Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress
Abstract
:1. Introduction
2. Results
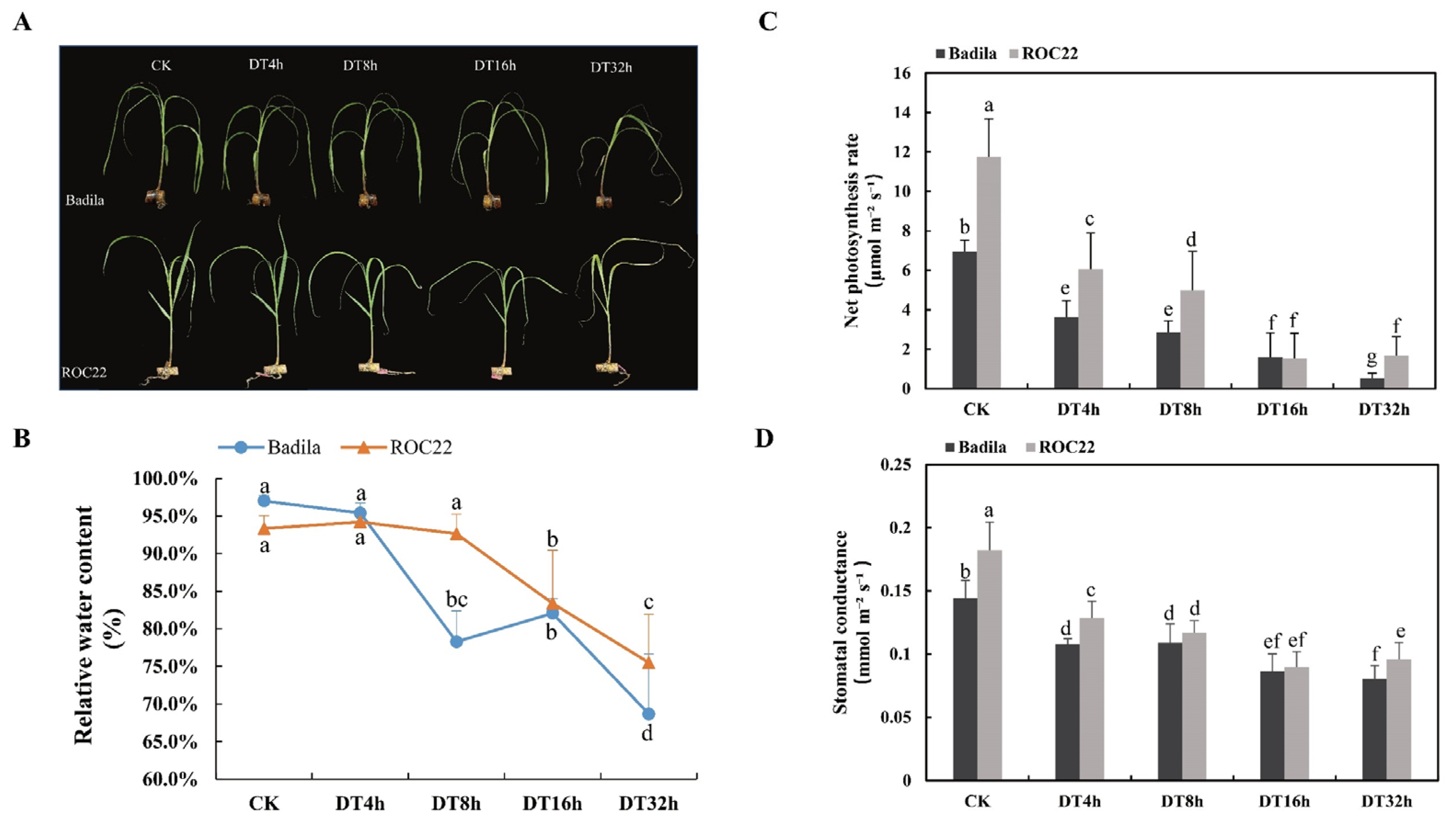
2.1. Morphological and Phenotypic Changes of Sugarcane Seedlings Exposed to Drought
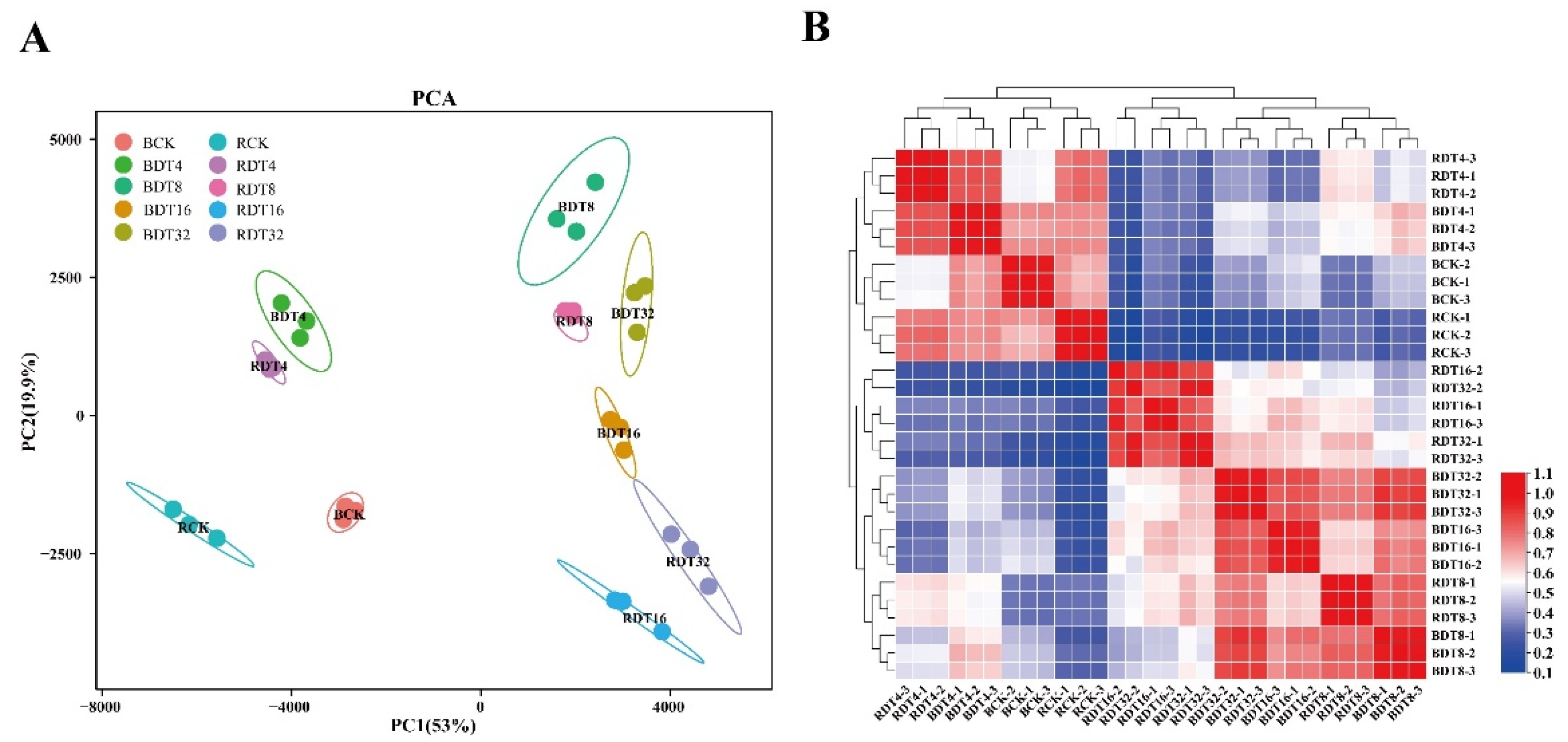
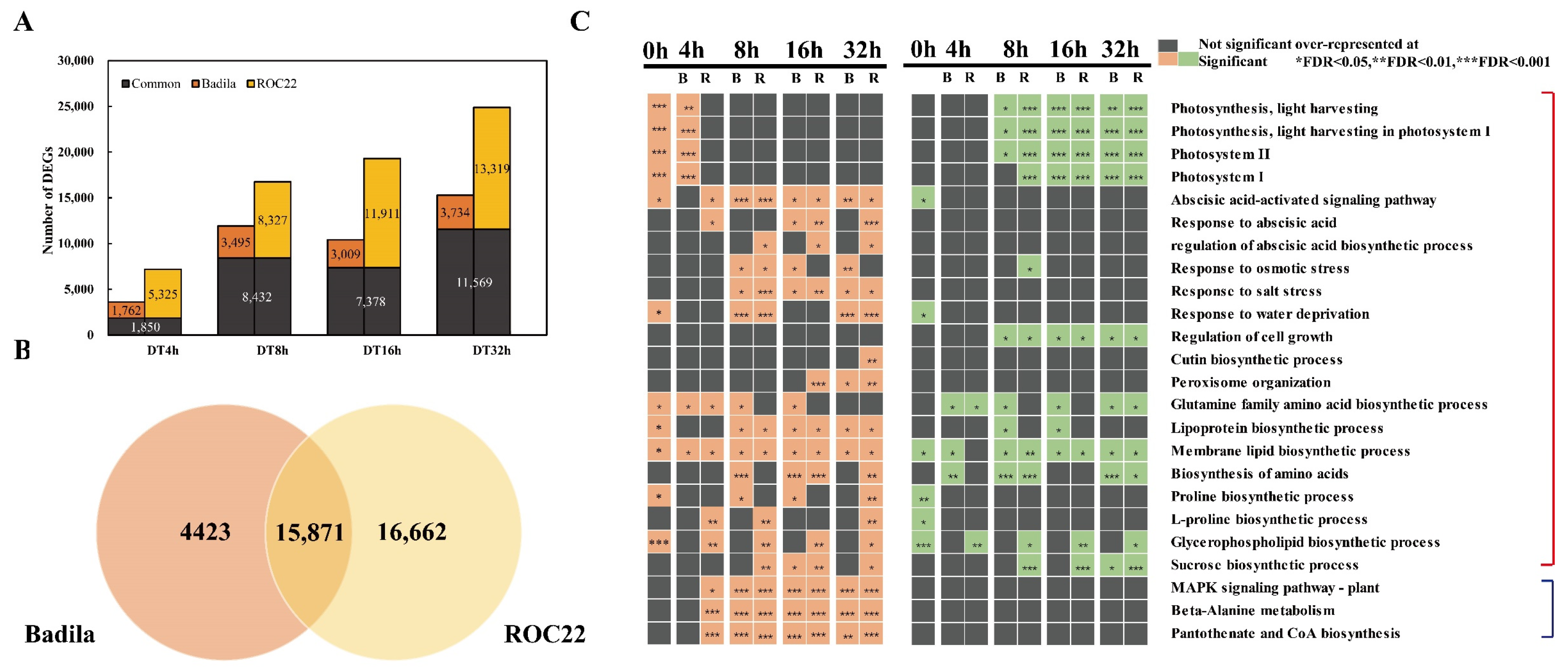
2.2. Overview of Transcriptome Divergence between Wild and Cultivated Sugarcane
2.3. Temporal Variation of Transcriptome of Different Drought Resistant Materials under Drought Stress
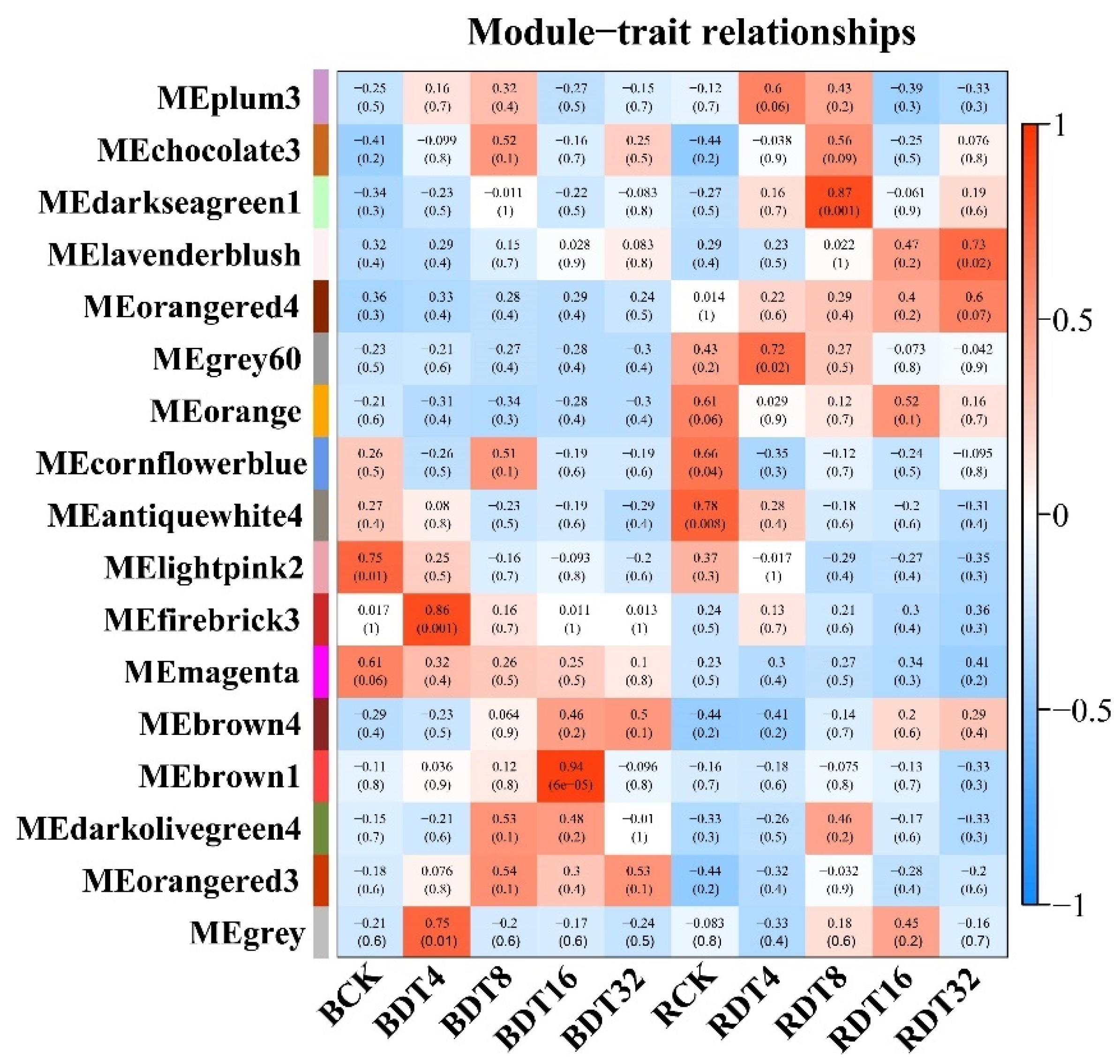
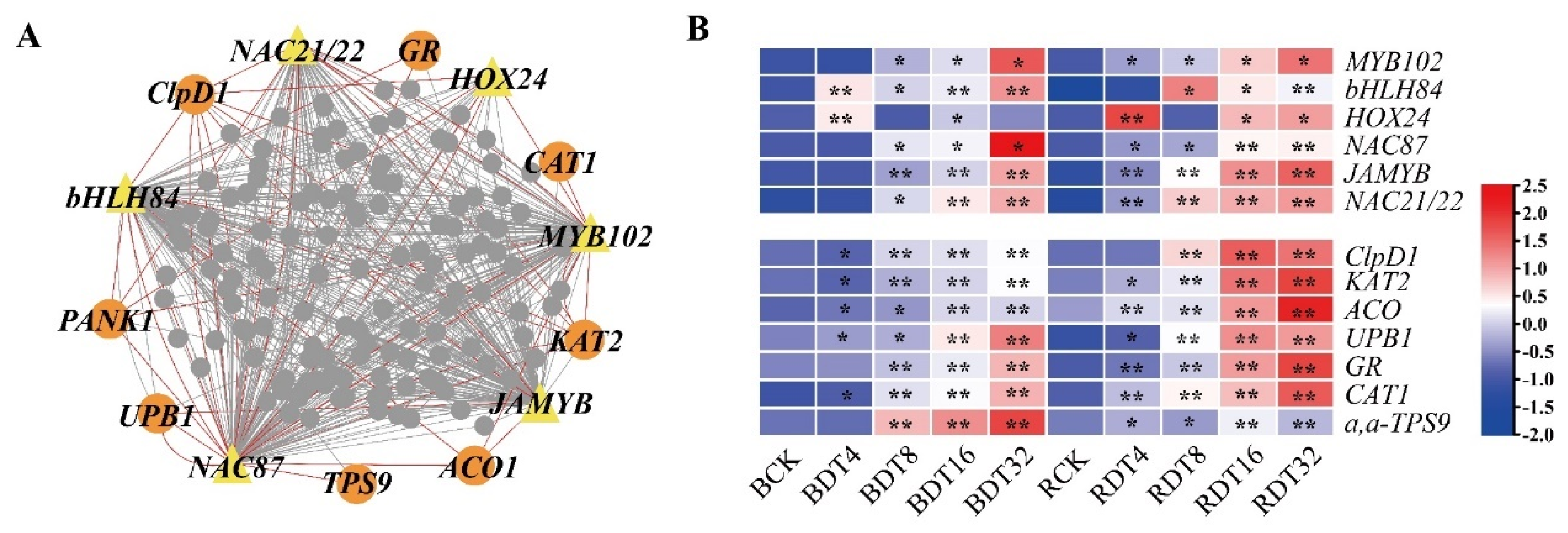
2.4. Co-Expression Network Revealed Modules with Different Expression Patterns under Drought Treatment
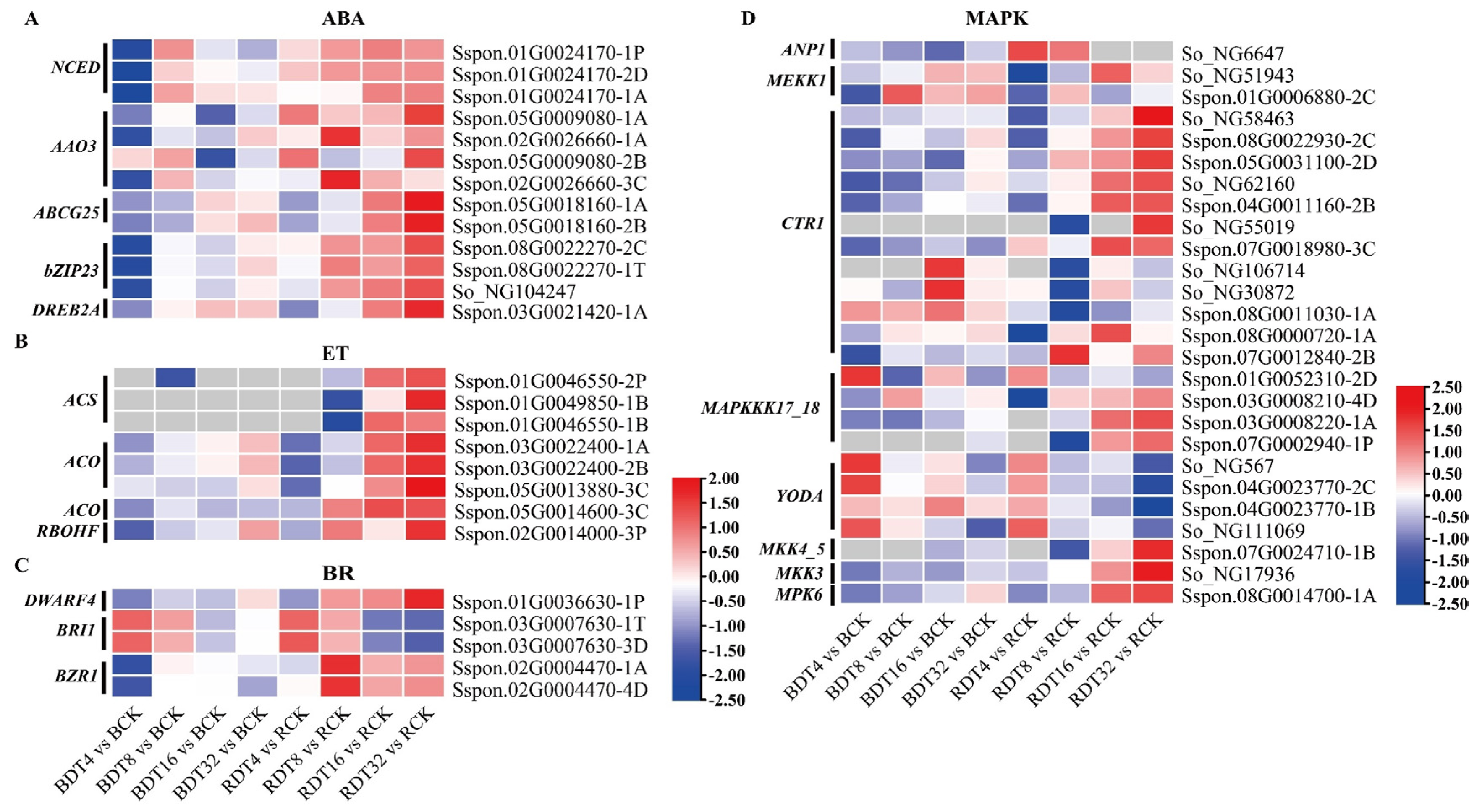
2.5. Complex Signal Transduction Processes Caused by Drought Stress
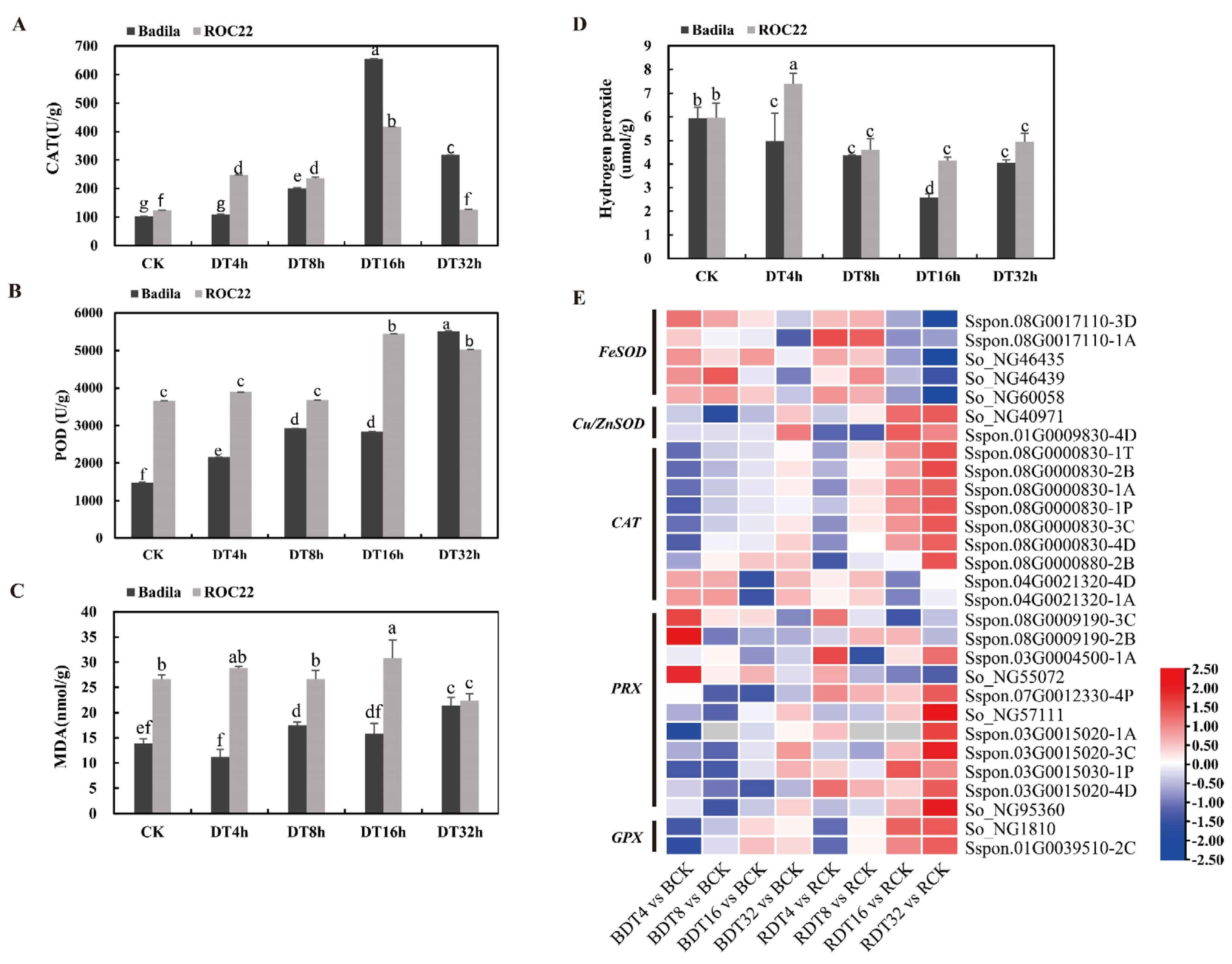
2.6. Enhancement of Antioxidant Capacity of Sugarcane under Drought Stress
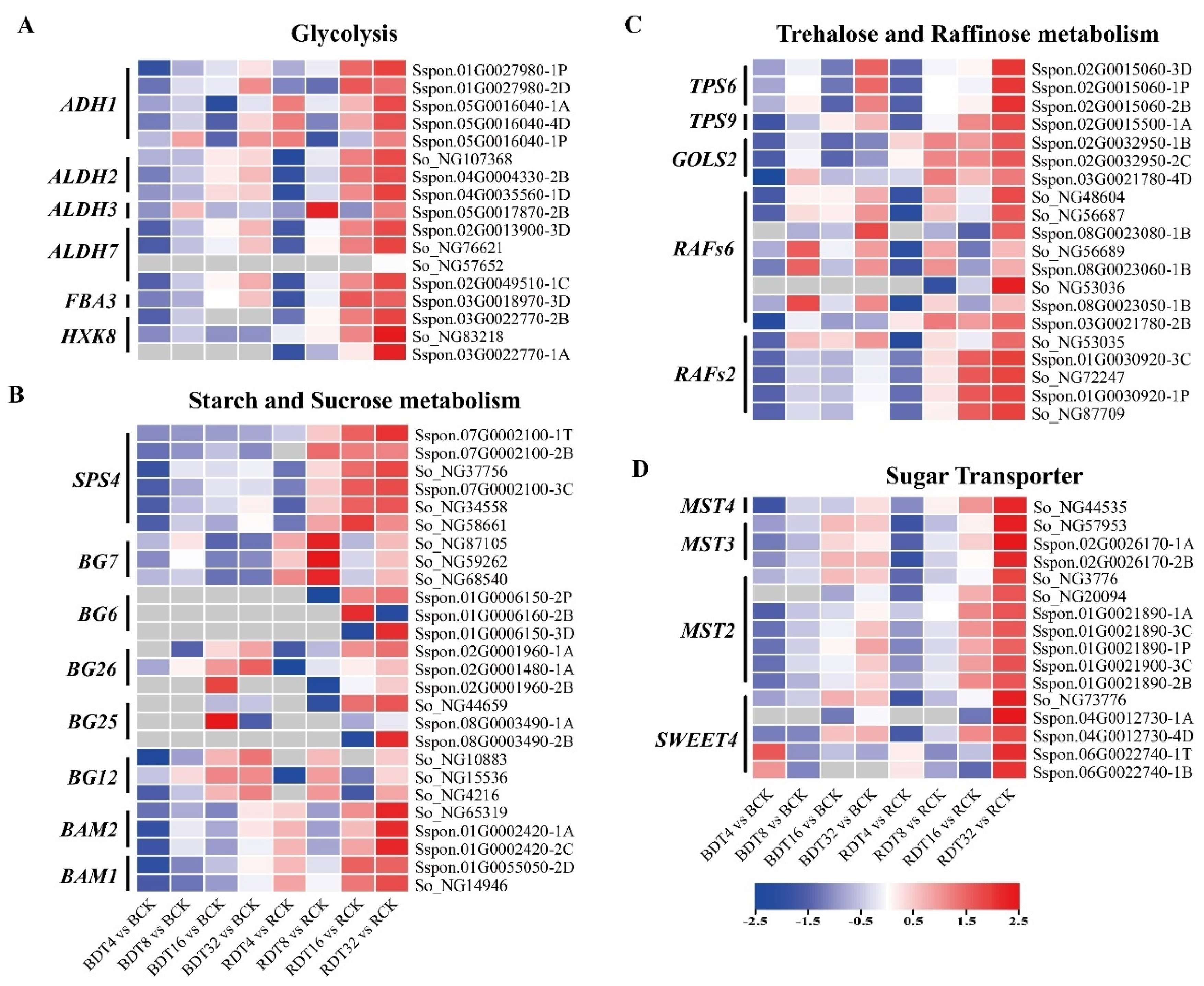
2.7. Sugarcane Carbohydrate Metabolism under Drought Stress
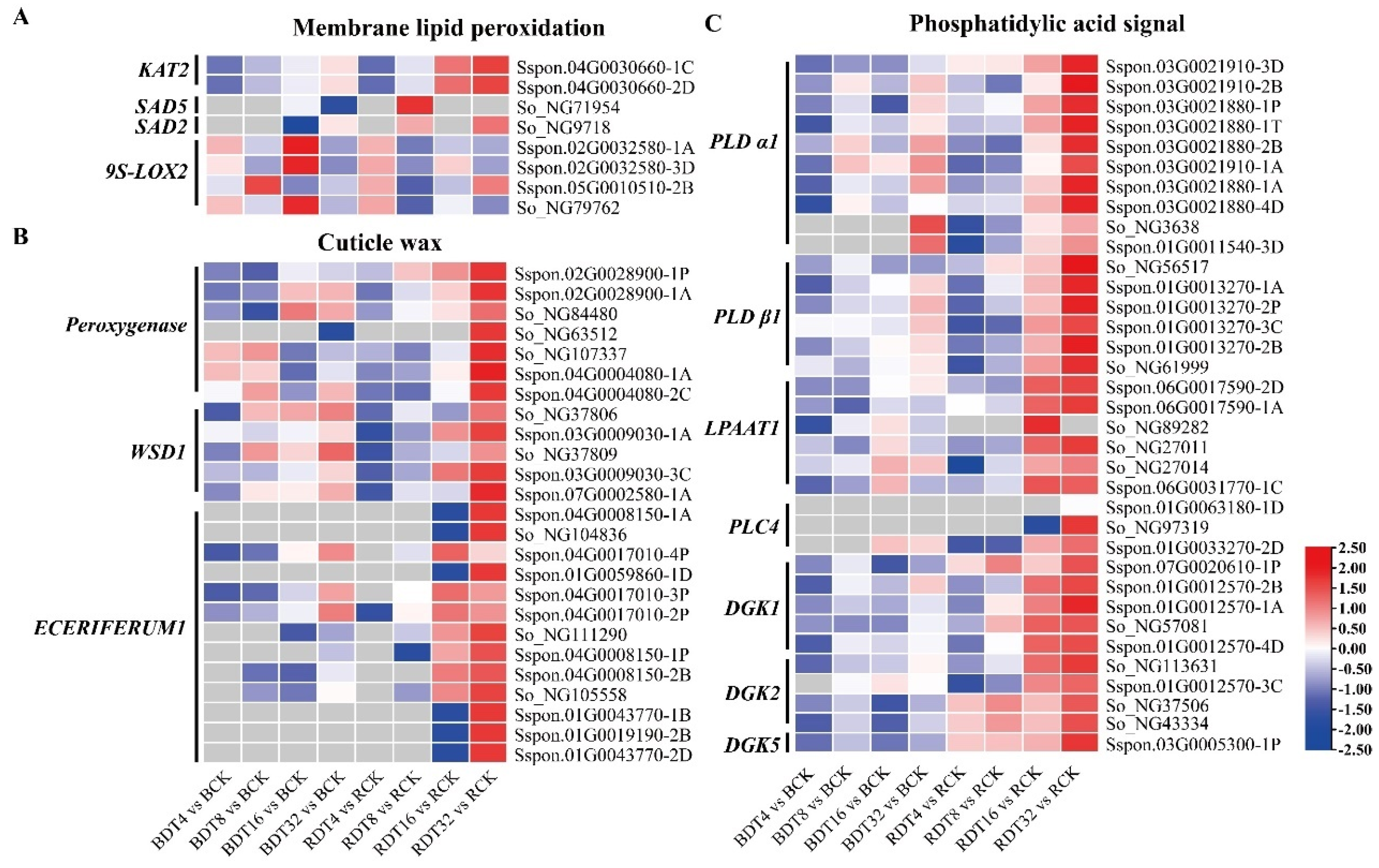
2.8. Sugarcane Lipid Metabolism under Drought Stress
2.9. Regulatory Network of Sugarcane in Response to Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Library Preparation
4.2. RNA-Seq and Data Analyses
4.3. Co-Expression Network Construction
4.4. Physiological Indicator Measurements
4.4.1. Relative Water Content of Leaves
4.4.2. Net Photosynthetic Rate and Stomatal Conductance
4.4.3. POD and CAT Enzyme Activities and MDA and H2O2 Content
