Galectins in Early Pregnancy and Pregnancy-Associated Pathologies
Abstract
1. Introduction
2. Galectin Expression Pattern in Placenta
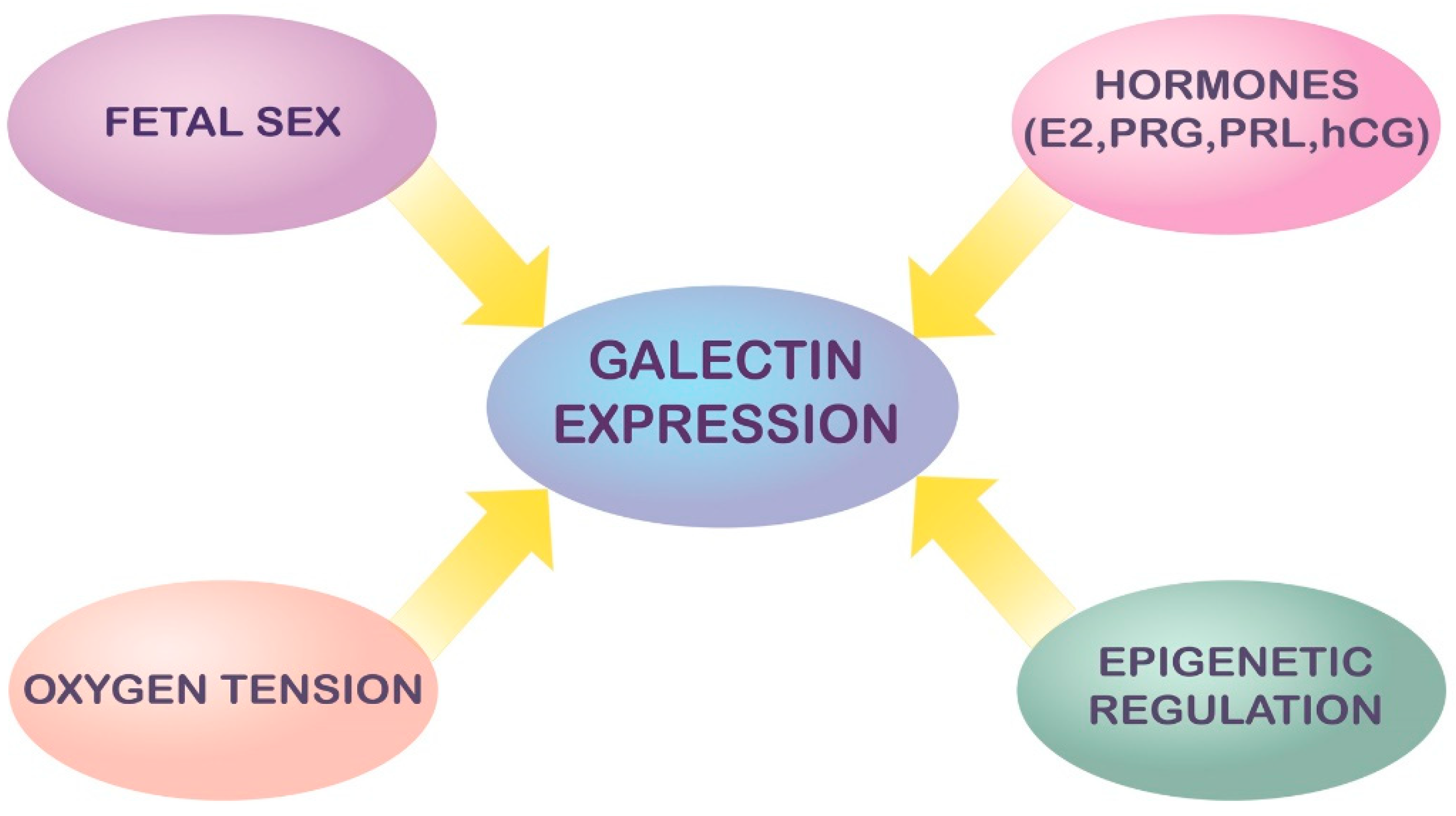
3. Modulation and Regulation of Galectin Expression in Pregnancy
4. Galectins and Human Trophoblast Cell Function
5. Galectins in Pregnancy-Related Disorders
5.1. Galectins in Pregnancy Loss
5.2. Galectins and GDM
5.3. Galectins in Inflammation/Infection in Pregnancy
5.4. Galectins in PE
5.5. Galectins and IUGR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Blois, S.M.; Conrad, M.L.; Freitag, N.; Barrientos, G. Galectins in angiogenesis: Consequences for gestation. J. Reprod. Immunol. 2015, 108, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Ilarregui, J.M.; Tometten, M.; Garcia, M.; Orsal, A.S.; Cordo-Russo, R.; Toscano, M.A.; Bianco, G.A.; Kobelt, P.; Handjiski, B.; et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007, 13, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Kim, C.J.; McGowen, M.R.; Papp, Z.; Wildman, D.E. Galectins: Guardians of eutherian pregnancy at the maternal–fetal interface. Trends Endocrinol. Metab. 2012, 23, 23–31. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Toth, E.; Romero, R.; Parej, K.; Csala, D.; Szenasi, N.L.; Hajdu, I.; Juhasz, K.; Kovacs, A.F.; Meiri, H.; et al. Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response. Front. Immunol. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Liu, F. Intracellular functions of galectins. Biochim. Biophys. Acta-Gen. Subj. 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Blois, S.M.; Dveksler, G.; Vasta, G.R.; Freitag, N.; Blanchard, V.; Barrientos, G. Pregnancy Galectinology: Insights into a Complex Network of Glycan Binding Proteins. Front. Immunol. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [PubMed]
- Bevan, B.H.; Kilpatrick, D.C.; Liston, W.A.; Hirabayashi, J.; Kasai, K. Immunohistochemical localization of a beta-D-galactoside-binding lectin at the human maternofetal interface. Histochem. J. 1994, 26, 582–586. [Google Scholar] [CrossRef]
- Vicovac, L.; Jankovic, M.; Cuperlovic, M. Galectin-1 and -3 in cells of the first trimester placental bed. Hum. Reprod. 1998, 13, 730–735. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Wang, X.; Gabius, H.-J.; Strowitzki, T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol. Hum. Reprod. 2004, 11, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Maquoi, E.; van den Brûle, F.; Castronovo, V.; Foidart, J. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 1997, 18, 433–439. [Google Scholar] [CrossRef]
- Jeschke, U.; Hutter, S.; Heublein, S.; Vrekoussis, T.; Andergassen, U.; Unverdorben, L.; Papadakis, G.; Makrigiannakis, A. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 2013, 34, 863–872. [Google Scholar] [CrossRef]
- Hutter, S.; Knabl, J.; Andergassen, U.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Placental Expression Patterns of Galectin-1, Galectin-2, Galectin-3 and Galectin-13 in Cases of Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2016, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Menkhorst, E.; Koga, K.; Van Sinderen, M.; Dimitriadis, E. Galectin-7 serum levels are altered prior to the onset of pre-eclampsia. Placenta 2014, 35, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Kolundžić, N.; Bojić-Trbojević, Ž.; Radojčić, L.; Petronijević, M.; Vićovac, L. Galectin-8 is expressed by villous and extravillous trophoblast of the human placenta. Placenta 2011, 32, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Heusschen, R.; Freitag, N.; Tirado-González, I.; Barrientos, G.; Moschansky, P.; Muñoz-Fernández, R.; Leno-Durán, E.; Klapp, B.F.; Thijssen, V.L.J.L.; Blois, S.M. Profiling Lgals9 Splice Variant Expression at the Fetal-Maternal Interface: Implications in Normal and Pathological Human Pregnancy1. Biol. Reprod. 2013, 88, 22. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Jeschke, U.; Santoso, L.; Hofmann, S.; Kuhn, C.; Arck, P.; Hutter, S. Comparative analyses on expression of galectins1-4, 7-10 and 12 in first trimester placenta, decidua and isolated trophoblast cells in vitro. Histol. Histopathol. 2016, 31, 1095–1111. [Google Scholar] [CrossRef]
- Kliman, H.J.; Sammar, M.; Grimpel, Y.I.; Lynch, S.K.; Milano, K.M.; Pick, E.; Bejar, J.; Arad, A.; Lee, J.J.; Meiri, H.; et al. Placental Protein 13 and Decidual Zones of Necrosis: An Immunologic Diversion That May be Linked to Preeclampsia. Reprod. Sci. 2012, 19, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Pick, E.; Bellyei, S.; Szigeti, A.; Burger, O.; Berente, Z.; Janaky, T.; Boronkai, A.; Kliman, H.; Meiri, H.; et al. Functional analyses of placental protein13/galectin-13. Eur. J. Biochem. 2004, 271, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Chiariotti, L.; Salvatore, P.; Frunzio, R.; Bruni, C.B. Galectin genes: Regulation of expression. Glycoconj. J. 2002, 19, 441–449. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Erez, O.; Weckle, A.; Tarca, A.L.; Hotra, J.; Abbas, A.; Han, Y.M.; Kim, S.-S.; Kusanovic, J.P.; et al. Emergence of hormonal and redox regulation of galectin-1 in placental mammals: Implication in maternal-fetal immune tolerance. Proc. Natl. Acad. Sci. USA 2008, 105, 15819–15824. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.E.; Muench, K.L.; Robinson, B.G.; Wang, A.; Palmer, T.D.; Winn, V.D. Examining Sex Differences in the Human Placental Transcriptome during the First Fetal Androgen Peak. Reprod. Sci. 2021, 28, 801–818. [Google Scholar] [CrossRef]
