Systematic Analysis of Clemastine, a Candidate Apicomplexan Parasite-Selective Tubulin-Targeting Agent
Abstract
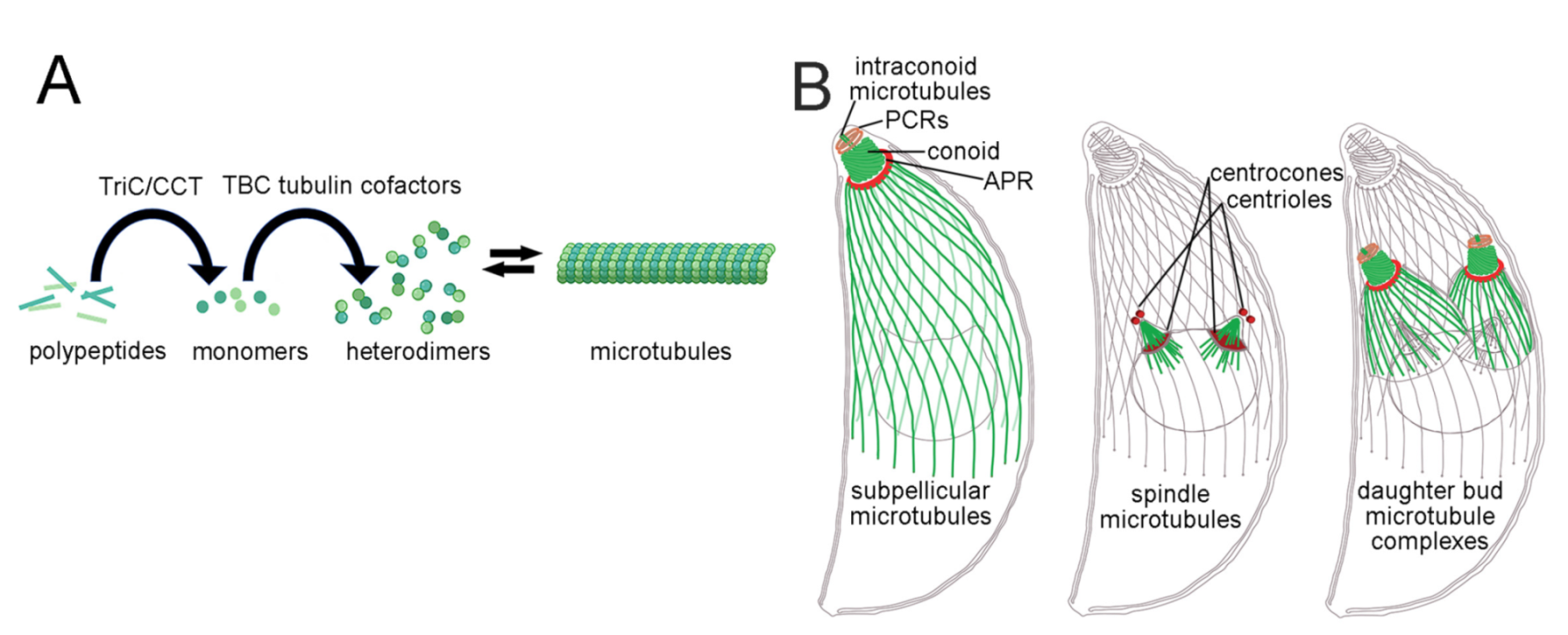
:1. Introduction
2. Results
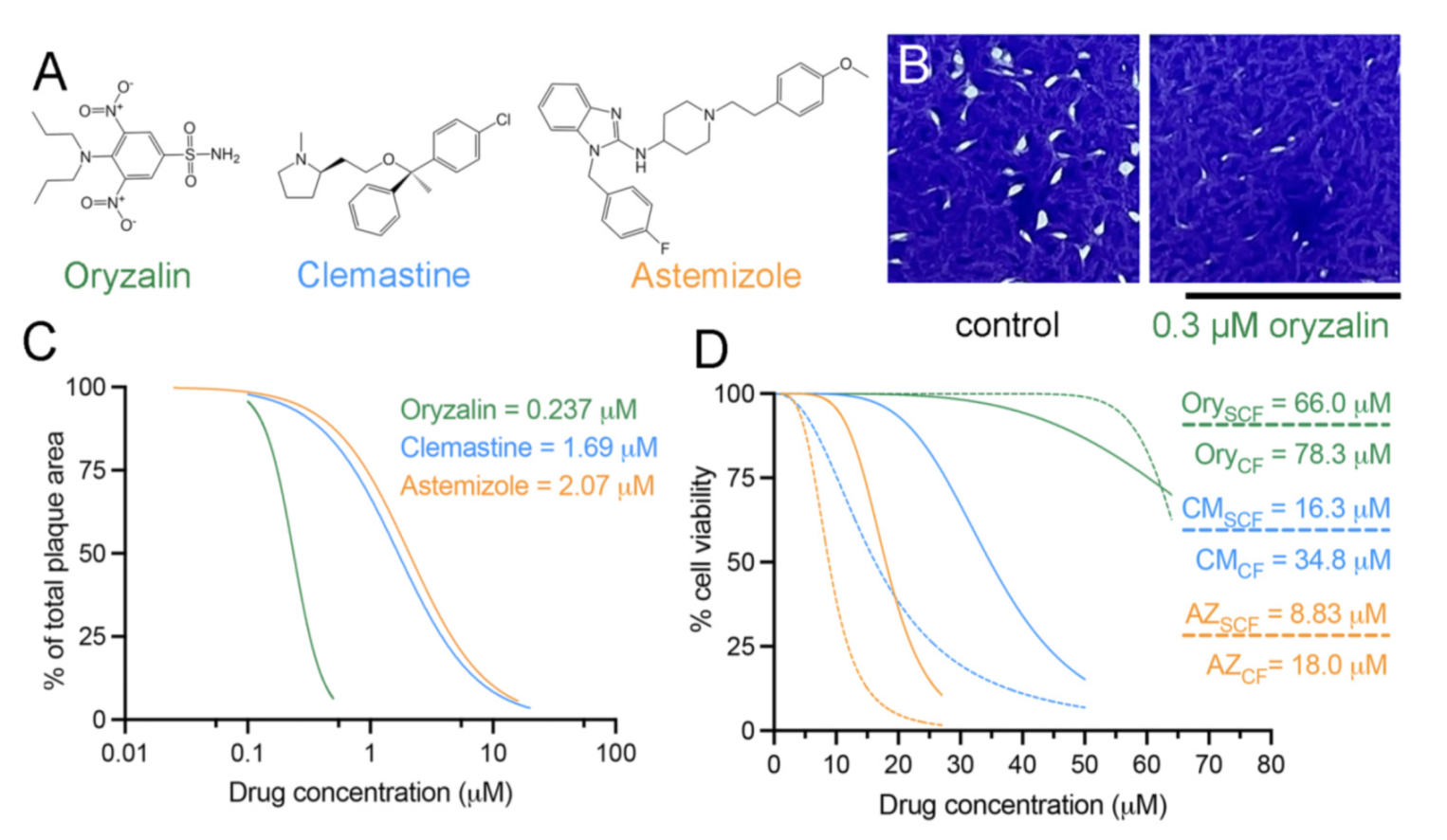
2.1. Clemastine and Astemizole Selectively Inhibit Tachyzoite Growth
2.2. Clemastine and Astemizole Vertebrate Cytotoxicity Is above Their Efficacy Range
| Drug | Toxoplasma RH Strain | HFF CC50subconfluent | HFF CC50confluent | Tg Therapeutic Index Range | Plasmodium spp. |
|---|---|---|---|---|---|
| Oryzalin | 0.24 μM | 66.0 µM | 78.3 µM | 275–326 | 4.3 μM [31] |
| Clemastine | 1.69 μM | 16.3 µM | 34.8 µM | 9.65–20.6 | 1 µM [20] |
| Astemizole | 2.07 μM | 8.83 µM | 18.0 µM | 4.27–8.70 | 0.23 µM Pf RC [22] 0.086 µM Pb RC [23] 0.66 µM Pb EE [23] |
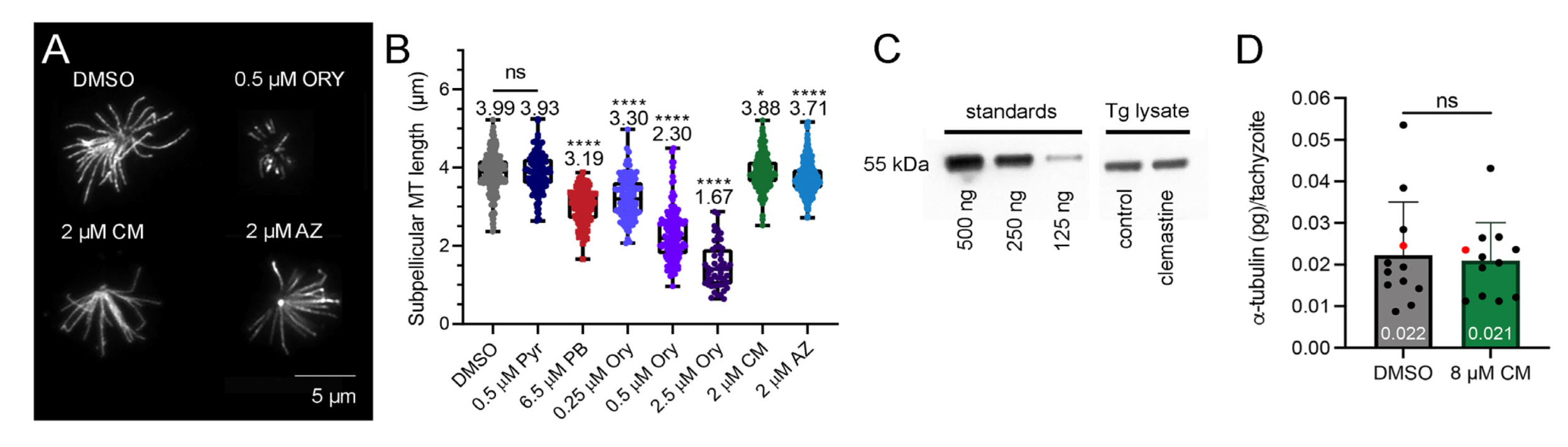
2.3. Clemastine and Astemizole Shorten Toxoplasma Subpellicular Microtubules
3. Discussion
4. Materials and Methods
4.1. Parasite and Tissue Culture
4.2. Drug Stocks and Use in Assays
4.3. Determination of EC50 Values
4.4. Invasion Assay
4.5. Tachyzoite Replication Assay
4.6. MTT Toxicity Assay
4.7. Tubulin Immunofluorescence and Microtubule Quantification
4.8. Quantification of Tubulin Protein
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AZ | Astemizole |
| CC50 | 50% cytotoxic concentration |
| CCT | Chaperonin containing tailless complex polypeptide 1 (TCP1) |
| CM | Clemastine |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EC50 | 50% effective concentration |
| HFF | Human foreskin fibroblast |
| Ory | Oryzalin |
| PB | Parabulin |
| PBS | Phosphate buffered saline |
| Pyr | Pyrimethamine |
| RH-GFP | GFP-expressing Toxoplasma gondii |
| TRiC | Tailless complex polypeptide 1 ring complex |
| TTA | Tubulin-targeting agent |
References
- Morrissette, N. Targeting Toxoplasma tubules: Tubulin, microtubules, and associated proteins in a human pathogen. Eukaryot. Cell 2015, 14, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.P.; Lupovitch, A.; Katase, R.Y. Daraprim: A Folic Acid Antagonist: The Detection of Early Toxicity. Am. J. Ophthalmol. 1964, 58, 608–611. [Google Scholar] [CrossRef]
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Microbiol. Spectr. 2016, 4, 409–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef]
- Francis, J.W.; Newman, L.E.; Cunningham, L.A.; Kahn, R.A. A Trimer Consisting of the Tubulin-specific Chaperone D (TBCD), Regulatory GTPase ARL2, and beta-Tubulin Is Required for Maintaining the Microtubule Network. J. Biol. Chem. 2017, 292, 4336–4349. [Google Scholar] [CrossRef] [Green Version]