4.5. RT-qPCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, S.; Zhao, X.; Liu, Y.; Xing, Y.; Dao, J.; Wei, B.; Peng, Y.; Duan, W.; Wang, Z. Drought Sensitivity of Sugarcane Cultivars Shapes Rhizosphere Bacterial Community Patterns in Response to Water Stress. Front. Microbiol. 2021, 12, 732989. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [Green Version]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; Lopez, J.D.D.B.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory Gene Networks in Drought Stress Responses and Resistance in Plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Dai, X.; Zhang, W.-H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moenga, S.M.; Gai, Y.; Carrasquilla-Garcia, N.; Perilla-Henao, L.M.; Cook, D.R. Gene co-expression analysis reveals tran-scriptome divergence between wild and cultivated chickpea under drought stress. Plant J. 2020, 104, 1195–1214. [Google Scholar] [CrossRef]
- Yiqiang, C. Screening and Analysis of the Sugarcane Drought Resistant Germplasm and the Inter-Generic Hybrids from Crossing of Saccharum L. and E. arundinaceus. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China, 2005. [Google Scholar]
- Kim, J.-S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE Promoter Sequence is Involved in Osmotic Stress-Responsive Expression of the DREB2A Gene, Which Encodes a Transcription Factor Regulating Drought-Inducible Genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and Regulation of Mitochondrial Respiration in Plants. Annu. Rev. Plant Biol. 2011, 62, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Watanabe, S.; Shimada, H.; Sakamoto, A. Dynamics of the leaf endoplasmic reticulum modulate β-glucosidase-mediated stress-activated ABA production from its glucosyl ester. J. Exp. Bot. 2020, 71, 2058–2071. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Lang, S.; Wang, D.; Xue, H.; Wang, X.-F. Functional characterization of galactinol synthase and raffinose synthase in desiccation tolerance acquisition in developing Arabidopsis seeds. J. Plant Physiol. 2018, 230, 109–121. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2021, 171, 620–637. [Google Scholar] [CrossRef]
- Germain, V.; Rylott, E.L.; Larson, T.R.; Sherson, S.M.; Bechtold, N.; Carde, J.; Bryce, J.H.; Graham, I.A.; Smith, S.M. Require-ment for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 2001, 28, 1–12. [Google Scholar] [CrossRef]
- Parvini, F.; Sicardo, M.D.; Hosseini-Mazinani, M.; Martínez-Rivas, J.M.; Hernández, M.L. Transcriptional Analysis of Stea-royl-Acyl Carrier Protein Desaturase Genes from Olive (Olea europaea) in Relation to the Oleic Acid Content of the Virgin Olive Oil. J. Agric. Food Chem. 2016, 64, 7770–7781. [Google Scholar] [CrossRef]
- Alves, M.L.; Bento-Silva, A.; Gaspar, D.; Paulo, M.; Brites, C.; Mendes-Moreira, P.; Bronze, M.D.R.; Malosetti, M.; Van Eeuwijk, F.; Patto, M.C.V. Volatilome–Genome-Wide Association Study on Wholemeal Maize Flour. J. Agric. Food Chem. 2020, 68, 7809–7818. [Google Scholar] [CrossRef]
- Lequeu, J.; Fauconnier, M.-L.; Chammaï, A.; Bronner, R.; Blée, E. Formation of plant cuticle: Evidence for the occurrence of the peroxygenase pathway. Plant J. 2003, 36, 155–164. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Lam, P.; Bird, D.; Zheng, H.; Samuels, L.; Jetter, R.; Kunst, L. Identification of the Wax Ester Syn-thase/Acyl-Coenzyme A:Diacylglycerol Acyltransferase WSD1 Required for Stem Wax Ester Biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-K.; Huang, B.