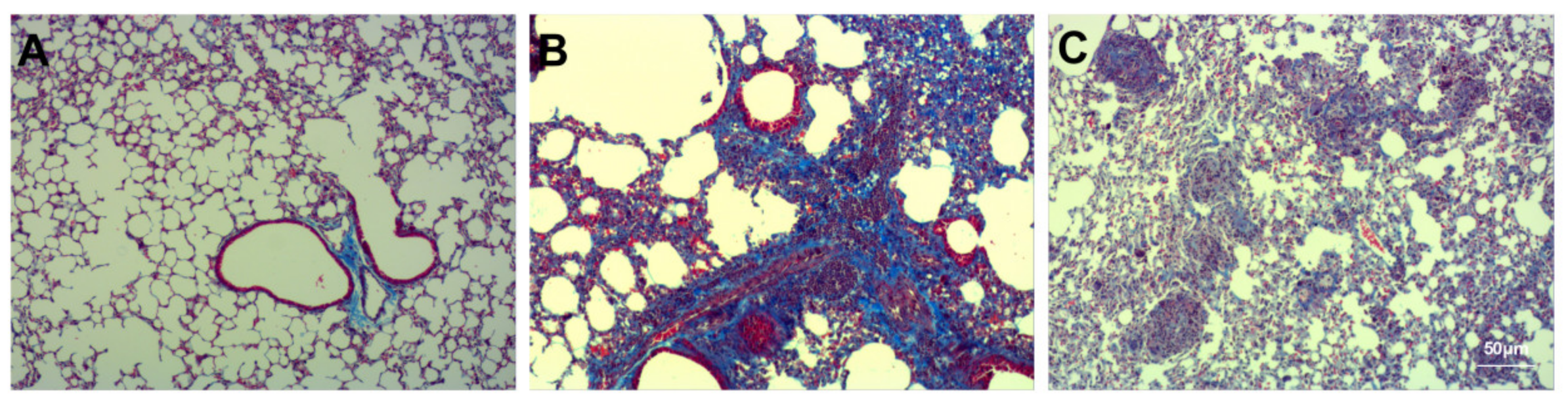
2.6. Histopathology
2.7. Collection of Bronchoalveolar Lavage Fluid (BALF) and Tissue Samples
2.8. Determination of Pulmonary Oedema
2.9. Determination of Oxidative Stress Index in Lung Homogenates
2.10. Cytokines
2.11. qRT–PCR
2.12. Statistical Analysis
3. Results and Discussion
3.1. Biological Characteristics of Mouse GMSCs
3.2. Assessment of Lung Injury and Neutrophil Infiltration
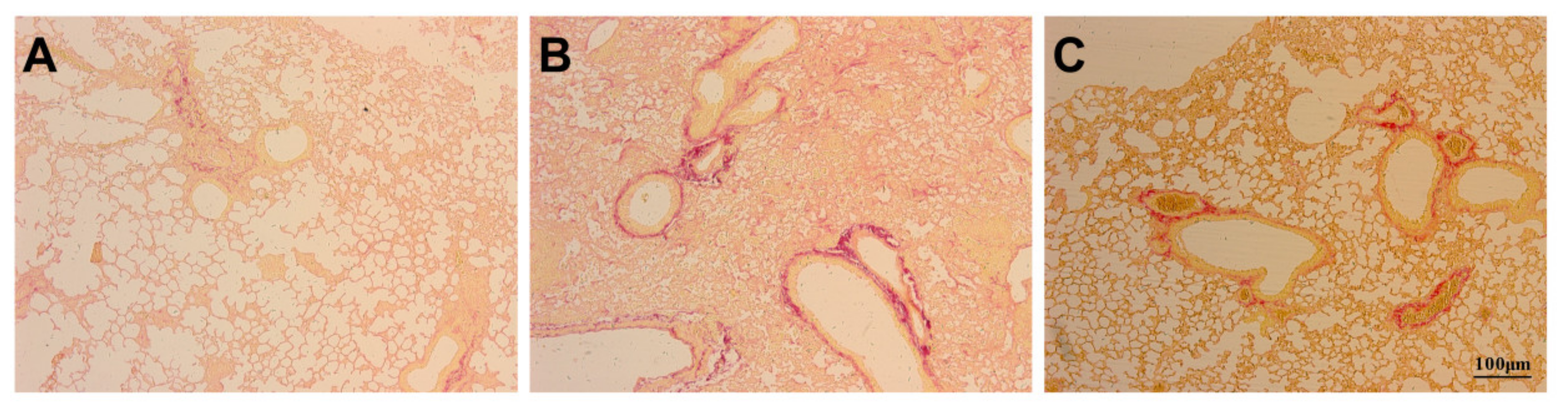
3.3. Assessment of Pulmonary Fibrosis
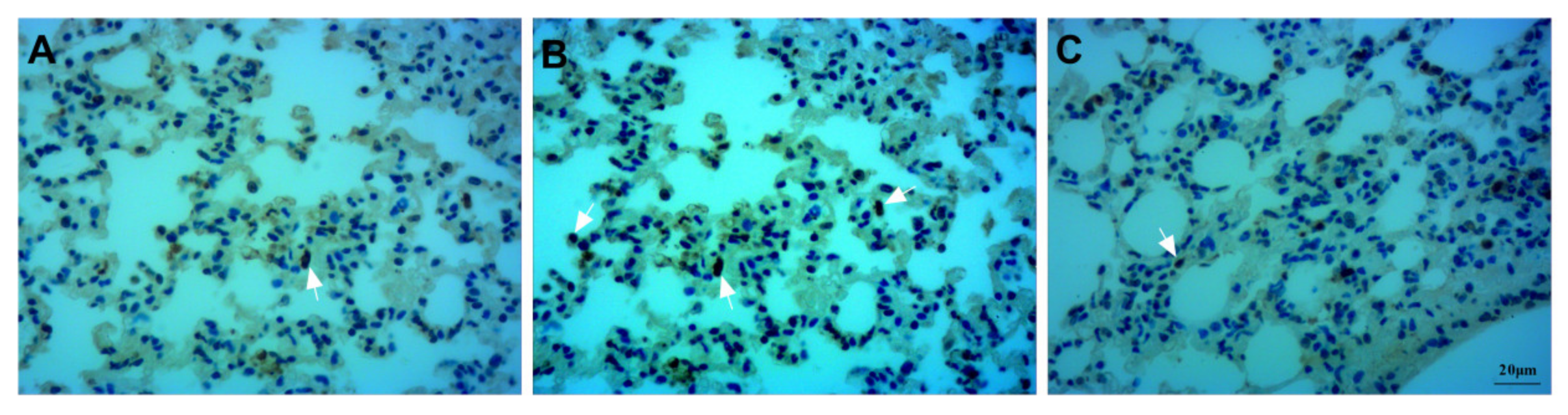
3.4. Assessment of Apoptosis
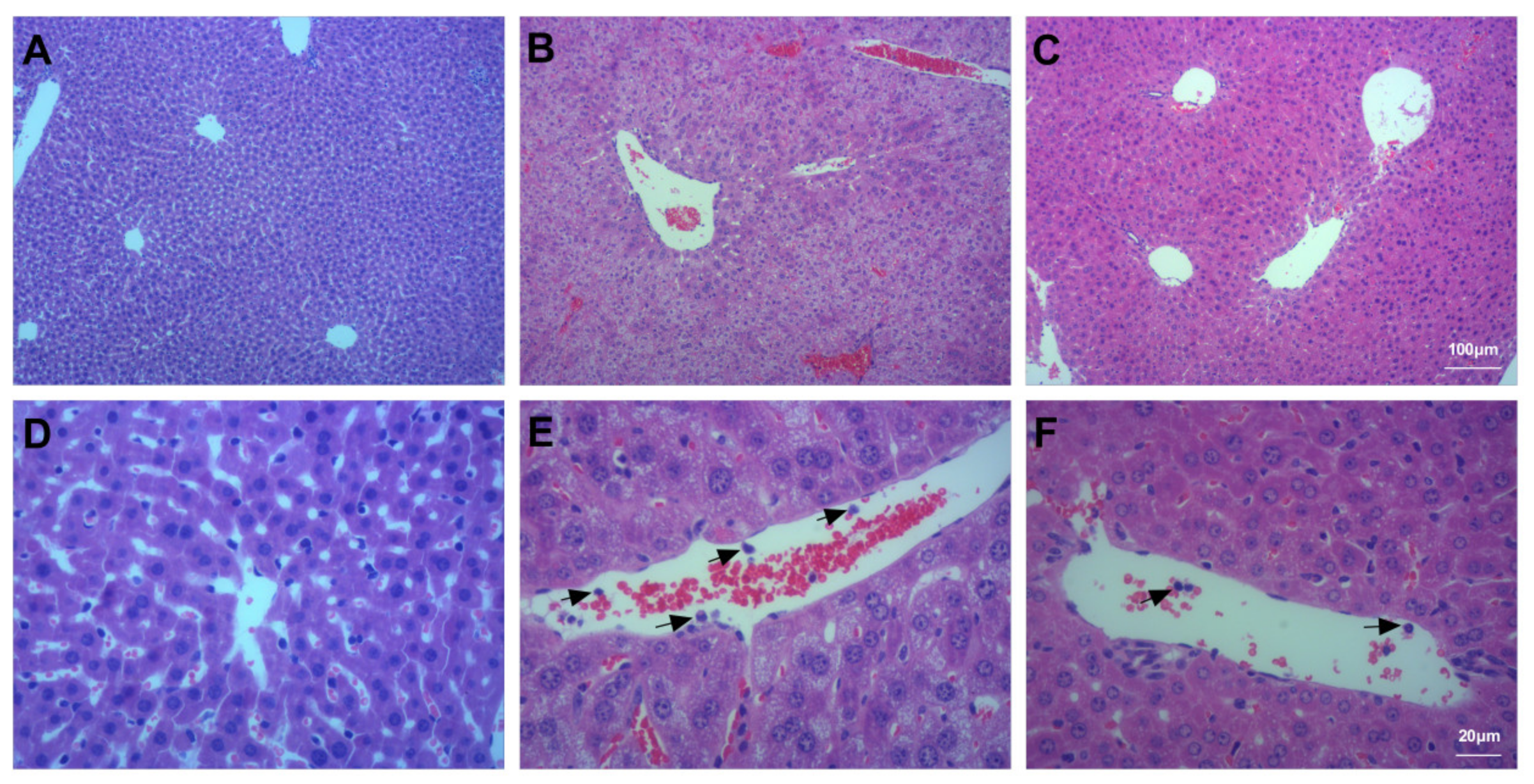
3.5. Assessment of Liver Injury
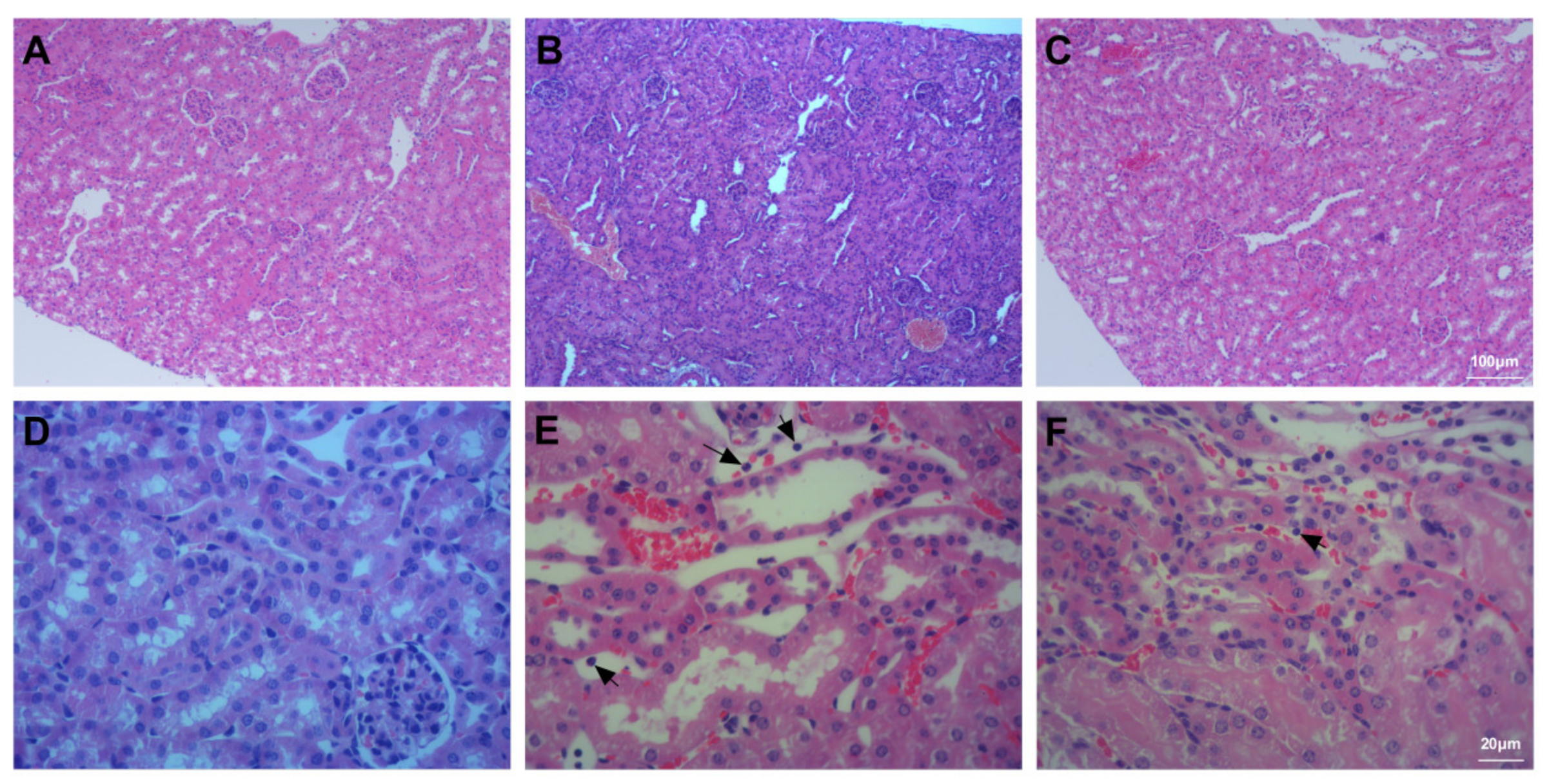
3.6. Assessment of Kidney Injury
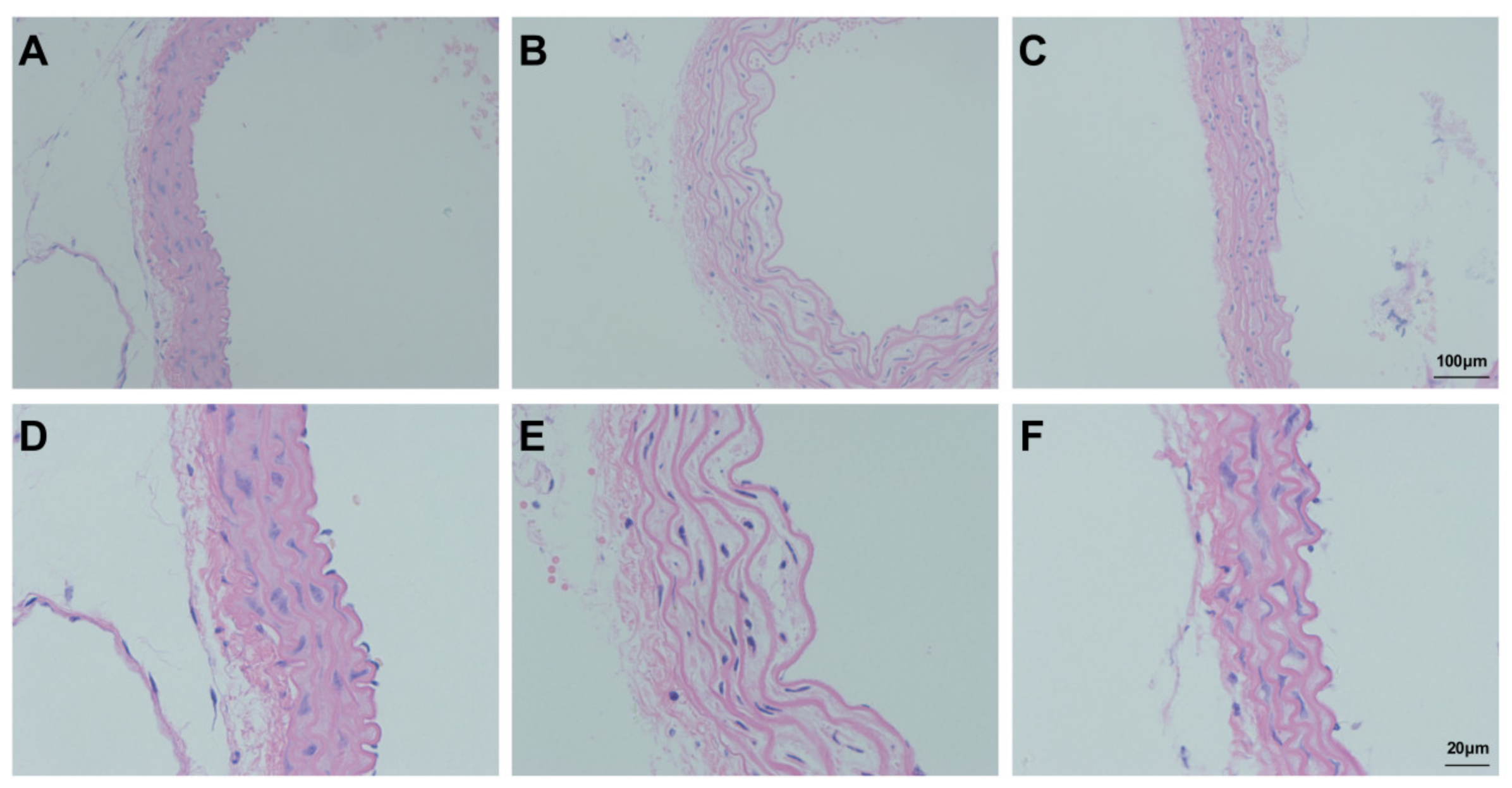
3.7. Assessment of Aortic Injury
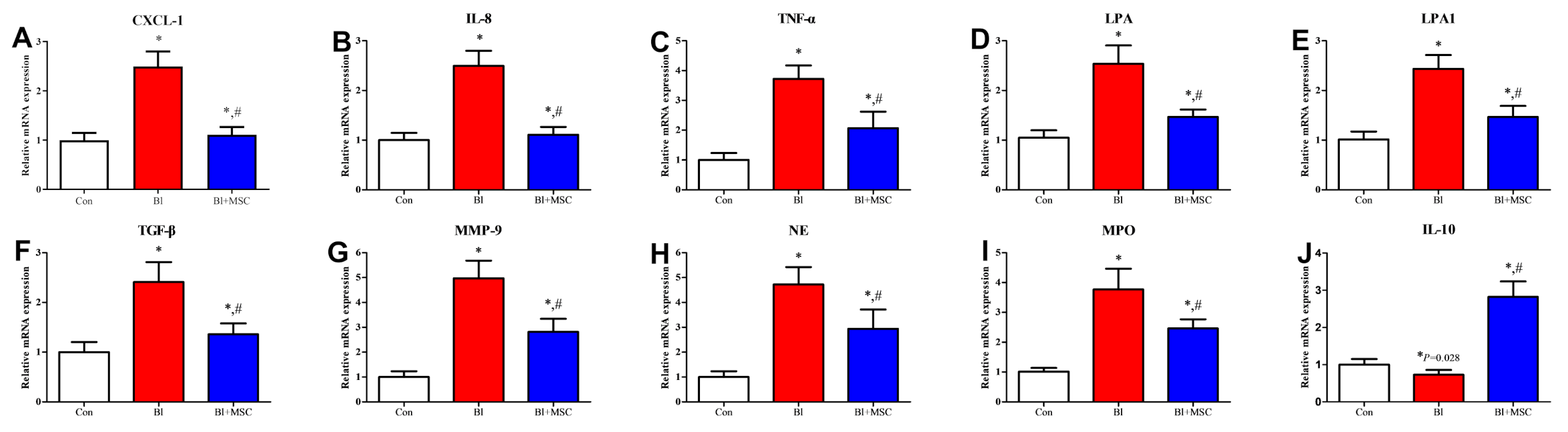
3.8. Assessment of mRNA Expression in Lung Tissue
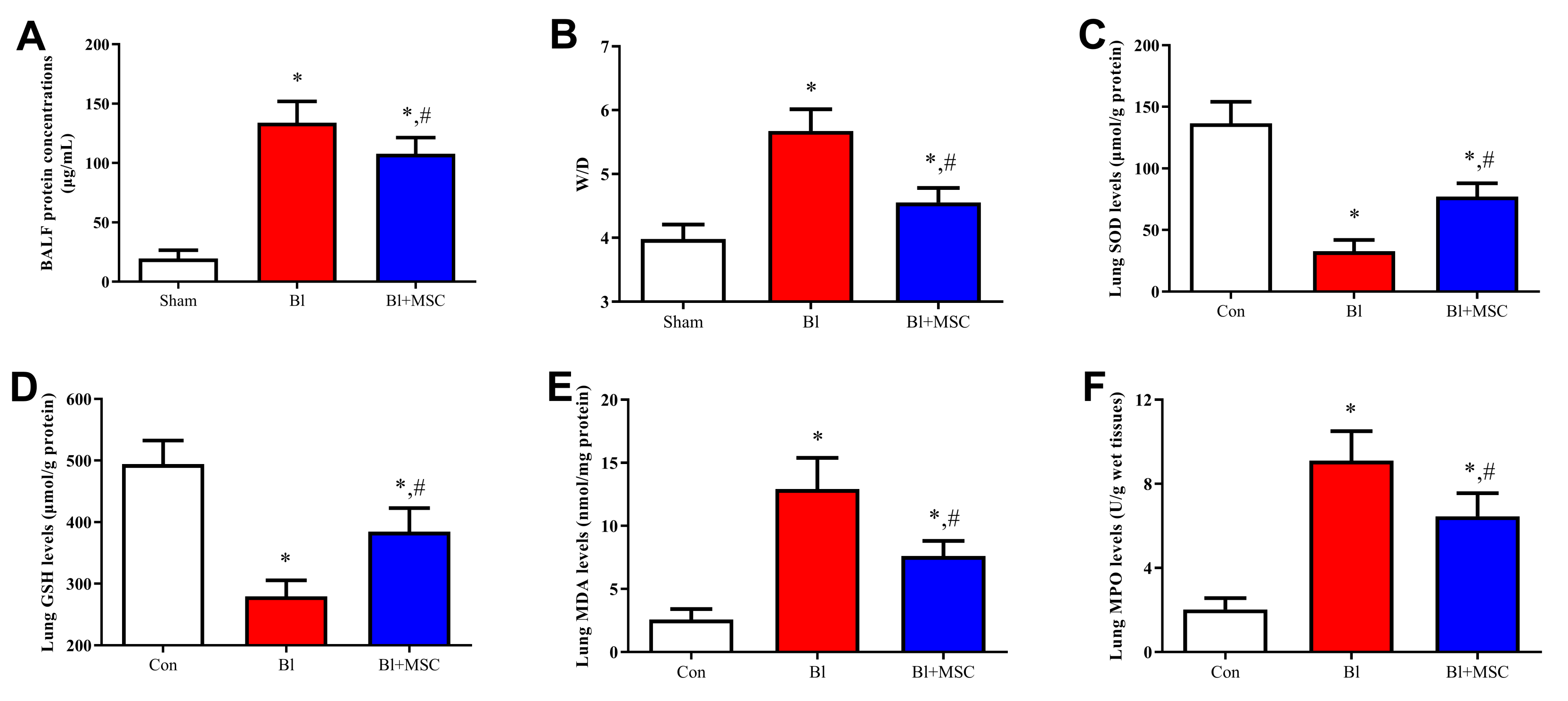
3.9. Assessment of Pulmonary Oedema
3.10. Assessment of Oxidative Stress-Related Indicators in Lung Tissue
3.11. Assessment of BALF Cell Count
3.12. Assessment of the Levels of Inflammatory Cytokines in BALF
