Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells
Abstract
:1. Introduction
2. Results
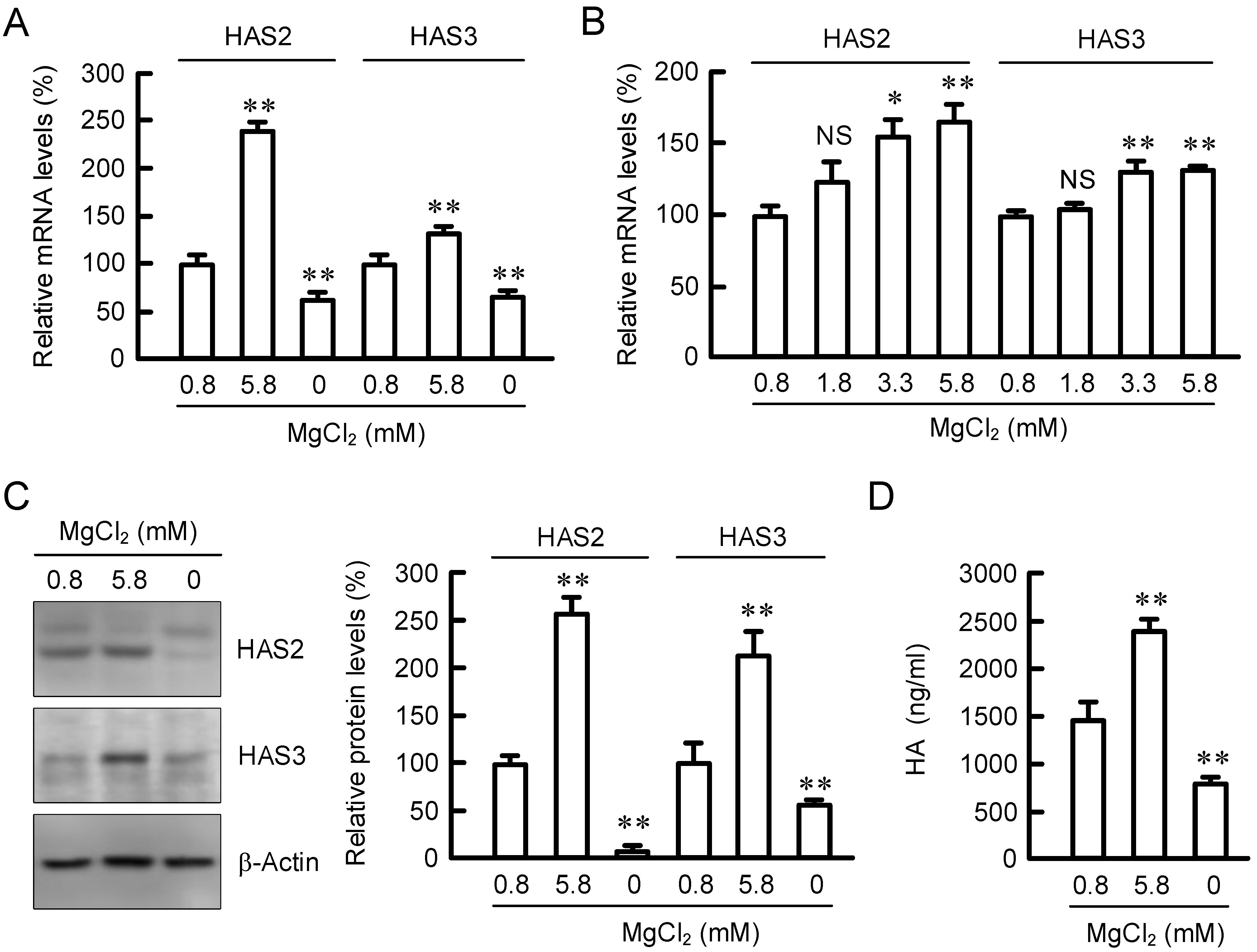
2.1. Effect of Extracellular MgCl2 Concentration on Expression of HAS2/3
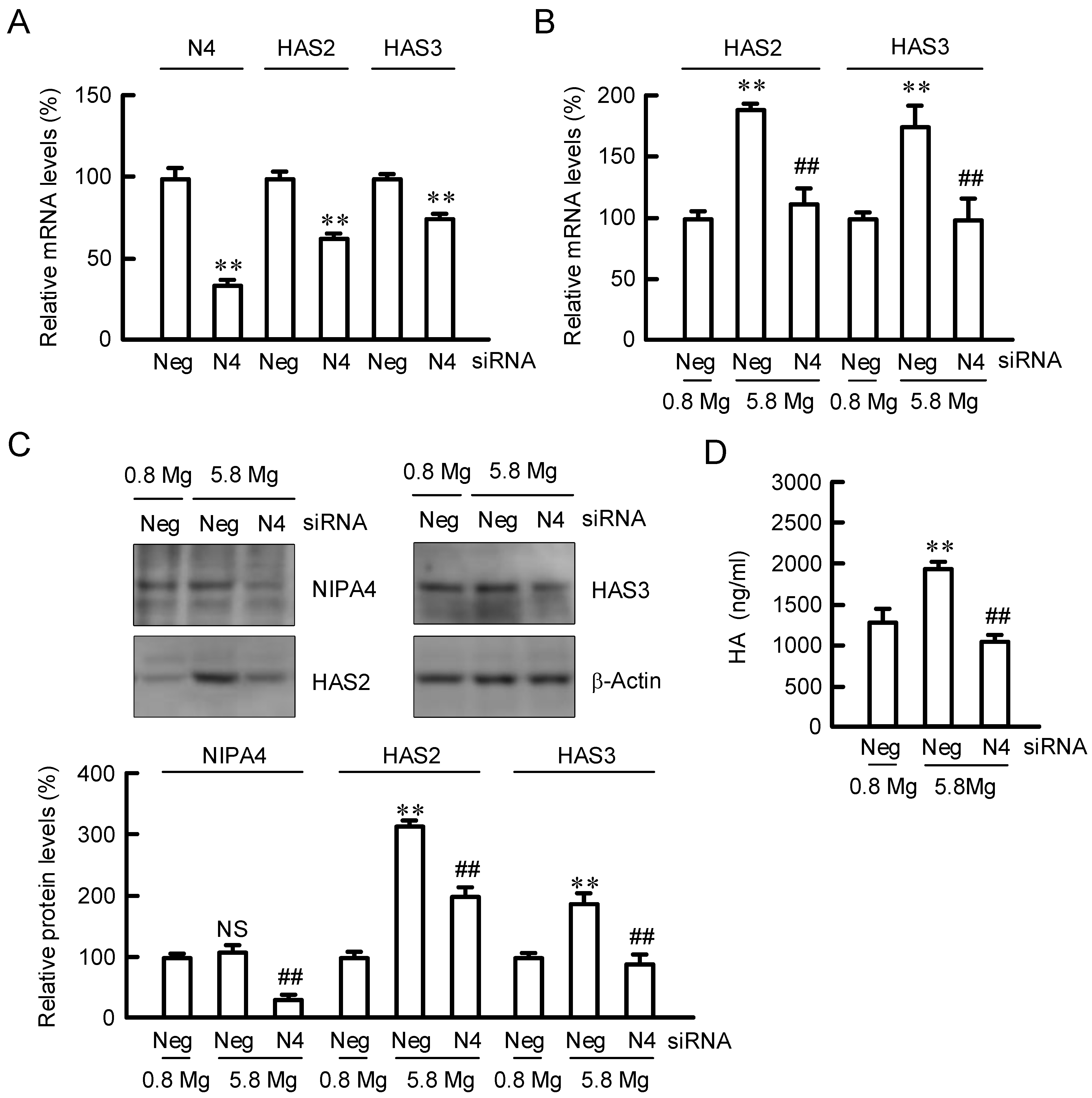
2.2. Effect of NIPAL4 siRNA on the Expressions of HAS2/3
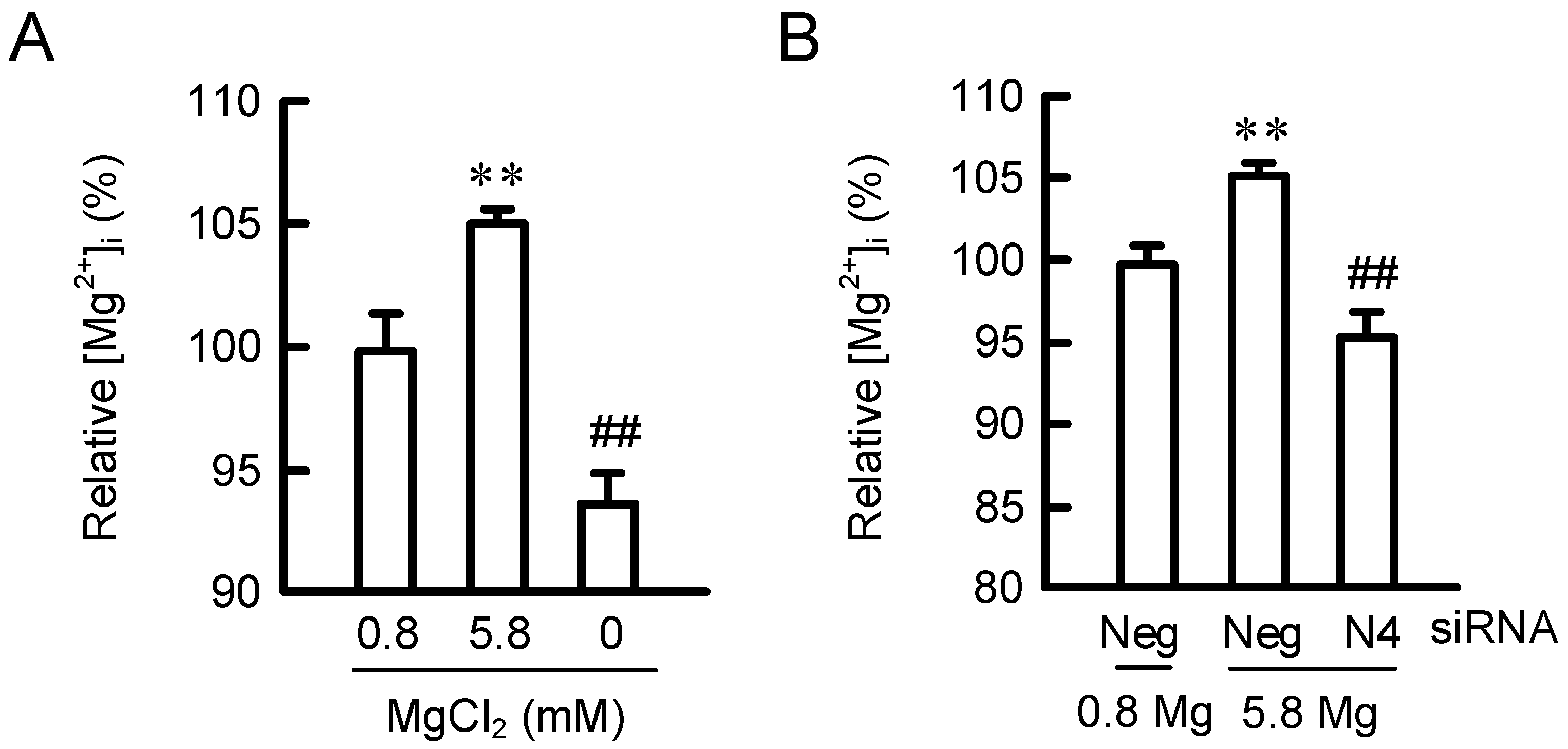
2.3. Effects of MgCl2 Supplementation and NIPAL4 siRNA on [Mg2+]i
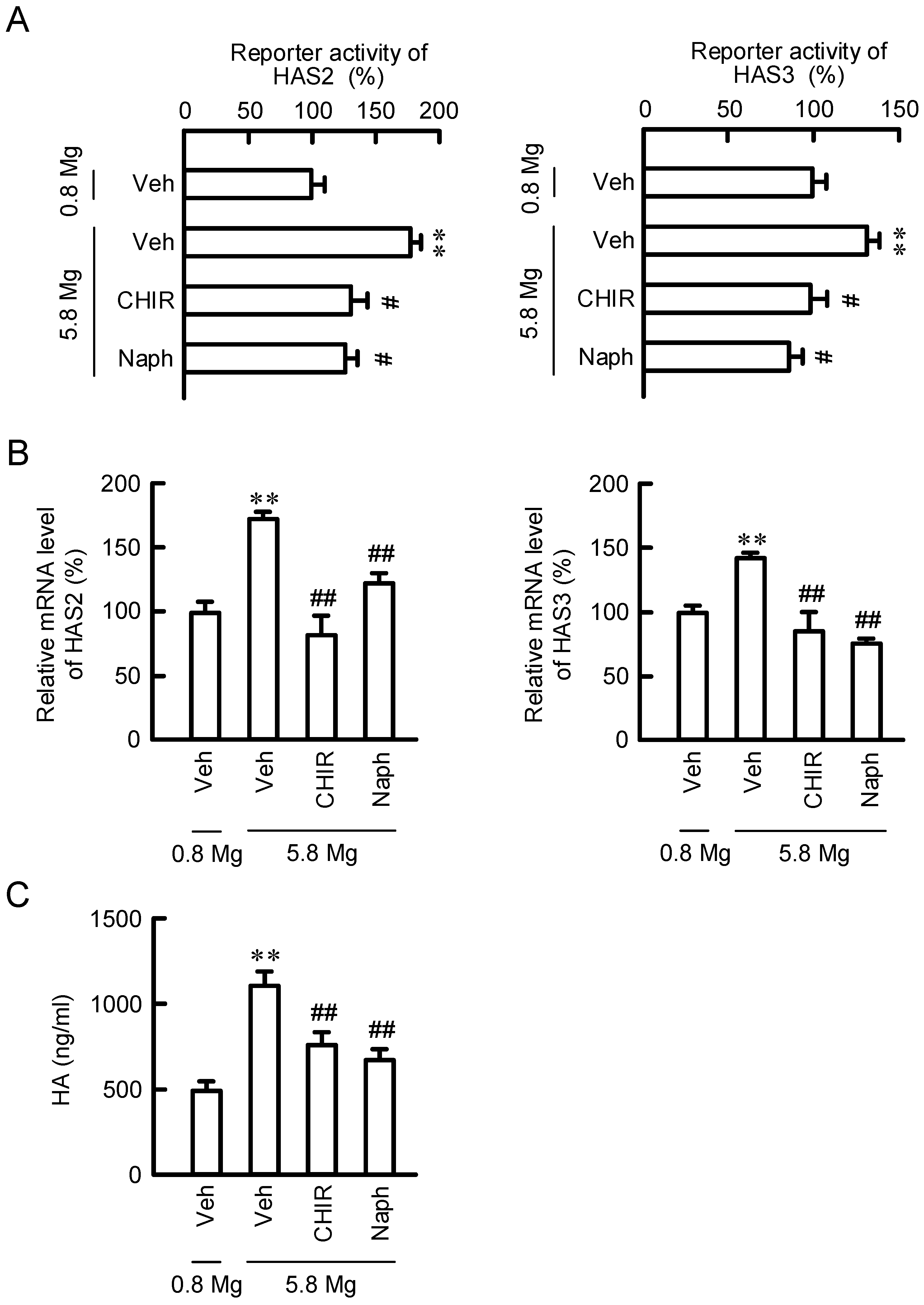
2.4. Effect of MgCl2 Supplementation and NIPAL4 siRNA on Reporter Activities of HAS2/3
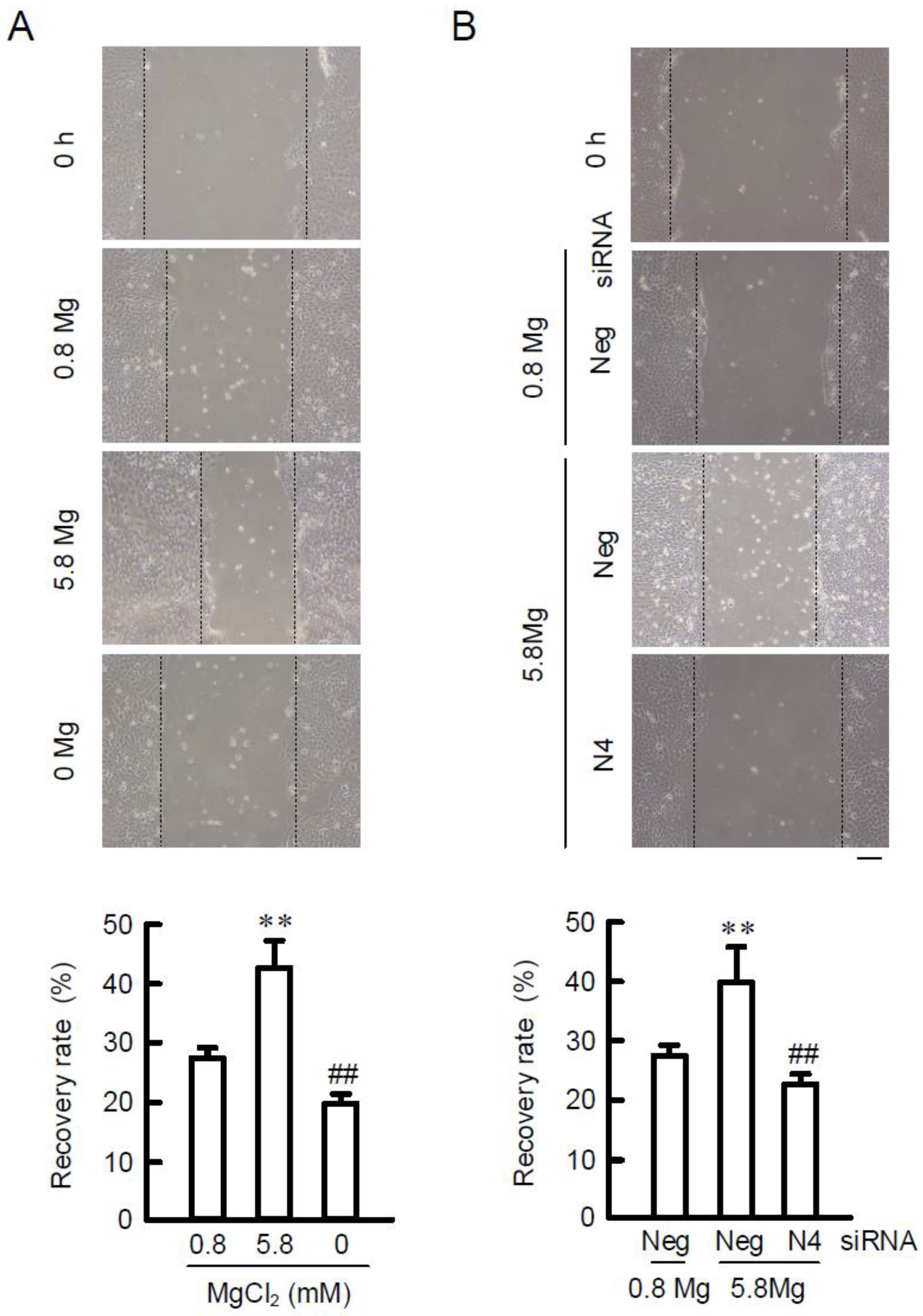
2.5. Effects of MgCl2 Supplementation and NIPAL4 siRNA on Cell Migration
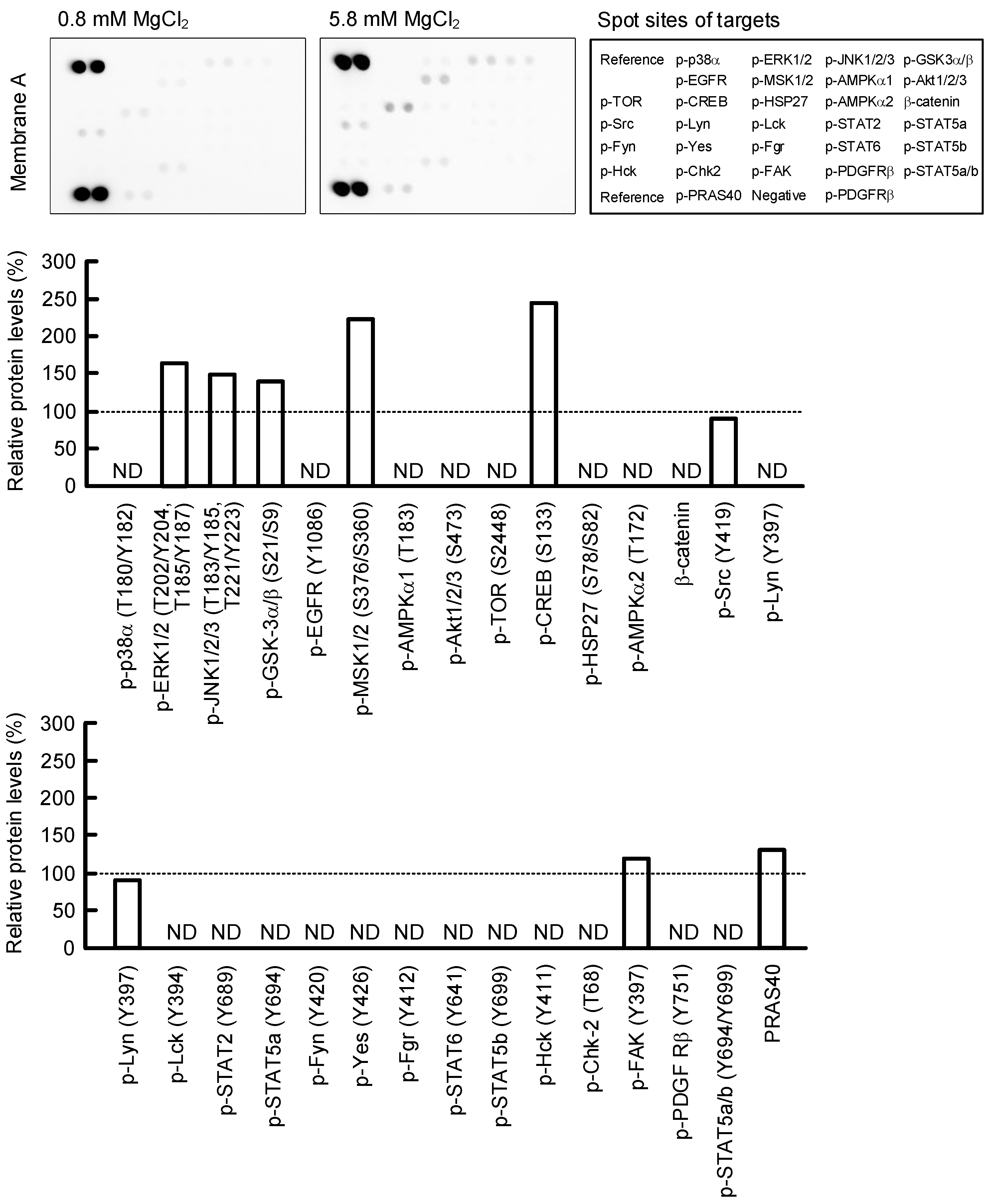
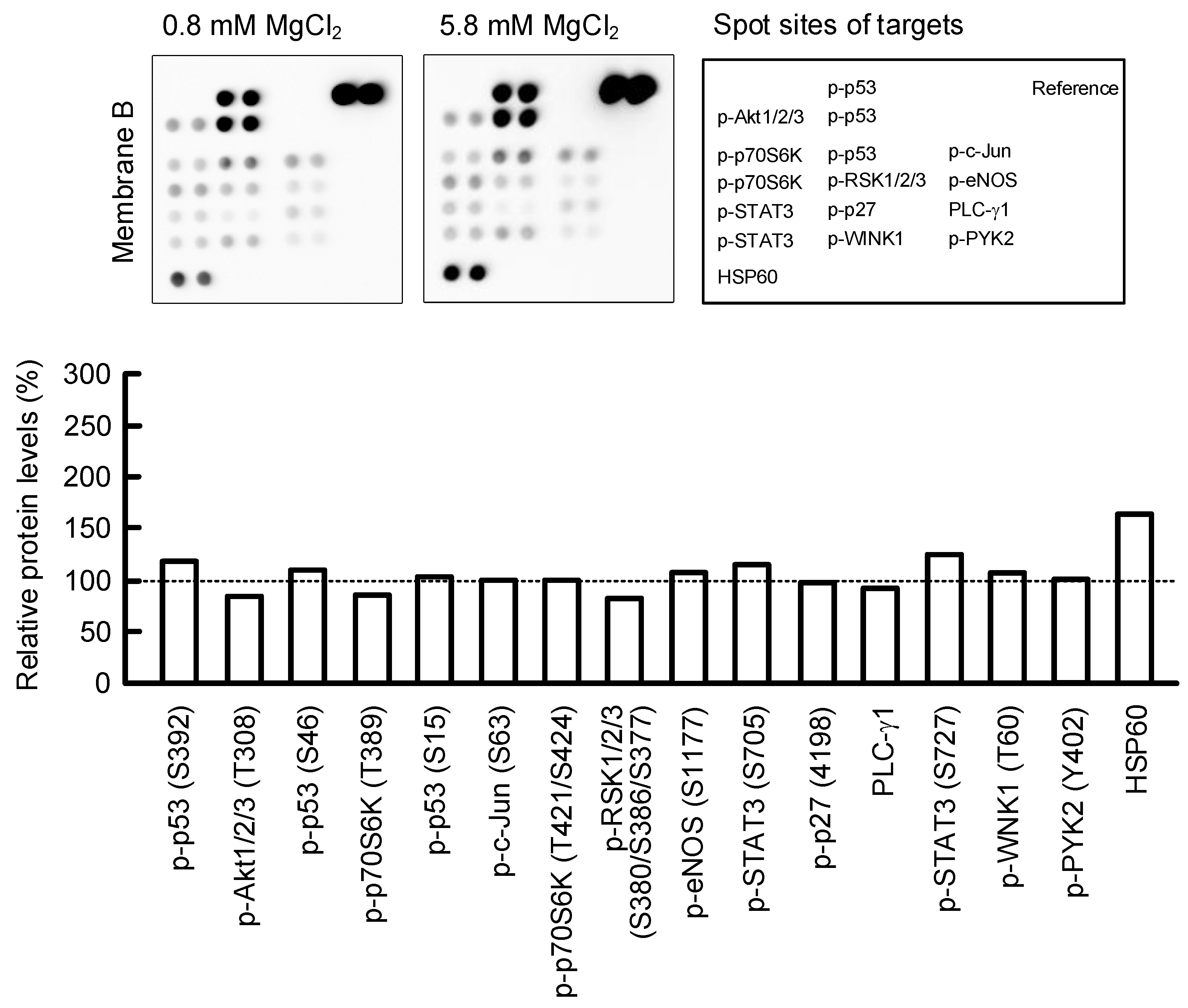
2.6. Effect of MgCl2 Supplementation on Intracellular Signaling Pathways
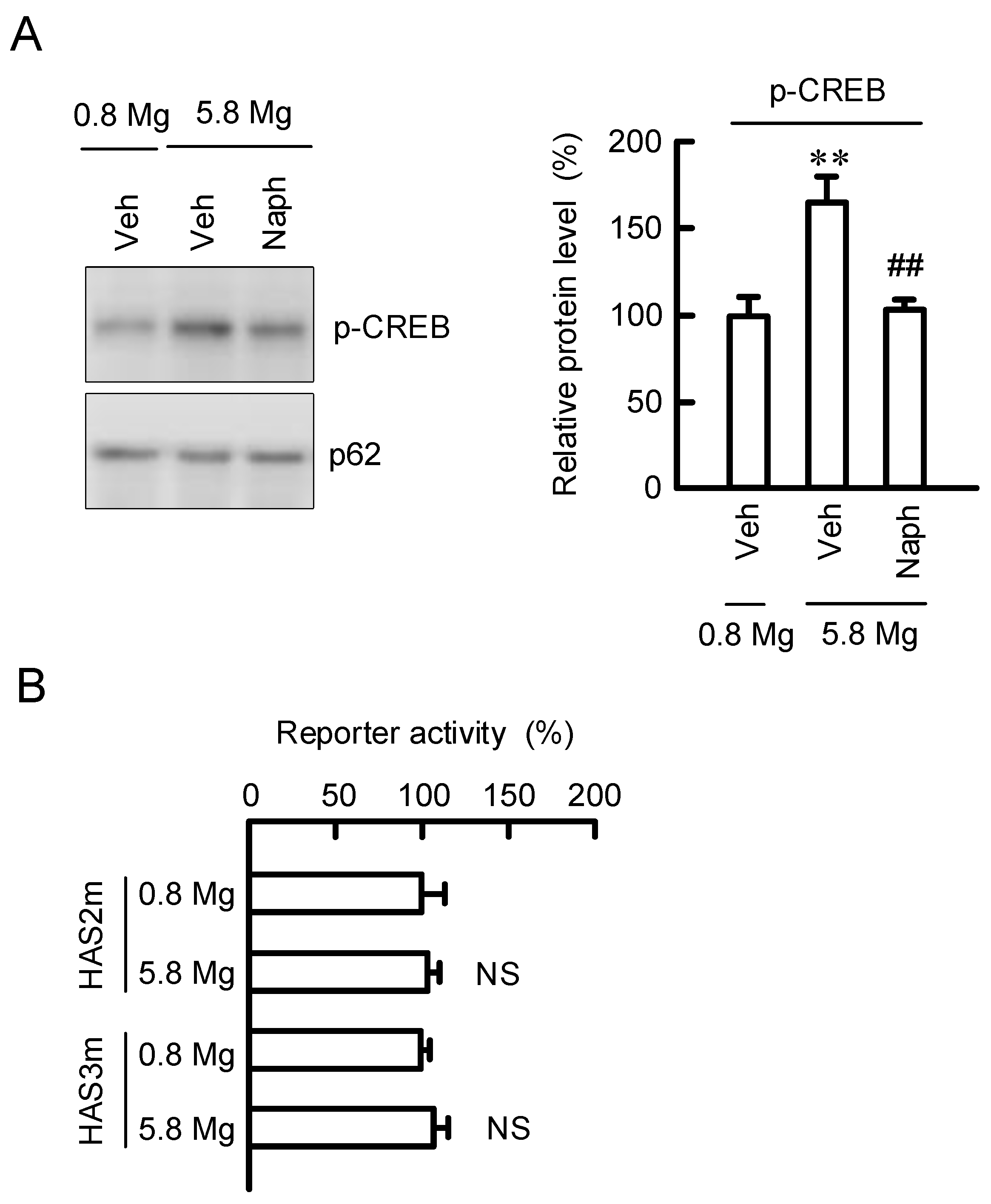
2.7. Regulation of Transcription Activities of HAS2/3 by CREB
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. Transfection of siRNA and Reporter Assay