- Lacey, E.; Gill, J.H. Biochemistry of benzimidazole resistance. Acta Trop. 1994, 56, 245–262. [Google Scholar] [CrossRef]
- Kohler, P. The biochemical basis of anthelmintic action and resistance. Int. J. Parasitol. 2001, 31, 336–345. [Google Scholar] [CrossRef]
- Stokkermans, T.J.; Schwartzman, J.D.; Keenan, K.; Morrissette, N.S.; Tilney, L.G.; Roos, D.S. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 1996, 84, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrissette, N.S.; Mitra, A.; Sept, D.; Sibley, L.D. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell 2004, 15, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Abbott, S.; Sackett, D.L.; Wloga, D.; Gaertig, J.; Morgan, R.E.; Werbovetz, K.A.; Morrissette, N.S. Alpha-Tubulin mutations alter oryzalin affinity and microtubule assembly properties to confer dinitroaniline resistance. Eukaryot. Cell 2010, 9, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.E.; Werbovetz, K.A. Selective Lead Compounds against Kinetoplastid Tubulin. In Drug Targets in Kinetoplastid Parasites; Majumder, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 33–47. [Google Scholar] [CrossRef]
- Chan, M.M.; Fong, D. Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science 1990, 249, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Endeshaw, M.M.; Li, C.; de Leon, J.; Yao, N.; Latibeaudiere, K.; Premalatha, K.; Morrissette, N.; Werbovetz, K.A. Synthesis and evaluation of oryzalin analogs against Toxoplasma gondii. Bioorg. Med. Chem. Lett. 2010, 20, 5179–5183. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Tran, J.; Gu, F.; Ochoa, R.; Li, C.; Sept, D.; Werbovetz, K.; Morrissette, N. Dinitroaniline activity in Toxoplasma gondii expressing wild-type or mutant alpha-tubulin. Antimicrob. Agents Chemother. 2010, 54, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, N.; Sharma, A.; Abbaali, I.; Liu, T.; Shilliday, F.; Cook, A.D.; Ehrhard, V.; Bangera, M.; Roberts, A.J.; Moores, C.A.; et al. Inhibiting parasite proliferation using a rationally designed anti-tubulin agent. EMBO Mol. Med. 2021, 13, e13818. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, N.S.; Sibley, L.D. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J. Cell Sci. 2002, 115, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, S.; Kanchanawong, P. An integrated enhancement and reconstruction strategy for the quantitative extraction of actin stress fibers from fluorescence micrographs. BM.MC Bioinform. 