3.13. Detection of GMSCs Homing in Lung Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Srour, N.; Thébaud, B. Mesenchymal Stromal Cells in Animal Bleomycin Pulmonary Fibrosis Models: A Systematic Review. STEM CELLS Transl. Med. 2015, 4, 1500–1510. [Google Scholar] [CrossRef] [Green Version]
- Periera-Simon, S.; Xia, X.; Catanuto, P.; Coronado, R.; Kurtzberg, J.; Bellio, M.; Lee, Y.; Khan, A.; Smith, R.; Elliot, S.J.; et al. Anti-fibrotic effects of different sources of MSC in bleomycin-induced lung fibrosis in C57BL6 male mice. Respirology 2021, 26, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakayama, T.; Hashimoto, N.; Miyata, Y.; Egashira, K.; Nakao, N.; Nishiwaki, S.; Hasegawa, M.; Hasegawa, Y.; Naoe, T. Mesenchymal Stem Cells Stably Transduced with a Dominant-Negative Inhibitor of CCL2 Greatly Attenuate Bleomycin-Induced Lung Damage. Am. J. Pathol. 2011, 179, 1088–1094. [Google Scholar] [CrossRef]
- Chen, S.; Cui, G.; Peng, C.; Lavin, M.F.; Sun, X.; Zhang, E.; Yang, Y.; Guan, Y.; Du, Z.; Shao, H. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res. Ther. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [Green Version]
- Coccè, V.; Farronato, D.; Brini, A.T.; Masia, C.; Giannì, A.B.; Piovani, G.; Sisto, F.; Alessandri, G.; Angiero, F.; Pessina, A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 9376. [Google Scholar] [CrossRef] [PubMed]