-H.; Ho, S.-W.; Huang, M.-Y.; Wang, J.-C.; Gao, J.; Liao, P.-C. Molecular genetic and biochemical evidence for adaptive evolution of leaf abaxial epicuticular wax crystals in the genus Lithocarpus (Fagaceae). BMC Plant Biol. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C.; Wang, X. Phosphatidic acid: An emerging versatile class of cellular mediators. Essays Biochem. 2020, 64, 533–546. [Google Scholar] [CrossRef]
- Liu, S.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic ex-pression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2019, 102, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Windari, E.A.; Ando, M.; Mizoguchi, Y.; Shimada, H.; Ohira, K.; Kagaya, Y.; Higashiyama, T.; Takayama, S.; Watanabe, M.; Suwabe, K. Two aquaporins, SIP1;1 and PIP1;2, mediate water transport for pollen hydration in the Arabidopsis pistil. Plant Biotechnol. 2021, 38, 77–87. [Google Scholar] [CrossRef]
- Shukla, S.; Zhao, C.; Shukla, D. Dewetting Controls Plant Hormone Perception and Initiation of Drought Resistance Signaling. Structure 2019, 27, 692–702.e3. [Google Scholar] [CrossRef] [Green Version]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yin, Y.; Fei, S. Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci. 2015, 234, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Yoshida, R.; Ichimura, K.; Mizoguchi, T.; Seo, S.; Yonezawa, M.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The Mitogen-Activated Protein Kinase Cascade MKK3–MPK6 Is an Important Part of the Jasmonate Signal Transduction Pathway in Arabidopsis. Plant Cell 2007, 19, 805–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Woo, D.-H.; Kim, J.