- Hutter, S.; Knabl, J.; Andergassen, U.; Mayr, D.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Fetal gender specific expression of tandem-repeat galectins in placental tissue from normally progressed human pregnancies and intrauterine growth restriction (IUGR). Placenta 2015, 36, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Gitt, M.A.; Barondes, S.H. Genomic sequence and organization of two members of a human lectin gene family. Biochemistry 1991, 30, 82–89. [Google Scholar] [CrossRef]
- Salvatore, P.; Contursi, C.; Benvenuto, G.; Bruni, C.B.; Chiariotti, L. Characterization and functional dissection of the galactin-1 gene promoter. FEBS Lett. 1995, 373, 159–163. [Google Scholar] [CrossRef][Green Version]
- Choe, Y.S.; Shim, C.; Choi, D.; Lee, C.S.; Lee, K.-K.; Kim, K. Expression of galectin-1 mRNA in the mouse uterus is under the control of ovarian steroids during blastocyst implantation. Mol. Reprod. Dev. 1997, 48, 261–266. [Google Scholar] [CrossRef]
- Bojic-Trbojevic, Z.; Bozic, M.; Vicovac, L. Steroid hormones modulate galectin-1 in the trophoblast HTR-8/SVneocell line. Arch. Biol. Sci. 2008, 60, 11–23. [Google Scholar] [CrossRef]
- Cujic, D.; Bojic-Trbojevic, Z.; Tosic, N.; Pavlovic, S.; Vicovac, L. Effect of steroids on transcription and secretion of Gal-1 by the human trophoblast cell line in vitro. Arch. Biol. Sci. 2013, 65, 1331–1337. [Google Scholar] [CrossRef]
- Stefanoska, I.; Jovanović Krivokuća, M.; Vasilijić, S.; Ćujić, D.; Vićovac, L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 2013, 34, 775–783. [Google Scholar] [CrossRef]
- Yang, H.; Taylor, H.S.; Lei, C.; Cheng, C.; Zhang, W. Hormonal regulation of galectin 3 in trophoblasts and its effects on endometrium. Reprod. Sci. 2011, 18, 1118–1127. [Google Scholar] [CrossRef]
- Yang, H.; Lei, C.; Cheng, C.; Feng, Y.; Zhang, W.; Petracco, R.G.; Sak, S. The Antiapoptotic Effect of Galectin-3 in Human Endometrial Cells under the Regulation of Estrogen and Progesterone1. Biol. Reprod. 2012, 87, 39. [Google Scholar] [CrossRef]
- Yang, H.; Lei, C.X.; Zhang, W. Human chorionic gonadotropin (hCG) regulation of galectin-3 expression in endometrial epithelial cells and endometrial stromal cells. Acta Histochem. 2013, 115, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yin, J.; Ficarrotta, K.; Hsu, S.H.; Zhang, W.; Cheng, C. Aberrant expression and hormonal regulation of Galectin-3 in endometriosis women with infertility. J. Endocrinol. Investig. 2016, 39, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Ramhorst, R.E.; Giribaldi, L.; Fraccaroli, L.; Toscano, M.A.; Stupirski, J.C.; Romero, M.D.; Durand, E.S.; Rubinstein, N.; Blaschitz, A.; Sedlmayr, P.; et al. Galectin-1 confers immune privilege to human trophoblast: Implications in recurrent fetal loss. Glycobiology 2012, 22, 1374–1386. [Google Scholar] [CrossRef]
- Inagaki, Y.; Sohma, Y.; Horie, H.; Nozawa, R.; Kadoya, T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur. J. Biochem. 2000, 267, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, T.; Horie, H. Structural and Functional Studies of Galectin-1: A Novel Axonal Regeneration-Promoting Activity for Oxidized Galectin-1. Curr. Drug Targets 2005, 6, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in innate immunity: Dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Kolundžić, N.; Bojić-Trbojević, Ž.; Kovačević, T.; Stefanoska, I.; Kadoya, T.; Vićovac, L. Galectin-1 Is Part of Human Trophoblast Invasion Machinery—A Functional Study In Vitro. PLoS ONE 2011, 6, e28514. [Google Scholar] [CrossRef]
- Kolundžić, N.; Ćujić, D.; Abu Rabi, T.; Bojić-Trbojević, Ž.; Kadoya, T.; Vićovac, L. Galectin signature of the choriocarcinoma JAr cells: Galectin-1 as a modulator of invasiveness in vitro. Mol. Reprod. Dev. 2015, 82, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Karsten, U.; Wiest, I.; Schulze, S.; Kuhn, C.; Friese, K.; Walzel, H. Binding of galectin-1 (gal-1) to the Thomsen-Friedenreich (TF) antigen on trophoblast cells and inhibition of proliferation of trophoblast tumor cells in vitro by gal-1 or an anti-TF antibody. Histochem. Cell Biol. 2006, 126, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Redel, S.; Hofmann, S.; Kuhn, C.; Friese, K.; Walzel, H.; Jeschke, U. Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta 2010, 31, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Morales-Prieto, D.M.; Andergassen, U.; Tschakert, L.; Kuhn, C.; Hofmann, S.; Markert, U.R.; Jeschke, U. Gal-1 silenced trophoblast tumor cells (BeWo) show decreased syncytium formation and different miRNA production compared to non-target silenced BeWo cells. Cell Adh. Migr. 2016, 10, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Reimer, T.; Bergemann, C.; Wiest, I.; Schulze, S.; Friese, K.; Walzel, H. Binding of galectin-1 (gal-1) on trophoblast cells and inhibition of hormone production of trophoblast tumor cells in vitro by gal-1. Histochem. Cell Biol. 2004, 121, 501–508. [Google Scholar] [CrossRef]
- Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Kolundžić, N.; Petronijević, M.; Vrzić-Petronijević, S.; Golubović, S.; Vićovac, L. Galectin-1 binds mucin in human trophoblast. Histochem. Cell Biol. 2014, 142, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Bojić-Trbojević, Z.; Jovanović Krivokuća, M.; Stefanoska, I.; Kolundzić, N.; Vilotić, A.; Kadoya, T.; Vićovac, L. Integrin β1 is bound to galectin-1 in human trophoblast. J. Biochem. 2018, 163, 39–50. [Google Scholar] [CrossRef]
- Zhou, Q.; Cummings, R. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch Biochem Biophys 1993, 300, 6–17. [Google Scholar] [PubMed]