4.4. Isolation of Total RNA and Quantitative Real-Time PCR
4.5. SDS-Polyacrylamide Gel Electrophoresis and Western Blotting
4.6. Measurement of [Mg2+]i
4.7. Measurement of HA Content
4.8. Wound-Healing Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oji, V.; Tadini, G.; Akiyama, M.; Bardon, C.B.; Bodemer, C.; Bourrat, E.; Coudiere, P.; DiGiovanna, J.J.; Elias, P.; Fischer, J.; et al. Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Soreze 2009. J. Am. Acad. Dermatol. 2010, 63, 607–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wajid, M.; Kurban, M.; Shimomura, Y.; Christiano, A.M. NIPAL4/ichthyin is expressed in the granular layer of human epidermis and mutated in two Pakistani families with autosomal recessive ichthyosis. Dermatology 2010, 220, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Kitamura, T.; Naganuma, T.; Abe, T.; Ohno, Y.; Sassa, T.; Kihara, A. Decreased Skin Barrier Lipid Acylceramide and Differentiation-Dependent Gene Expression in Ichthyosis Gene Nipal4-Knockout Mice. J. Investig. Dermatol. 2018, 138, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar]
- Dabrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salwowska, N.M.; Bebenek, K.A.; Zadlo, D.A.; Wcislo-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pienimaki, J.P.; Rilla, K.; Fulop, C.; Sironen, R.K.; Karvinen, S.; Pasonen, S.; Lammi, M.J.; Tammi, R.; Hascall, V.C.; Tammi, M.I. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J. Biol. Chem. 2001, 276, 20428–20435. [Google Scholar] [CrossRef] [Green Version]
- Heldin, P.; Lin, C.Y.; Kolliopoulos, C.; Chen, Y.H.; Skandalis, S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019, 78–79, 100–117. [Google Scholar] [CrossRef]
- Chen, H.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Kahweol Exerts Skin Moisturizing Activities by Upregulating STAT1 Activity. Int. J. Mol. Sci. 2021, 22, 8864. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, Y.A.; Yu, S.; Park, S.Y.; Kim, K.H.; Kang, N.J. 3,6-Anhydro-L-galactose increases hyaluronic acid production via the EGFR and AMPKalpha signaling pathway in HaCaT keratinocytes. J. Dermatol. Sci. 2019, 96, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.M.; Park, J.; Cho, J.Y. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Jia, T.; Qiao, W.; Gu, H.; Kaku, K. Unsaturated fatty acid-enriched extract from Hippophae rhamnoides seed reduces skin dryness through up-regulating aquaporins 3 and hyaluronan synthetases 2 expressions. J. Cosmet. Dermatol. 2021, 20, 321–329. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruna-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Terazawa, S.; Nakano, M.; Yamamoto, A.; Imokawa, G. Mycosporine-like amino acids stimulate hyaluronan secretion by up-regulating hyaluronan synthase 2 via activation of the p38/MSK1/CREB/c-Fos/AP-1 axis. J. Biol. Chem. 2020, 295, 7274–7288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiol, C.J.; Williams, J.S.; Chou, C.H.; Wang, Q.M.; Roach, P.J.; Andrisani, O.M. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J. Biol. Chem. 1994, 269, 32187–32193. [Google Scholar] [CrossRef]
- Goytain, A.; Hines, R.M.; Quamme, G.A. Functional characterization of NIPA2, a selective Mg2+ transporter. Am. J. Physiol. Cell Physiol. 2008, 295, C944–C953. [Google Scholar] [CrossRef]
- Li, H.; Lorie, E.P.; Fischer, J.; Vahlquist, A.; Torma, H. The expression of epidermal lipoxygenases and transglutaminase-1 is perturbed by NIPAL4 mutations: Indications of a common metabolic pathway essential for skin barrier homeostasis. J. Investig. Dermatol. 2012, 132, 2368–2375. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Maeda-Sano, K.; Gotoh, M.; Morohoshi, T.; Someya, T.; Murofushi, H.; Murakami-Murofushi, K. Cyclic phosphatidic acid and lysophosphatidic acid induce hyaluronic acid synthesis via CREB transcription factor regulation in human skin fibroblasts. Biochim. Biophys. Acta 2014, 1841, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Yang, H.; Qiu, P.; Cong, Z.; Zou, Y.; Song, L.; Guo, J.; Anastassiades, T.P. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int. J. Mol. Sci. 2019, 20, 3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monslow, J.; Govindaraju, P.; Pure, E. Hyaluronan—A functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 2015, 6, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef]
- Chen, J.Q.; Man, X.Y.; Li, W.; Zhou, J.; Landeck, L.; Cai, S.Q.; Zheng, M. Regulation of involucrin in psoriatic epidermal keratinocytes: The roles of ERK1/2 and GSK-3beta. Cell Biochem. Biophys. 2013, 66, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yan, N.; Li, Z.; Hua, Y.; Chen, W. FGF19 sustains the high proliferative ability of keratinocytes in psoriasis through the regulation of Wnt/GSK-3beta/beta-catenin signalling via FGFR4. Clin. Exp. Pharmacol. Physiol. 2019, 46, 761–769. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhang, X.; Feng, C.; Fan, C.; Yang, X.; Zhang, R.; Zhu, F.; Zhou, Y.; Xu, Y.; et al. DC591017, a phosphodiesterase-4 (PDE4) inhibitor with robust anti-inflammation through regulating PKA-CREB signaling. Biochem. Pharmacol. 2020, 177, 113958. [Google Scholar] [CrossRef] [PubMed]
- Blach, J.; Nowacki, W.; Mazur, A. Magnesium in skin allergy. Postepy Hig. Med. Dosw. 2007, 61, 548–554. [Google Scholar]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marunaka, K.; Kobayashi, M.; Shu, S.; Matsunaga, T.; Ikari, A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2019, 20, 3869. [Google Scholar] [CrossRef] [Green Version]

| Name | Direction | Sequence |
|---|---|---|
| HAS2m | Forward | 5′-TAGCTGCAGCTCAGAAACTTTTGAGTT-3′ |
| Reverse | 5′-AGTGTCAAAGCCTTTCTCAT-3′ | |
| HAS3m | Forward | 5′-ATGCCACCGAGGCGGGGCGCCAGCG-3′ |
| Reverse | 5′-GCCGGAGGCGGCGCCCACCAG-3′ |
| Name | Direction | Sequence |
|---|---|---|
| HAS2 | Forward | 5′-CTGGGCTATGCAACAAAATACA-3′ |
| Reverse | 5′-TTCTCGGAAGTAGGACTTGCTC-3′ | |
| HAS3 | Forward | 5′-AGAAGTTCCTAGGCAGCAAGTG-3′ |
| Reverse | 5′-GGAGGTACTTAGTGGGGGTCTC-3′ | |
| NIPAL4 | Forward | 5′-ACATGCTCCTGAGGAAGAGAAG-3′ |
| Reverse | 5′-GCAATGACAAAGATGAGGATGA-3′ | |
| β-Actin | Forward | 5′-CCTGAGGCACTCTTCCAGCCTT-3′ |
| Reverse | 5′-TGCGGATGTCCACGTCACACTTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marunaka, K.; Shu, S.; Kobayashi, M.; Goto, M.; Katsuta, Y.; Yoshino, Y.; Ikari, A. Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2022, 23, 71. https://doi.org/10.3390/ijms23010071
Marunaka K, Shu S, Kobayashi M, Goto M, Katsuta Y, Yoshino Y, Ikari A. Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells. International Journal of Molecular Sciences. 2022; 23(1):71. https://doi.org/10.3390/ijms23010071
Chicago/Turabian StyleMarunaka, Kana, Shokoku Shu, Mao Kobayashi, Makiko Goto, Yuji Katsuta, Yuta Yoshino, and Akira Ikari. 2022. "Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells" International Journal of Molecular Sciences 23, no. 1: 71. https://doi.org/10.3390/ijms23010071
APA StyleMarunaka, K., Shu, S., Kobayashi, M., Goto, M., Katsuta, Y., Yoshino, Y., & Ikari, A. (2022). Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells. International Journal of Molecular Sciences, 23(1), 71. https://doi.org/10.3390/ijms23010071