-M.; Lee, S.-Y.; Chung, W.S.; Moon, Y.-H. Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem. Biophys. Res. Commun. 2011, 412, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Lu, Y.; Wang, Q.; La, Y.; Hong, X.; Tan, F.; Niu, J.; Xia, H.; Wu, Y.; Zhou, S.; et al. Increased Drought Resistance 1 Mutation Increases Drought Tolerance of Upland Rice by Altering Physiological and Morphological Traits and Limiting ROS Levels. Plant Cell Physiol. 2021, 62, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, L.-L.; Gong, S.-Y.; Sun, X.; Li, P.; Qin, L.-X.; Zhou, Y.; Xu, W.-L.; Li, X.-B. A cotton mitogen-activated protein kinase (GhMPK6) is involved in ABA-induced CAT1 expression and H2O2 production. J. Genet. Genom. 2011, 38, 557–565. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Y.-L.; Wu, H.-T.; Shalmani, A.; Liu, W.-T.; Li, W.-Q.; Xu, J.-W.; Chen, K.-M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Ouyang, B.; Shi, C.; Demidchik, V.; Hao, Z.; Yu, M.; Shabala, S. NADPH oxidases and the evolution of plant salinity tolerance. Plant Cell Environ. 2020, 43, 2957–2968. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- Cai, B.; Li, Q.; Xu, Y.-C.; Yang, L.; Bi, H.; Ai, X. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol. Biochem. 2016, 108, 251–265. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Yao, Y.; Wei, Y.; Chan, Z. Alcohol dehydrogenase 1 (ADH1 ) confers both abiotic and biotic stress resistance in Arabidopsis. Plant Sci. 2017, 262, 24–31. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Andrade, M.O.; Gomes, A.P.S.; DaMatta, F.M.; Baracat-Pereira, M.C.; Fontes, E.P. Arabidopsis and tobacco plants ectopically expressing the soybean antiquitin-like ALDH7 gene display enhanced tolerance to drought, salinity, and oxidative stress. J. Exp. Bot. 2006, 57, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Lugassi, N.; Yadav, B.S.; Egbaria, A.; Wolf, D.; Kelly, G.; Neuhaus, E.; Raveh, E.; Carmi, N.; Granot, D. Expression of Ara-bidopsis Hexokinase in Tobacco Guard Cells Increases Water-Use Efficiency and Confers Tolerance to Drought and Salt Stress. Plants 2019, 8, 613. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Lee, K.H.; Dong, T.; Jeong, J.C.; Jin, J.B.; Kanno, Y.; Kim, D.H.; Kim, S.Y.; Seo, M.; Bressan, R.A.; et al. A Vacuolar β-Glucosidase Homolog That Possesses Glucose-Conjugated Abscisic Acid Hydrolyzing Activity Plays an Important Role in Osmotic Stress Responses in Arabidopsis. Plant Cell 2012, 24, 2184–2199. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, S.; Dong, Y.; Ren, R.; Chen, D.; Chen, X. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 2020, 226, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, X.-F.; Wang, X.-F.; Zhang, D.-P. Arabidopsis 3-Ketoacyl-CoA Thiolase-2 (KAT2), an Enzyme of Fatty Acid β-Oxidation, is Involved in ABA Signal Transduction. Plant Cell Physiol. 2011, 52, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Zeisler-Diehl, V.V.; Schreiber, L.; Bi, Y.-M.; Rothstein, S.J.; Ranathunge, K. Overexpression of ANAC046 Pro-motes Suberin Biosynthesis in Roots of Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmita, S.; Ekatiyar, A.; Chinnusamy, V.; Epandey, D.M.; Bansal, K.C. Transcriptional Regulatory Network Analysis of MYB Transcription Factor Family Genes in Rice. Front. Plant Sci. 2015, 6, 1157. [Google Scholar] [CrossRef] [Green Version]