- Ozeki, Y.; Matsui, T.; Yamamoto, Y.; Funahashi, M.; Hamako, J.; Titani, K. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology 1995, 5, 255–261. [Google Scholar] [CrossRef]
- Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Vilotić, A.; Kolundžić, N.; Stefanoska, I.; Zetterberg, F.; Nilsson, U.J.; Leffler, H.; Vićovac, L. Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep. 2019, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Cao, Z.; Leffler, H.; Nilsson, U.J.; Panjwani, N. Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis. Investig. Opthalmol. Vis. Sci. 2017, 58, 9. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Tirado-González, I.; Barrientos, G.; Cohen, M.; Daher, S.; Goldman-Wohl, D.; Mincheva-Nilsson, L.; John, C.M.; Jeschke, U.; Blois, S.M. The chimera-type galectin-3 is a positive modulator of trophoblast functions with dysregulated expression in gestational diabetes mellitus. Am. J. Reprod. Immunol. 2020, 84, e13311. [Google Scholar] [CrossRef]
- Hu, R.; Jin, H.; Zhou, S.; Yang, P.; Li, X. Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta 2007, 28, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Menkhorst, E.M.; Gamage, T.; Cuman, C.; Kaitu’U-Lino, T.J.; Tong, S.; Dimitriadis, E. Galectin-7 acts as an adhesion molecule during implantation and increased expression is associated with miscarriage. Placenta 2014, 35, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Menkhorst, E.; Zhou, W.; Santos, L.L.; Delforce, S.; So, T.; Rainczuk, K.; Loke, H.; Syngelaki, A.; Varshney, S.; Williamson, N.; et al. Galectin-7 Impairs Placentation and Causes Preeclampsia Features in Mice. Hypertension 2020, 76, 1185–1194. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Q.; Wang, Y.; Wang, L.; Li, Z.; Mor, G.; Liao, A. Newly characterized decidual Tim-3+ Treg cells are abundant during early pregnancy and driven by IL-27 coordinately with Gal-9 from trophoblasts. Hum. Reprod. 2020, 35, 2454–2466. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhou, W.-H.; Tao, Y.; Wang, S.-C.; Jiang, Y.-L.; Zhang, D.; Piao, H.-L.; Fu, Q.; Li, D.-J.; Du, M.-R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal–fetal interface in early pregnancy. Cell. Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Ban, Y.; Gao, W.; Song, B.; Wang, Y.; Zhang, Y.; Shao, Q.; Kong, B.; Qu, X. Tim-3 Is Upregulated in NK Cells during Early Pregnancy and Inhibits NK Cytotoxicity toward Trophoblast in Galectin-9 Dependent Pathway. PLoS ONE 2016, 11, e0147186. [Google Scholar] [CrossRef]
- Li, M.; Peng, X.; Qian, J.; Sun, F.; Chen, C.; Wang, S.; Zhang, J.; Du, M. Galectin-9 regulates HTR8/SVneo function via JNK signaling. Reproduction 2021, 161, 1–10. [Google Scholar] [CrossRef]
- Than, N.G.; Balogh, A.; Romero, R.; Kárpáti, É.; Erez, O.; Szilágyi, A.; Kovalszky, I.; Sammar, M.; Gizurarson, S.; Matkó, J.; et al. Placental Protein 13 (PP13)-A Placental Immunoregulatory Galectin Protecting Pregnancy. Front. Immunol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Vokalova, L.; Balogh, A.; Toth, E.; Van Breda, S.V.; Schäfer, G.; Hoesli, I.; Lapaire, O.; Hahn, S.; Than, N.G.; Rossi, S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Wang, P.; Xu, Y.; Jin, P.; Wu, Z.; Qian, Y.; Bai, L.; Dong, M. Galectin-14 Promotes Trophoblast Migration and Invasion by Upregulating the Expression of MMP-9 and N-Cadherin. Front. Cell Dev. Biol. 2021, 9, 645658. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-X.; Jin, F.; Zhang, W.-W.; Zhou, T.-H.; Zhou, C.-Y.; Yao, W.-M.; Qian, Y.-L.; Huang, H.-F. Proteomic Analysis on the Alteration of Protein Expression in the Placental Villous Tissue of Early Pregnancy Loss. Biol. Reprod. 2006, 75, 414–420. [Google Scholar] [CrossRef]
- Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. Int. J. Mol. Sci. 2016, 17, 644. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Powell, K.L.; Boehm-Sturm, P.; Koch, S.P.; Hecher, K.; Staff, A.C.; Arck, P.C.; Diemert, A.; et al. Galectin-3 deficiency in pregnancy increases the risk of fetal growth restriction (FGR) via placental insufficiency. Cell Death Dis. 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zeng, F.; Tang, G.; Lei, C.; Zou, X.; Liu, X.; Peng, B.; Qin, S.; Li, H. Expression of galectin-3 and apoptosis in placental villi from patients with missed abortion during early pregnancy. Exp. Ther. Med. 2019, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fang, A. Expression and Influence of Galectin-3 on Missed Abortion. J. Reprod. Contracept. 2014, 25, 227–234. [Google Scholar] [CrossRef]
- Blois, S.M.; Gueuvoghlanian-Silva, B.Y.; Tirado-Gonzalez, I.; Torloni, M.R.; Freitag, N.; Mattar, R.; Conrad, M.L.; Unverdorben, L.; Barrientos, G.; Knabl, J.; et al. Getting too sweet: Galectin-1 dysregulation in gestational diabetes mellitus. Mol. Hum. Reprod. 2014, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Hepp, P.; Unverdorben, L.; Hutter, S.; Kuhn, C.; Ditsch, N.; Groß, E.; Mahner, S.; Jeschke, U.; Knabl, J.; Heidegger, H.H. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. Int. J. Mol. Sci. 2020, 21, 2404. [Google Scholar] [CrossRef] [PubMed]