- Górski, B. Gingiva as a new and the most accessible source of mesenchymal stem cells from the oral cavity to be used in regenerative therapies. Postepy Hig. Med. Dosw. 2016, 70, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.P.; Larjava, H.; Häkkinen, L. Gingiva as a Source of Stem Cells with Therapeutic Potential. Stem Cells Dev. 2013, 22, 3157–3177. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.; Zhao, S.; Guan, W.; Gao, Y. Gingival-Derived Mesenchymal Stem Cells Protect Against Sepsis and Its Complications. Infect. Drug Resist. 2021, 14, 3341–3355. [Google Scholar] [CrossRef]
- Bellaye, P.-S.; Yanagihara, T.; Granton, E.; Sato, S.; Shimbori, C.; Upagupta, C.; Imani, J.; Hambly, N.; Ask, K.; Gauldie, J.; et al. Macitentan reduces progression of TGF-β1-induced pulmonary fibrosis and pulmonary hypertension. Eur. Respir. J. 2018, 52, 1701857. [Google Scholar] [CrossRef]
- Dadrich, M.; Nicolay, N.H.; Flechsig, P.; Bickelhaupt, S.; Hoeltgen, L.; Roeder, F.; Hauser, K.; Tietz, A.; Jenne, J.; Lopez, R.; et al. Combined inhibition of TGFbeta and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology 2015, 5, e1123366. [Google Scholar] [CrossRef] [PubMed]
- Olianas, M.C.; Dedoni, S.; Onali, P. Antidepressants induce profibrotic responses via the lysophosphatidic acid receptor LPA1. Eur. J. Pharmacol. 2020, 873, 172963. [Google Scholar] [CrossRef] [PubMed]