- Piao, W.; Kim, S.-H.; Lee, B.-D.; An, G.; Sakuraba, Y.; Paek, N.-C. Rice transcription factor OsMYB102 delays leaf senescence by down-regulating abscisic acid accumulation and signaling. J. Exp. Bot. 2019, 70, 2699–2715. [Google Scholar] [CrossRef]
- Yokotani, N.; Ichikawa, T.; Kondou, Y.; Iwabuchi, M.; Matsui, M.; Hirochika, H.; Oda, K. Role of the rice transcription factor JAmyb in abiotic stress response. J. Plant Res. 2012, 126, 131–139. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2−ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
| Module Name | Number of Genes | GO-Enriched (FDR ≤ 0.05) | Expression Trends (Average) |
|---|---|---|---|
| antiquewhite4 cornflowerblue RCK | 2809 36 | phospholipid biosynthetic process (GO:0046474) membrane lipid biosynthetic process (GO:0046467) glycerophospholipid biosynthetic process (GO:0046474) hydrogen peroxide biosynthetic process (GO:0050665) glycolytic process (GO:0006096) |  |
|
lightpink2
BCK | 1390 | phospholipid biosynthetic process (GO:0008654) membrane lipid biosynthetic process (GO:0046467) glycerophospholipid biosynthetic process (GO:0046474) galactolipid biosynthetic process (GO:0019375) galactolipid metabolic process (GO:0019374) regulation of cell growth (GO:0001558) cellular developmental process (GO:0048869) |  |
| grey60 RDT4 | 1110 | plant-type cell wall cellulose biosynthetic process (GO:0052324) cellulose biosynthetic process (GO:0030244) cellulose microfibril organization (GO:0010215) |  |
| firebrick3 grey BDT4 | 67 52 | glucose-6-phosphate dehydrogenase activity (GO:0004345) |  |