- Talmor-Barkan, Y.; Chezar-Azerrad, C.; Kruchin, B.; Leshem-Lev, D.; Levi, A.; Hadar, E.; Kornowski, R.; Tenenbaum-Gavish, K.; Porter, A. Elevated galectin-3 in women with gestational diabetes mellitus, a new surrogate for cardiovascular disease in women. PLoS ONE 2020, 15, e0234732. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, X.; Guo, Y.; Zhang, J.; Xie, J.; Shao, S.; Xiang, Y.; Chen, G.; Yu, X. Association of circulating galectin-3 with gestational diabetes mellitus, progesterone, and insulin resistance. J. Diabetes 2021, 13, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Heusler, I.; Biron-Shental, T.; Farladansky-Gershnabel, S.; Pasternak, Y.; Kidron, D.; Vulih-Shuitsman, I.; Einbinder, Y.; Cohen-Hagai, K.; Benchetrit, S.; Zitman-Gal, T. Enhanced expression of Galectin-3 in gestational diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Boutsikou, T.; Giotaki, M.; Boutsikou, M.; Briana, D.D.; Baka, S.; Piatopoulou, D.; Hassiakos, D.; Gourgiotis, D.; Malamitsi-Puchner, A. Cord blood galectin-1 and -3 concentrations in term pregnancies with normal restricted and increased fetal growth. J. Perinat. Med. 2015, 43, 305–309. [Google Scholar] [CrossRef]
- Unverdorben, L.; Hüttenbrenner, R.; Knabl, J.; Jeschke, U.; Hutter, S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta 2015, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Han, X.; Meng, Q.; Luo, Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. J. Diabetes Investig. 2018, 9, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Erez, O.; Wildman, D.E.; Tarca, A.L.; Edwin, S.S.; Abbas, A.; Hotra, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.S.; et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J. Matern. Neonatal Med. 2008, 21, 429–442. [Google Scholar] [CrossRef]
- Stefanoska, I.; Tadić, J.; Vilotić, A.; Jovanović Krivokuća, M.; Abu Rabi, T.; Vićovac, L. Histological chorioamnionitis in preterm prelabor rupture of the membranes is associated with increased expression of galectin-3 by amniotic epithelium. J. Matern. Neonatal Med. 2017, 30, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal Infection as a Possible Risk Factor for Preterm Low Birth Weight. J. Periodontol. 1996, 67 (Suppl. 10), 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Reyes, L.; von Deneen, K.; Reinhard, M.K.; Progulske-Fox, A.; Brown, M.B. Colonization of maternal and fetal tissues by Porphyromonas gingivalis is strain-dependent in a rodent animal model. Am. J. Obstet. Gynecol. 2008, 199, 86.e1–86.e7. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. TIM3: Dual role in immunity. Nat. Rev. Immunol. 2008, 8, 4. [Google Scholar] [CrossRef]
- Chabtini, L.; Mfarrej, B.; Mounayar, M.; Zhu, B.; Batal, I.; Dakle, P.J.; Smith, B.D.; Boenisch, O.; Najafian, N.; Akiba, H.; et al. TIM-3 Regulates Innate Immune Cells To Induce Fetomaternal Tolerance. J. Immunol. 2013, 190, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Meggyes, M.; Bogar, B.; Schmitz, N.; Barakonyi, A.; Varnagy, A.; Farkas, B.; Tamas, P.; Bodis, J.; Szekeres-Bartho, J.; et al. Involvement of Galectin-9/TIM-3 Pathway in the Systemic Inflammatory Response in Early-Onset Preeclampsia. PLoS ONE 2013, 8, e71811. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, L.-L.; Liu, H.; Muyayalo, K.P.; Huang, X.-B.; Mor, G.; Liao, A.-H. Galectin-9 Alleviates LPS-Induced Preeclampsia-Like Impairment in Rats via Switching Decidual Macrophage Polarization to M2 Subtype. Front. Immunol. 2019, 9, 3142. [Google Scholar] [CrossRef]
- Lutomski, D.; Joubert-Caron, R.; Lefebure, C.; Salama, J.; Belin, C.; Bladier, D.; Michel, C. Anti-galectin-1 autoantibodies in serum of patients with neurological diseases. Clin. Chim. Acta 1997, 262, 131–138. [Google Scholar] [CrossRef]
- Romero, M.D.; Muin~o, J.C.; Bianco, G.A.; Ferrero, M.; Juarez, C.P.; Luna, J.D.; Rabinovich, G.A. Circulating Anti-galectin-1 Antibodies Are Associated with the Severity of Ocular Disease in Autoimmune and Infectious Uveitis. Investig. Opthalmol. Vis. Sci. 2006, 47, 1550. [Google Scholar] [CrossRef]
- Montiel, J.; Monsiváis-Urenda, A.; Figueroa-Vega, N.; Moctezuma, J.; Burgos-Vargas, R.; González-Amaro, R.; Rosenstein, Y. Anti-CD43 and anti-galectin-1 autoantibodies in patients with systemic lupus erythematosus. Scand. J. Rheumatol. 2010, 39, 50–57. [Google Scholar] [CrossRef]
- Sarter, K.; Janko, C.; Andre, S.; Munoz, L.E.; Schorn, C.; Winkler, S.; Rech, J.; Kaltner, H.; Lorenz, H.-M.; Schiller, M.; et al. Autoantibodies against galectins are associated with antiphospholipid syndrome in patients with systemic lupus erythematosus. Glycobiology 2013, 23, 12–22. [Google Scholar] [CrossRef]
- Kang, E.; Moon, K.; Lee, E.; Lee, Y.; Lee, E.; Ahn, C.; Song, Y. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 2009, 18, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tan, G.; Meng, Z.; Yu, M.; Li, K.; Yin, J.; Wei, K.; Luo, Y.; Jia, S.; Zhang, S.; et al. Association of Anti-Acidic Ribosomal Protein P0 and Anti-Galectin 3 Antibodies With the Development of Skin Lesions in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, D.-Y.; Lee, S.; Park, S.-Y.; Kim, J.; Cho, B.; Lee, H.; Kim, H.-Y.; Lee, E.; Song, Y.W.; et al. Identification of autoantibodies associated with systemic lupus erythematosus. Biochem. Biophys. Res. Commun. 2002, 295, 119–124. [Google Scholar] [CrossRef]
- Massardo, L.; Metz, C.; Pardo, E.; Mezzano, V.; Babul, M.; Jarpa, E.; Guzmán, A.; André, S.; Kaltner, H.; Gabius, H.; et al. Autoantibodies against galectin-8: Their specificity, association with lymphopenia in systemic lupus erythematosus and detection in rheumatoid arthritis and acute inflammation. Lupus 2009, 18, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Mayr, D.; Schiessl, B.; Mylonas, I.; Schulze, S.; Kuhn, C.; Friese, K.; Walzel, H. Expression of Galectin-1, -3 (gal-1, gal-3) and the Thomsen–Friedenreich (TF) Antigen in Normal, IUGR, Preeclamptic and HELLP Placentas. Placenta 2007, 28, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.M.; Moodley, J.; Naicker, T. The role of Galectin-1 in HIV associated preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 138–144. [Google Scholar] [CrossRef]