- Sattikar, A.; Dowling, M.R.; Rosethorne, E.M. Endogenous lysophosphatidic acid (LPA1) receptor agonists demonstrate ligand bias between calcium and ERK signalling pathways in human lung fibroblasts. Br. J. Pharmacol. 2017, 174, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Takemasa, A.; Ishii, Y.; Fukuda, T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur. Respir. J. 2012, 40, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbel, M.; Belleguic, C.; Boichot, E.; Lagente, V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol. Toxicol. 2002, 18, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; Lopes-Pacheco, M.; De Castro, L.L.; Kitoko, J.; Trivelin, S.A.; Amorim, N.R.; Capelozzi, V.L.; Morales, M.M.; Gutfilen, B.; De Souza, S.A.L.; et al. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Pan, X.; Rui, Y.; Li, F. Matrine attenuates heterotopic ossification by suppressing TGF-β induced mesenchymal stromal cell migration and osteogenic differentiation. Biomed. Pharmacother. 2020, 127, 110152. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dou, Y.-N.; Zhao, Q.-W.; Zhang, J.; Yang, Y.; Wang, T.; Xia, Y.-F.; Dai, Y.; Wei, Z.-F. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 2016, 37, 794–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lu, W.; Zhang, X.; Lu, J.; Xu, S.; Chen, S.; Zhong, Z.; Zhou, T.; Wang, Q.; Chen, J.; et al. Cryptotanshinone protects against pulmonary fibrosis through inhibiting Smad and STAT3 signaling pathways. Pharmacol. Res. 2019, 147, 104307. [Google Scholar] [CrossRef]
- Cheng, C.; Yi, J.; Wang, R.; Cheng, L.; Wang, Z.; Lu, W. Protection of Spleen Tissue of γ-ray Irradiated Mice against Immunosuppressive and Oxidative Effects of Radiation by Adenosine 5′-Monophosphate. Int. J. Mol. Sci. 2018, 19, 1273. [Google Scholar] [CrossRef] [Green Version]
- Meshkibaf, M.H.; Maleknia, M.; Noroozi, S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund’s adjuvant inflammation-induced male rats. J. Inflamm. Res. 2019, 12, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, S.; Sadeghi, M.A.; Yousefi-Manesh, H.; Eslamiyeh, M.; Vafaei, A.; Foroutani, L.; Donyadideh, G.; Dehpour, A.; Rezaei, N. Protective Effects of Leukadherin1 in a Rat Model of Targeted Experimental Autoimmune Encephalomyelitis (EAE): Possible Role of P47phox and MDA Downregulation. J. Inflamm. Res. 2020, 13, 411–420. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Yanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Fan, H. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol. Ther. 2018, 26, 1375–1384. [Google Scholar] [CrossRef] [Green Version]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71, 112–127. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sironi, M.; Vecchi, A.; Colotta, F.; Mantovani, A.; Locati, M. IL-8 induces a specific transcriptional profile in human neutrophils: Synergism with LPS for IL-1 production. Eur. J. Immunol. 2004, 34, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Schnyder-Candrian, S.; Quesniaux, V.F.J.; Di Padova, F.; Maillet, I.; Noulin, N.; Couillin, I.; Moser, R.; Erard, F.; Vargaftig, B.B.; Ryffel, B.; et al. Dual Effects of p38 MAPK on TNF-Dependent Bronchoconstriction and TNF-Independent Neutrophil Recruitment in Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome. J. Immunol. 2005, 175, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Drummond, R.A.; Swamydas, M.; Oikonomou, V.; Zhai, B.; Dambuza, I.M.; Schaefer, B.C.; Bohrer, A.C.; Mayer-Barber, K.D.; Lira, S.A.; Iwakura, Y.; et al. CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat. Immunol. 2019, 20, 559–570. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Myllärniemi, M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid. Redox Signal. 2008, 10, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, M.; Zhang, H.; Ni, W.; Pierce, R.W.; Zhou, H.J.; Min, W. BMX Represses Thrombin-PAR1–Mediated Endothelial Permeability and Vascular Leakage During Early Sepsis. Circ. Res. 2020, 126, 471–485. [Google Scholar] [CrossRef] [PubMed]
| Group | Con | Bl | Bl + MSC | p |
|---|---|---|---|---|
| ALT (U/L) | 33.82 ± 8.87 | 124.33 ± 23.78 * | 84.27 ± 15.48 # | <0.05 |
| AST (U/L) | 119.64 ± 14.96 | 180.67 ± 26.45 * | 130.25 ± 25.31 # | <0.05 |
| Cre (μmol/L) | 55.91 ± 8.98 | 214.21 ± 10.35 * | 134.35 ± 15.49 # | <0.05 |
| BUN (mmol/L) | 7.99 ± 1.14 | 13.56 ± 2.31 * | 10.59 ± 2.16 # | <0.05 |
| Group | Con | Bl | Bl + MSC | p |
|---|---|---|---|---|
| Total cells | 1.25 ± 1.86 | 8.28 ± 2.14 * | 6.58 ± 0.94 # | <0.05 |
| Neutrophils | 0.03 ± 0.01 | 7.35 ± 1.23 * | 5.84 ± 2.38 # | <0.05 |
| Lymphocytes | 0.05 ± 0.03 | 0.15 ± 0.14 | 0.15 ± 0.16 | ns |
| Macrophages | 1.23 ± 0.14 | 1.12 ± 0.60 | 0.86 ± 0.60 | ns |
| Eosinophils | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.04 ± 0.05 | ns |
| Group | Con | Bl | Bl + MSC | p |
|---|---|---|---|---|
| IL-1β (ng/mL) | 12.64 ± 2.14 | 706.91 ± 113.55 * | 563.14 ± 116.98 # | <0.05 |
| CXCL-1 (ng/mL) | 24.22 ± 3.15 | 503.77 ± 56.11 * | 348.14 ± 40.33 # | <0.05 |