2017, 18, 268. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.-Y.; Quan, B.; Sylvester, K.; Srivastava, T.; Fitzgerald, M.C.; Derbyshire, E.R. Plasmodium chaperonin TRiC/CCT identified as a target of the antihistamine clemastine using parallel chemoproteomic strategy. Proc. Natl. Acad. Sci. USA 2020, 117, 5810. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Chong, C.R.; Chen, X.; Shi, L.; Liu, J.O.; Sullivan, D.J., Jr. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2006, 2, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Okombo, J.; Mambwe, D.; Taylor, D.; Lawrence, N.; Reader, J.; van der Watt, M.; Fontinha, D.; Sanches-Vaz, M.; Bezuidenhout, B.C.; et al. Multistage Antiplasmodium Activity of Astemizole Analogues and Inhibition of Hemozoin Formation as a Contributor to Their Mode of Action. ACS Infect. Dis. 2019, 5, 303–315. [Google Scholar] [CrossRef]
- Mambwe, D.; Kumar, M.; Ferger, R.; Taylor, D.; Njoroge, M.; Coertzen, D.; Reader, J.; van der Watt, M.; Birkholtz, L.M.; Chibale, K. Structure-Activity Relationship Studies Reveal New Astemizole Analogues Active against Plasmodium falciparum In Vitro. ACS Med. Chem. Lett. 2021, 12, 1333–1341. [Google Scholar] [CrossRef]
- Pfefferkorn, E.R.; Nothnagel, R.F.; Borotz, S.E. Parasiticidal effect of clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob. Agents Chemother. 1992, 36, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparas, S.D.; Schlesinger, R.W. Plaque assay of Toxoplasma on monolayers of chick embryo fibroblasts. Proc. Soc. Exp. Biol. Med. 1959, 102, 431–437. [Google Scholar] [CrossRef]
- Ke, O.Y.; Krug, E.C.; Marr, J.J.; Berens, R.L. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob. Agents Chemother. 1990, 34, 1961–1965. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wu, M.; Hua, Q.; Lu, D.; Tian, Y.; Yu, H.; Cheng, L.; Chen, Y.; Cao, J.; Hu, X.; et al. Two old drugs, NVP-AEW541 and GSK-J4, repurposed against the Toxoplasma gondii RH strain. Parasites Vectors 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Eaton, M.S.; Schubert, W.; Wu, S.; Tang, J. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 2001, 113, 309–313. [Google Scholar] [CrossRef]
- Yam, A.Y.; Xia, Y.; Lin, H.T.; Burlingame, A.; Gerstein, M.; Frydman, J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 2008, 15, 1255–1262. [Google Scholar] [CrossRef]