- Molvarec, A.; Blois, S.M.; Stenczer, B.; Toldi, G.; Tirado-Gonzalez, I.; Ito, M.; Shima, T.; Yoneda, S.; Vásárhelyi, B.; Rigó, J.; et al. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin. Immunol. 2011, 139, 48–56. [Google Scholar] [CrossRef]
- Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Herse, F.; Thijssen, V.L.J.L.; Weedon-Fekjaer, S.M.; Schulz, H.; Wallukat, G.; Klapp, B.F.; Nevers, T.; et al. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc. Natl. Acad. Sci. USA 2013, 110, 11451–11456. [Google Scholar] [CrossRef]
- Hirashima, C.; Ohkuchi, A.; Nagayama, S.; Suzuki, H.; Takahashi, K.; Ogoyama, M.; Takahashi, H.; Shirasuna, K.; Matsubara, S. Galectin-1 as a novel risk factor for both gestational hypertension and preeclampsia, specifially its expression at a low level in the second trimester and a high level after onset. Hypertens. Res. 2018, 41, 45–52. [Google Scholar] [CrossRef]
- Hutter, S.; Martin, N.; von Schönfeldt, V.; Messner, J.; Kuhn, C.; Hofmann, S.; Andergassen, U.; Knabl, J.; Jeschke, U. Galectin 2 (gal-2) expression is downregulated on protein and mRNA level in placentas of preeclamptic (PE) patients. Placenta 2015, 36, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ruikar, K.; Aithal, M.; Shetty, P.; Dinesh, U.S.; Bargale, A.; Sadashiv, R.; Sarathkumar, E.; Khode, V.; Desai, R.; Patil, P. Placental Expression and Relative Role of Anti-inflammatory Annexin A1 and Animal Lectin Galectin-3 in the Pathogenesis of Preeclampsia. Indian J. Clin. Biochem. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Pankiewicz, K.; Szczerba, E.; Fijalkowska, A.; Szamotulska, K.; Szewczyk, G.; Issat, T.; Maciejewski, T. The association between serum galectin-3 level and its placental production in patients with preeclampsia. J. Physiol. Pharmacol. 2020, 71, 1–12. [Google Scholar] [CrossRef]
- Sattar Taha, A.; Zahraei, Z.; Al-Hakeim, H. Serum apelin and galectin-3 in preeclampsia in Iraq. Hypertens. Pregnancy 2020, 39, 379–386. [Google Scholar] [CrossRef]
- Than, N.G.; Abdul Rahman, O.; Magenheim, R.; Nagy, B.; Fule, T.; Hargitai, B.; Sammar, M.; Hupuczi, P.; Tarca, A.L.; Szabo, G.; et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008, 453, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Nisemblat, S.; Fleischfarb, Z.; Golan, A.; Sadan, O.; Meiri, H.; Huppertz, B.; Gonen, R. Placenta-bound and Body Fluid PP13 and its mRNA in Normal Pregnancy Compared to Preeclampsia, HELLP and Preterm Delivery. Placenta 2011, 32, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Cowans, N.J.; Spencer, K.; Goichman, S.; Meiri, H.; Harrington, K. First trimester markers for the prediction of pre-eclampsia in women with a priori high risk. Ultrasound Obstet. Gynecol. 2010, 35, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-X.; Ying, X.; Dong, M.-Y. Galectin-1 expression in the serum and placenta of pregnant women with fetal growth restriction and its significance. BMC Pregnancy Childbirth 2021, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Demmert, M.; Faust, K.; Bohlmann, M.K.; Tröger, B.; Göpel, W.; Herting, E.; Härtel, C. Galectin-3 in cord blood of term and preterm infants. Clin. Exp. Immunol. 2012, 167, 246–251. [Google Scholar] [CrossRef]
- Burger, O.; Pick, E.; Zwickel, J.; Klayman, M.; Meiri, H.; Slotky, R.; Mandel, S.; Rabinovitch, L.; Paltieli, Y.; Admon, A.; et al. Placental protein 13 (PP-13): Effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta 2004, 25, 608–622. [Google Scholar] [CrossRef]
- Chafetz, I.; Kuhnreich, I.; Sammar, M.; Tal, Y.; Gibor, Y.; Meiri, H.; Cuckle, H.; Wolf, M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2007, 197, 35.e1–35.e7. [Google Scholar] [CrossRef] [PubMed]
- Cowans, N.J.; Spencer, K.; Meiri, H. First-trimester maternal placental protein 13 levels in pregnancies resulting in adverse outcomes. Prenat. Diagn. 2008, 28, 121–125. [Google Scholar] [CrossRef]
- Frick, A.P. Advanced maternal age and adverse pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, P.; Cheng, L. Galectin-1 reduction and changes in T regulatory cells may play crucial roles in patients with unexplained recurrent spontaneous abortion. Int. J. Clin. Exp. Pathol. 2015, 8, 1973–1978. [Google Scholar]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Desoye, G.; Hauguel-de Mouzon, S. The Human Placenta in Gestational Diabetes Mellitus: The insulin and cytokine network. Diabetes Care 2007, 30, S120–S126. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984.e12. [Google Scholar] [CrossRef]
- Kuc, S.; Wortelboer, E.; Koster, M.; de Valk, H.; Schielen, P.; Visser, G. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG An Int. J. Obstet. Gynaecol. 2011, 118, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gruppi, A. Galectins as immunoregulators during infectious processes: From microbial invasion to the resolution of the disease. Parasite Immunol. 2005, 27, 103–114. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Liu, F.-T.; Hirashima, M.; Anderson, A. An Emerging Role for Galectins in Tuning the Immune Response: Lessons from Experimental Models of Inflammatory Disease, Autoimmunity and Cancer. Scand. J. Immunol. 2007, 66, 143–158. [Google Scholar] [CrossRef]