| IL-8 (ng/mL) | 126.9 ± 14.73 | 513.24 ± 65.21 * | 204.91 ± 21.72 # | <0.05 |
| TNF-α (ng/mL) | 13.38 ± 3.91 | 152.31 ± 28.64 * | 78.67 ± 18.97 # | <0.05 |
| TGF-β (ng/mL) | 21.35 ± 4.86 | 165.59 ± 43.54 * | 98.45 ± 24.68 # | <0.05 |
| MMP-9 (ng/mL) | 7.69 ± 0.62 | 30.11 ± 3.89 * | 18.66 ± 0.21 # | <0.05 |
| IL-10 (ng/mL) | 20.65 ± 2.36 | 12.82 ± 1.65 * | 35.69 ± 4.21 # | <0.05 |
| NE activity (nmol/mL) | 20.33 ± 0.17 | 64.89 ± 0.71 * | 46.55 ± 0.52 # | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, S.; Lai, J.; Guan, W.; Gao, Y. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. Int. J. Mol. Sci. 2022, 23, 99. https://doi.org/10.3390/ijms23010099
Wang X, Zhao S, Lai J, Guan W, Gao Y. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. International Journal of Molecular Sciences. 2022; 23(1):99. https://doi.org/10.3390/ijms23010099
Chicago/Turabian StyleWang, Xishuai, Shiyu Zhao, Junhui Lai, Weijun Guan, and Yang Gao. 2022. "Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice" International Journal of Molecular Sciences 23, no. 1: 99. https://doi.org/10.3390/ijms23010099
APA StyleWang, X., Zhao, S., Lai, J., Guan, W., & Gao, Y. (2022). Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. International Journal of Molecular Sciences, 23(1), 99. https://doi.org/10.3390/ijms23010099