- Fennell, B.J.; Naughton, J.A.; Dempsey, E.; Bell, A. Cellular and molecular actions of dinitroaniline and phosphorothioamidate herbicides on Plasmodium falciparum: Tubulin as a specific antimalarial target. Mol. Biochem. Parasitol. 2006, 145, 226–238. [Google Scholar] [CrossRef]
- Liu, P.; Qin, Y.; Wu, L.; Yang, S.; Li, N.; Wang, H.; Xu, H.; Sun, K.; Zhang, S.; Han, X.; et al. A phase I clinical trial assessing the safety and tolerability of combretastatin A4 phosphate injections. Anti-Cancer Drugs 2014, 25, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.Q.; Li, C.; Chyan, A.; Chung, L.; Morrissette, N.S. SPM1 stabilizes subpellicular microtubules in Toxoplasma gondii. Eukaryot. Cell 2012, 11, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, Y.; Benmerzouga, I.; Sullivan, W.J., Jr.; Morrissette, N.S.; Murray, J.M.; Hu, K. An ensemble of specifically targeted proteins stabilizes cortical microtubules in the human parasite Toxoplasma gondii. Mol. Biol. Cell 2016, 27, 549–571. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.G.; Oh, J.; Roos, D.S. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 2001, 45, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, E.; Prudencio, M.; Fennell, B.J.; Gomes-Santos, C.S.; Barlow, J.W.; Bell, A. Antimitotic herbicides bind to an unidentified site on malarial parasite tubulin and block development of liver-stage Plasmodium parasites. Mol. Biochem. Parasitol. 2013, 188, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Yakovich, A.J.; Ragone, F.L.; Alfonzo, J.D.; Sackett, D.L.; Werbovetz, K.A. Leishmania tarentolae: Purification and characterization of tubulin and its suitability for antileishmanial drug screening. Exp. Parasitol. 2006, 114, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, J.G.M.; Charlton, R.L.; Alpizar-Sosa, E.; Escrivani, D.O.; Brown, C.; Alqaisi, A.; Borsodi, M.P.G.; Figueiredo, C.P.; de Lima, E.V.; Dickie, E.A.; et al. Antileishmanial Chemotherapy through Clemastine Fumarate Mediated Inhibition of the Leishmania Inositol Phosphorylceramide Synthase. ACS Infect. Dis. 2021, 7, 47–63. [Google Scholar] [CrossRef]
- Planer, J.D.; Hulverson, M.A.; Arif, J.A.; Ranade, R.M.; Don, R.; Buckner, F.S. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2014, 8, e2977. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.E.; Naudzius, E.M.; Diaz, S.J.; Wismar, T.W.; Martchenko Shilman, M.; Schulz, D. Identification of clinically approved small molecules that inhibit growth and affect transcript levels of developmentally regulated genes in the African trypanosome. PLoS Negl. Trop. Dis. 2020, 14, e0007790. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Sugi, T.; Weiss, L.M.; Kato, K. Identification of compounds that suppress Toxoplasma gondii tachyzoites and bradyzoites. PLoS ONE 2017, 12, e0178203. [Google Scholar] [CrossRef] [Green Version]