- Blidner, A.G.; Rabinovich, G.A. ‘Sweetening’ Pregnancy: Galectins at the Fetomaternal Interface. Am. J. Reprod. Immunol. 2013, 69, 369–382. [Google Scholar] [CrossRef]
- Romero, R.; Gotsch, F.; Pineles, B.; Kusanovic, J.P. Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury. Nutr. Rev. 2008, 65, S194–S202. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and Infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef]
- Cotechini, T.; Graham, C.H. Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target? Placenta 2015, 36, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Molinero, L.L.; Toscano, M.A.; Ilarregui, J.M.; Rubinstein, N.; Fainboim, L.; Zwirner, N.W.; Rabinovich, G.A. Regulated expression of galectin-1 during T-cell activation involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase and p70 S6 kinase. Mol. Cell. Biochem. 2004, 267, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Wildman, D.E.; Erez, O.; Edwin, S.S.; Espinoza, J.; Kim, C.J.; Han, Y.M.; Mazaki-Tovi, S.; Kusanovic, J.P.; Hassan, S.; et al. Trophoblast, Galectin-1 and pre-eclampsia. Am. J. Obstet. Gynecol. 2006, 195, S138. [Google Scholar] [CrossRef]
- Bonney, E.A. Preeclampsia: A view through the danger model. J. Reprod. Immunol. 2007, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Romero, R.; Oh, S.Y.; Kim, C.J.; Kilburn, B.A.; Armant, D.R.; Nien, J.K.; Gomez, R.; Mazor, M.; Saito, S.; et al. Toll-like receptor 4: A potential link between “danger signals,” the innate immune system, and preeclampsia? Am. J. Obstet. Gynecol. 2005, 193, 921.e1–921.e8. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Pozsgay, J.; Matkó, J.; Dong, Z.; Kim, C.J.; Várkonyi, T.; Sammar, M.; Rigó, J.; Meiri, H.; Romero, R.; et al. Placental protein 13 (PP13/galectin-13) undergoes lipid raft-associated subcellular redistribution in the syncytiotrophoblast in preterm preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 2011, 205, 156.e1–156.e14. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Yung, H.-W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Giordanengo, L.; Gea, S.; Barbieri, G.; Rabinovich, G.A. Anti-galectin-1 autoantibodies in human Trypanosoma cruzi infection: Differential expression of this β-galactoside-binding protein in cardiac Chagas’ disease. Clin. Exp. Immunol. 2001, 124, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Redman, C.; Roberts, J.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Robillard, P.; Dekker, G.; Scioscia, M.; Bonsante, F.; Iacobelli, S.; Boukerrou, M.; Hulsey, T.C. Validation of the 34-week gestation as definition of late onset preeclampsia: Testing different cutoffs from 30 to 37 weeks on a population-based cohort of 1700 preeclamptics. Acta Obstet. Gynecol. Scand. 2020, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Shanmugalingam, R.; Chau, K.; Makris, A.; Hennessy, A. Galectin-1–Related Modulation of Trophoblast Endothelial Interactions by Integrins α1 and β1. Reprod. Sci. 2020, 27, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Chen, Y.; Yin, N.; Shan, N.; Luo, X.; Yuan, Y.; Luo, X.; Liu, Y.; Liu, X.; Qi, H. The Role of MGAT5 in Human Umbilical Vein Endothelial Cells. Reprod. Sci. 2017, 24, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern. Neonatal Med. 2021, 34, 2965–2970. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N.; Blazhev, A. Serum Galectin-3 Levels Are Unlikely to Be a Useful Predictive Marker for Early-onset Preeclampsia Development. Prague Med. Rep. 2020, 121, 172–180. [Google Scholar] [CrossRef]
- Sekizawa, A.; Purwosunu, Y.; Yoshimura, S.; Nakamura, M.; Shimizu, H.; Okai, T.; Rizzo, N.; Farina, A. PP13 mRNA Expression in Trophoblasts From Preeclamptic Placentas. Reprod. Sci. 2009, 16, 408–413. [Google Scholar] [CrossRef]
- Farina, A.; Zucchini, C.; Sekizawa, A.; Purwosunu, Y.; de Sanctis, P.; Santarsiero, G.; Rizzo, N.; Morano, D.; Okai, T. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10–14 weeks. Am. J. Obstet. Gynecol. 2010, 203, 575.e1–575.e7. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Meiri, H.; Gizurarson, S.; Osol, G.; Sammar, M. Placental protein 13 (PP13): A new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum. Reprod. Update 2013, 19, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Bindra, R.; Turan, O.M.; Chefetz, I.; Sammar, M.; Meiri, H.; Tal, J.; Cuckle, H.S. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet. Gynecol. 2005, 27, 13–17. [Google Scholar] [CrossRef]
- Wortelboer, E.; Koster, M.; Cuckle, H.; Stoutenbeek, P.; Schielen, P.; Visser, G. First-trimester placental protein 13 and placental growth factor: Markers for identification of women destined to develop early-onset pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1384–1389. [Google Scholar] [CrossRef]
- Cuckle, H.S. Screening for Pre-eclampsia–Lessons from Aneuploidy Screening. Placenta 2011, 32, S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, G.; Antsaklis, A.; Botet, F.; Chervenak, F.A.; Figueras, F.; Grunebaum, A.; Puerto, B.; Skupski, D.; Stanojevic, M. Intrauterine restriction (IUGR). J. Perinat. Med. 2008, 36, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, D.; Christou, H. Current Concepts in Intrauterine Growth Restriction. J. Intensive Care Med. 2004, 19, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Farahbakhsh, N.; Sharma, P. Intrauterine growth restriction–part 1. J. Matern. Neonatal Med. 2016, 29, 3977–3987. [Google Scholar] [CrossRef]
- Pankiewicz, K.; Maciejewski, T. Perinatal mortality and morbidity of growth restricted fetuses and newborns (own experience)-first report. Dev. Period Med. 2017, 29, 29–34. [Google Scholar]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci. Rep. 2021, 11, 7824. [Google Scholar] [CrossRef]