| darkseagreen1 RDT8 | 157 | regulation of brassinosteroid mediated signaling pathway (GO:1900457) negative regulation of brassinosteroid mediated signaling pathway (GO:1900458) steroid biosynthetic process (GO:0006694) |  |
| Brown1 BDT16 | 49 | tRNA metabolic process (GO:0006399) intra-Golgi vesicle-mediated transport (GO:0006891) cis-Golgi network membrane (GO:0033106) |  |
|
lavenderblush
RDT32 | 4231 | peroxisome (GO:0007031) glucose transport (GO:0015758) monosaccharide transport (GO:0015749) fatty acid beta-oxidation (GO:0006635) lipid oxidation (GO:0034440) |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Lin, P.; Zhao, Z.; Li, Z.; Liu, Y.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, Y.; et al. Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress. Int. J. Mol. Sci. 2022, 23, 569. https://doi.org/10.3390/ijms23010569
Li P, Lin P, Zhao Z, Li Z, Liu Y, Huang C, Huang G, Xu L, Deng Z, Zhang Y, et al. Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress. International Journal of Molecular Sciences. 2022; 23(1):569. https://doi.org/10.3390/ijms23010569
Chicago/Turabian StyleLi, Peiting, Pingping Lin, Zhenli Zhao, Zihong Li, Yanming Liu, Chaohua Huang, Guoqiang Huang, Liangnian Xu, Zuhu Deng, Yu Zhang, and et al. 2022. "Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress" International Journal of Molecular Sciences 23, no. 1: 569. https://doi.org/10.3390/ijms23010569
APA StyleLi, P., Lin, P., Zhao, Z., Li, Z., Liu, Y., Huang, C., Huang, G., Xu, L., Deng, Z., Zhang, Y., & Zhao, X. (2022). Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress. International Journal of Molecular Sciences, 23(1), 569. https://doi.org/10.3390/ijms23010569