- Roos, D.S.; Donald, R.G.; Morrissette, N.S.; Moulton, A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994, 45, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.R.; Morrissette, N.S.; LaChapelle, S.; Blader, I.J. Host cell invasion by Toxoplasma gondii is temporally regulated by the host microtubule cytoskeleton. Eukaryot. Cell 2010, 9, 1680–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, T.; Tokuda, H.; Yagita, K.; Koyama, T. Effects of Extracellular Potassium on Acid Release and Motility Initiation in Toxoplasma gondii. J. Protozool. 1987, 34, 291–295. [Google Scholar] [CrossRef]
- Zhang, Z.; Nishimura, Y.; Kanchanawong, P. Extracting microtubule networks from superresolution single-molecule localization microscopy data. Mol. Biol. Cell 2016, 28, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.K.; Parker, I.; Aswad, D.W. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Anal. Biochem. 2010, 397, 129–131. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaali, I.; Truong, D.A.; Day, S.D.; Haro-Ramirez, N.; Morrissette, N.S. Systematic Analysis of Clemastine, a Candidate Apicomplexan Parasite-Selective Tubulin-Targeting Agent. Int. J. Mol. Sci. 2022, 23, 68. https://doi.org/10.3390/ijms23010068
Abbaali I, Truong DA, Day SD, Haro-Ramirez N, Morrissette NS. Systematic Analysis of Clemastine, a Candidate Apicomplexan Parasite-Selective Tubulin-Targeting Agent. International Journal of Molecular Sciences. 2022; 23(1):68. https://doi.org/10.3390/ijms23010068
Chicago/Turabian StyleAbbaali, Izra, Danny A. Truong, Shania D. Day, Nancy Haro-Ramirez, and Naomi S. Morrissette. 2022. "Systematic Analysis of Clemastine, a Candidate Apicomplexan Parasite-Selective Tubulin-Targeting Agent" International Journal of Molecular Sciences 23, no. 1: 68. https://doi.org/10.3390/ijms23010068
APA StyleAbbaali, I., Truong, D. A., Day, S. D., Haro-Ramirez, N., & Morrissette, N. S. (2022). Systematic Analysis of Clemastine, a Candidate Apicomplexan Parasite-Selective Tubulin-Targeting Agent. International Journal of Molecular Sciences, 23(1), 68. https://doi.org/10.3390/ijms23010068