- Albu, A.R.; Anca, A.F.; Horhoianu, V.V.; Horhoianu, I.A. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. [Google Scholar] [PubMed]
- Sammar, M.; Drobnjak, T.; Mandala, M.; Gizurarson, S.; Huppertz, B.; Meiri, H. Galectin 13 (PP13) Facilitates Remodeling and Structural Stabilization of Maternal Vessels during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3192. [Google Scholar] [CrossRef] [PubMed]
- Scifres, C.M.; Nelson, D.M. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 2009, 587, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
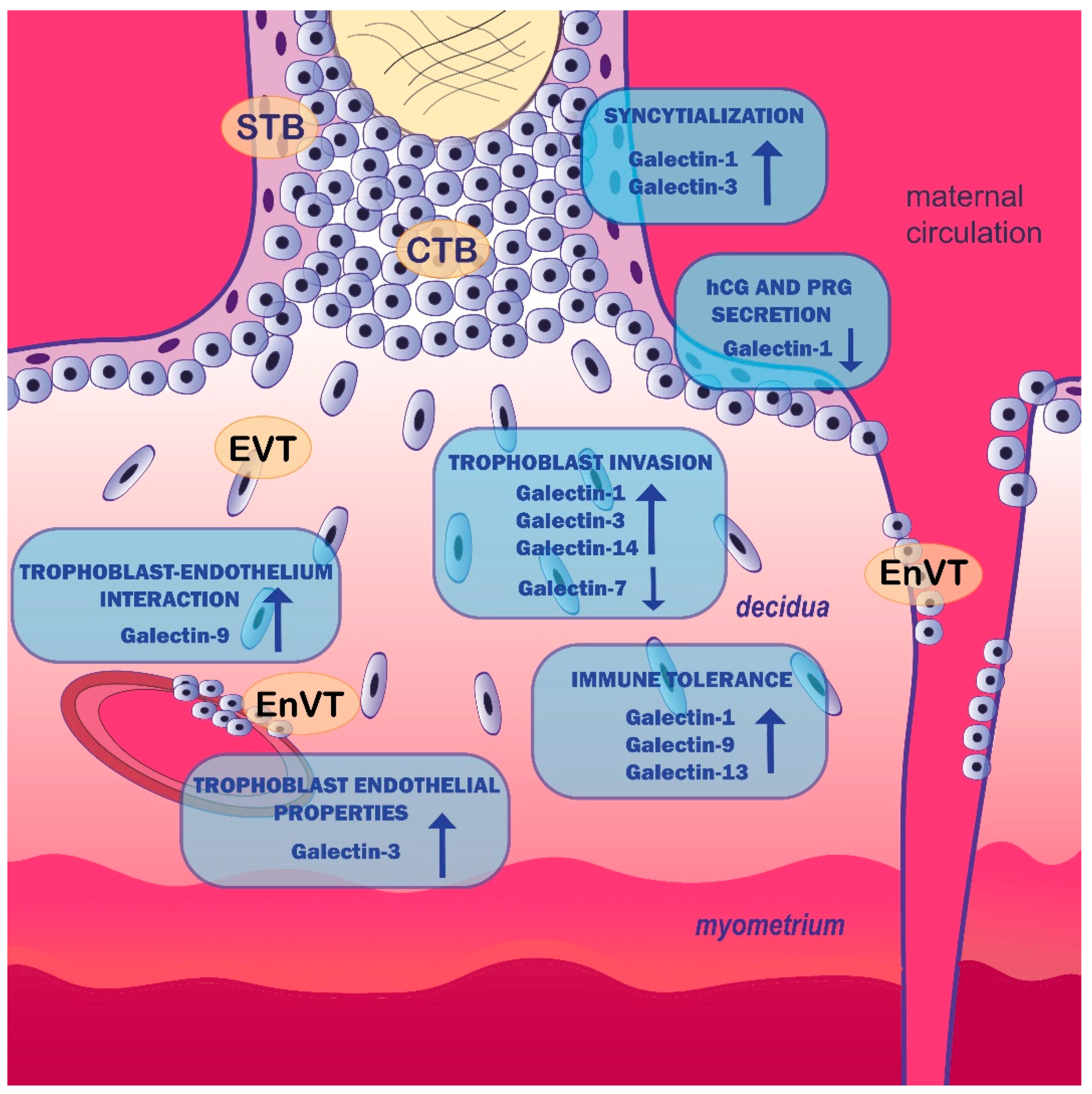
| Process/Pathology | Involvement of Galectins | Reference |
|---|---|---|
| Trophoblast cell function/placentation | Galectin-1 stimulates first-trimester cytotrophoblast, HTR-8/SVneo and JAR cell invasion | [41,42] |
| Galectin-1 decreases BeWo cell proliferation | [43] | |
| Galectin-1 modulates HLA-G expression on trophoblast cells, suggesting its role in immune tolerance | [44] | |
| Galectin-1 stimulates syncytialization of BeWo cells but decreases human chorionic gonadotropin and progesterone production | [45,47] | |
| Galectin-3 stimulates HTR-8/SVneo cell migration and HTR-8/SVneo cell, first-trimester CTB and HIPEC-65 cell invasion | [52,54] | |
| Galectin-3 increases endothelial-like properties of EVT cell line SGHPL-4 | [54] | |
| Galectin-3 stimulates syncytialization of BeWo cells | [54] | |
| Galectin-7 decreases first-trimester trophoblast cells’ and HTR-8/SVneo cells’ capacity to adhere to endometrial epithelial cells and first-trimester EVT outgrowth from placental explants | [16,57] | |
| Galectin-9 derived from trophoblast cells promotes immune tolerance at the feto-maternal interface | [58,59,60] | |
| Galectin-9 inhibits the apoptosis and proinflammatory cytokine production of HTR-8/SVneo cells and increases the interaction with endothelium in a JNK-dependent manner | [61] | |
| Galectin-13 induces apoptosis of activated T cells in vitro, diverts and kills T cells and macrophages in the maternal decidua and polarizes neutrophils towards permissive phenotype for placental growth | [62,63] | |
| Galectin-13 may induce angiogenesis at the feto-maternal interface | [6] | |
| Galectin-14 promotes trophoblast cell migration and invasion by stimulating the expression of MMP-9 and N-cadherin through Akt phosphorylation | [64] | |
| Galectin-14 may function in angiogenesis | [6] | |
| Pregnancy loss | Galectin-1 is significantly lower in placenta tissue after miscarriage and RPL | [65,66] |
| Galectin-2 is downregulated in VT and EVT after SA and RPL | [66] | |
| Maternal deficiency of galectin-3 is associated with structural alterations in placenta, with reduced trophoblast layers and a corresponding enlarged maternal decidua. The absence of galectin-3 also results in reduced total vessel length and vessel area, suggesting placental malperfusion. | [67] | |
| Excessive galectin-3 after 4th week secreted by VT leads to massive apoptosis of endometrial cells, which affects the normal development of villi in early pregnancy, and potentially leads to missed abortion.Imbalance between extracellular and intracellular galectin-3 levels can influence cell apoptosis in placental villi, leading to defects in early placental development and ultimately result in pregnancy loss. | [68] | |
| Galectin-3 is markedly decreased in serum, decidua and the villi in the group of women with missed abortions | [69] | |
| Expression of galectins-7 and -10 is decreased in VT after SA | [66] | |
| Gestational diabetes mellitus (GDM) | Serum galectin-1 levels are increased during gestation, whereas in GDM, its secretion pattern seems to be unchanged | [44,70] |
| In GDM patients, there is an inverse association between glucose and galectin-1 compared to normal pregnancies | [70] | |
| Possible relation of galectin-2 overexpression to pathophysiology of GDM | [71] | |
| Women in the first trimester had higher levels of galectin-3 and were more likely to develop GDM later in the pregnancy than women found to have low levels of galectin-3 | [72] | |
| Circulating galectin-3 levels are higher in subjects with GDM and also correspond to increased risk of GDM | [73] | |
| Galectin-3 mRNA and protein expression are increased in GDM maternal blood samples and placental tissue, and decreased in cord blood | [74] | |
| Cord blood galectin-3 is significantly increased in pregnancies with GDM | [75] | |
| Galectin-13 expression is markedly lower in the placenta of GDM pregnancies. Galectin-13 maternal serum levels at term are significantly lower, while in the early second trimester, significantly lower than in normal pregnancies. | [76,77] | |
| Inflammation /infection | Galectin-1 expression increases in chorioamniotic membranes, promoting weakening of the membranes and contributing to their rupture, or as a compensatory response to counteract inflammation and retain immunological tolerance | [78] |
| Galectin-3 is overexpressed in chorioamniotic membranes in women with PPROM, suggesting its role in the pathogenesis | [79] | |
| Galectin-3 expression increases in placenta and amniotic fluid upon Porphyromonas gingivalis placental invasion and development of inflammation, further potentiating local cytokine production and activation of myometrium | [80,81] | |
| Galectin 3 inhibits CD66a expression by intermediate trophoblast and endometrial epithelium/endothelium, thus contributing to placental abruption and preterm birth | ||
| Tim-3/galectin-9 axis impairment contributes to failure of immunotolerance by a shift towards the proinflammatory M1 phenotype of decidual macrophages, increased placental expression of TNF-α, IL-1β and iNOS, and reduced expression of TGF-β, IL-10 and Arginase-1, accompanied by inadequate trophoblast invasion, impaired spiral artery remodeling and fetal capillary development | [59,82,83,84,85] | |
| Anti-galectin-1 Abs are found in increased titers in autoimmune uveitis, and SLE | [86,87,88] | |
| Anti-galectin-2 Abs in SLE (highly associated with secondary anti-phospholipid syndrome) | [89] | |
| Anti-galectin-3 Abs in SLE, polymyositis/dermatomyositis | [90,91,92] | |
| Anti-galectin-4 in SLE and RA | [89] | |
| Anti-galectin-7 Abs in SLE | [89] | |
| Anti-galectin-8 and -9 in SLE and RA | [89,93] | |
| Pre-eclampsia (PE) | Galectin-1 overexpression in PE placentas, compared to placentas in normal pregnancy | [78,94] |
| Downregulation of galectin-1 in early-onset PE placentas | [95] | |
| Galectin-1-expressing peripheral blood T and NK cells proportion is decreased in women who developed PE compared to normal pregnancy | [96] | |
| Downregulation of galectin-1 in early-onset PE placentas | [95,97] | |
| Low serum galectin-1 levels during the second trimester might be a PE risk | [97,98] | |
| Galectin-2 is downregulated at protein and mRNA levels in EVTs in PE placentas Galectin-2 serum levels are lower in PE patients compared to patients with uncomplicated pregnancies | [99] | |
| Galectin-3 mRNA and protein levels are increased in PE placental tissue in comparison to normal pregnancy placentas | [100] | |
| Galectin-3 is overexpressed in EVT and STB of PE placentas in comparison to normal pregnancy placentas | [94,100,101] | |
| Galectin-3 serum levels are higher in PE patients compared to patients with uncomplicated pregnancies | [101,102] | |
| Galectin-7 serum levels are higher in women who developed PE in comparison to uncomplicated pregnancies | [16] | |
| Altered expression of galectin-9 is detected on peripheral blood lymphocytes in early-onset pre-eclamptic women Galectin-13 mRNA and protein levels are decreased in placenta tissue in early- and late-onset PE in comparison to healthy pregnancies | [84][103,104] | |
| Galectin-13 is overexpressed in STB microvillous membrane in PE compared to healthy controls Low galectin-13 serum protein level during first trimester, with rapid increase starting with second trimester in women who subsequently develop PE Galectin-13 in combination with other (bio)markers is a promising tool in PE prediction | [103][62][105] | |
| Intrauterine growth restriction (IUGR) | Galectin-1 low expression in the serum and placenta of pregnant women with IUGR | [106] |
| Galectin-2 expression decreased in male IUGR placentas in all compartments when compared to controls | [15] | |
| Galectin-3 expression is significantly higher in cord blood of small-for-gestational-age infants compared to appropriate-for-gestational-age infants | [107] | |
| Galectin-3 is significantly downregulated in the EVT of IUGR placentas | [15] | |
| Placental galectin-3 expression is downregulated in human pregnancies complicated with IUGR | [67] | |
| Galectin-1 and galectin-3 expression in the EVT is unchanged in IUGR placentas compared with normal controls | [94] | |
| Significant downregulation of galectin-4, -8 and -9 in the IUGR trophoblast of male fetuses | [25] | |
| In IUGR pregnancies with female fetus, galectin-9 and galectin-12 are upregulated in the EVT and in endothelial cells in the case of galectin-12; decreased/increased expression in placenta (gender-specific) | [25] | |
| Galectin-13 levels, lower than normal, are found in IUGR in the first trimester. In the 2nd and 3rd trimesters, higher than normal concentrations are found in IUGR. | [108] | |
| Low levels of first-trimester galectin-13 are associated with preterm birth in women with IUGR | [109] | |
| Decreased levels of galectin-13 are not significantly correlated with the studied adverse pregnancy outcomes of IUGR | [110] | |
| Galectin-13 expression is strongly decreased in VT and EVT in IUGR-complicated pregnancies of male fetal gender | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović Krivokuća, M.; Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Ćujić, D.; Legner, J.; Dekanski, D.; Bojić-Trbojević, Ž. Galectins in Early Pregnancy and Pregnancy-Associated Pathologies. Int. J. Mol. Sci. 2022, 23, 69. https://doi.org/10.3390/ijms23010069
Jovanović Krivokuća M, Vilotić A, Nacka-Aleksić M, Pirković A, Ćujić D, Legner J, Dekanski D, Bojić-Trbojević Ž. Galectins in Early Pregnancy and Pregnancy-Associated Pathologies. International Journal of Molecular Sciences. 2022; 23(1):69. https://doi.org/10.3390/ijms23010069
Chicago/Turabian StyleJovanović Krivokuća, Milica, Aleksandra Vilotić, Mirjana Nacka-Aleksić, Andrea Pirković, Danica Ćujić, Janko Legner, Dragana Dekanski, and Žanka Bojić-Trbojević. 2022. "Galectins in Early Pregnancy and Pregnancy-Associated Pathologies" International Journal of Molecular Sciences 23, no. 1: 69. https://doi.org/10.3390/ijms23010069
APA StyleJovanović Krivokuća, M., Vilotić, A., Nacka-Aleksić, M., Pirković, A., Ćujić, D., Legner, J., Dekanski, D., & Bojić-Trbojević, Ž. (2022). Galectins in Early Pregnancy and Pregnancy-Associated Pathologies. International Journal of Molecular Sciences, 23(1), 69. https://doi.org/10.3390/ijms23010069







