Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model
Abstract
:1. Introduction
2. Results
2.1. Characterization of Gelatin/Nano-Hydroxyapatite/Metformin Scaffold (GHMS)
2.1.1. Morphology of GHMS
2.1.2. Crystal Phase Identification
2.1.3. Functional Group Identification
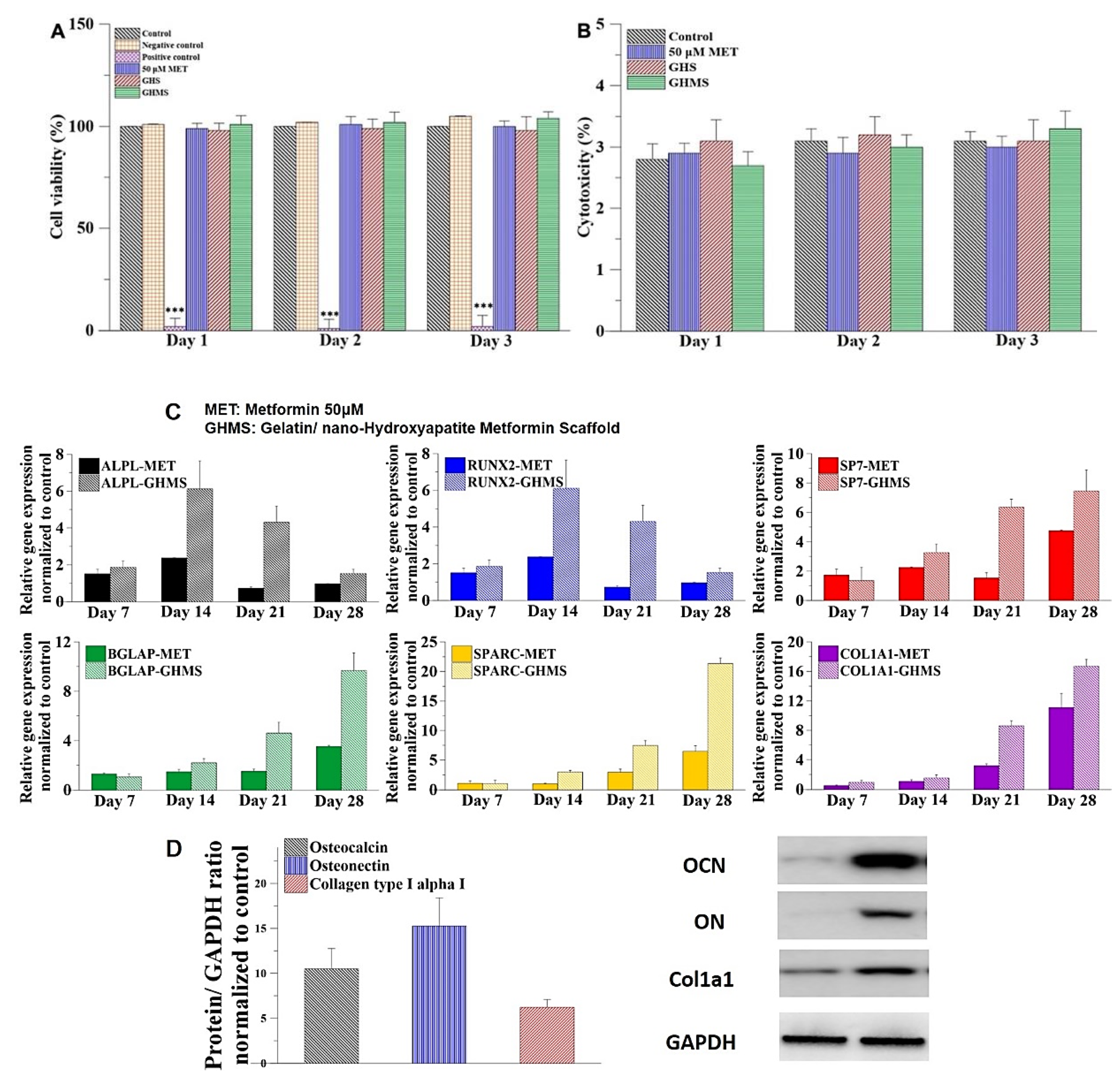
2.2. Biocompatibility of Gelatin/Nano-Hydroxyapatite/Metformin Scaffold (GHMS)
2.3. Osteogenic Genes Expression and Protein Synthesis
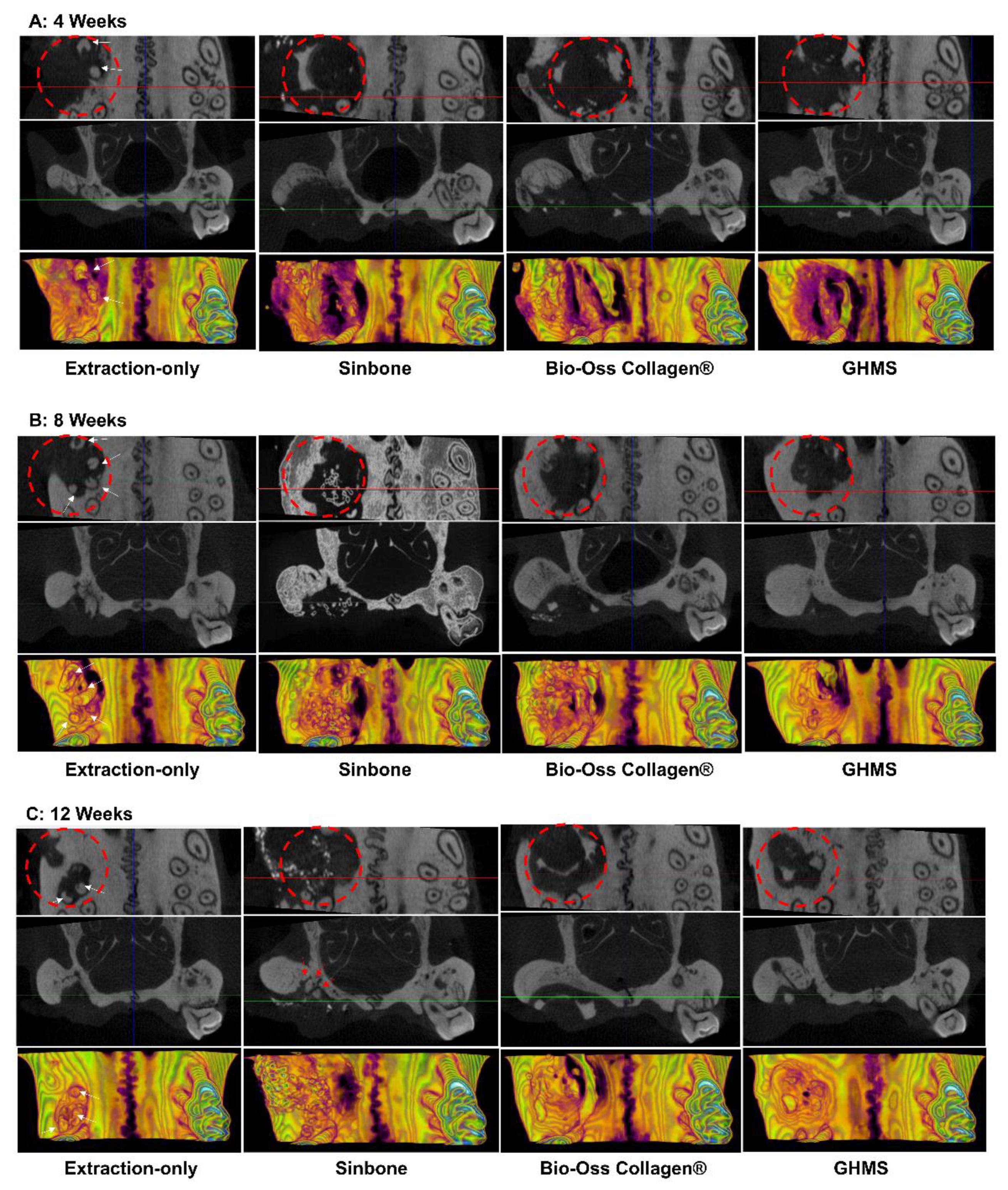
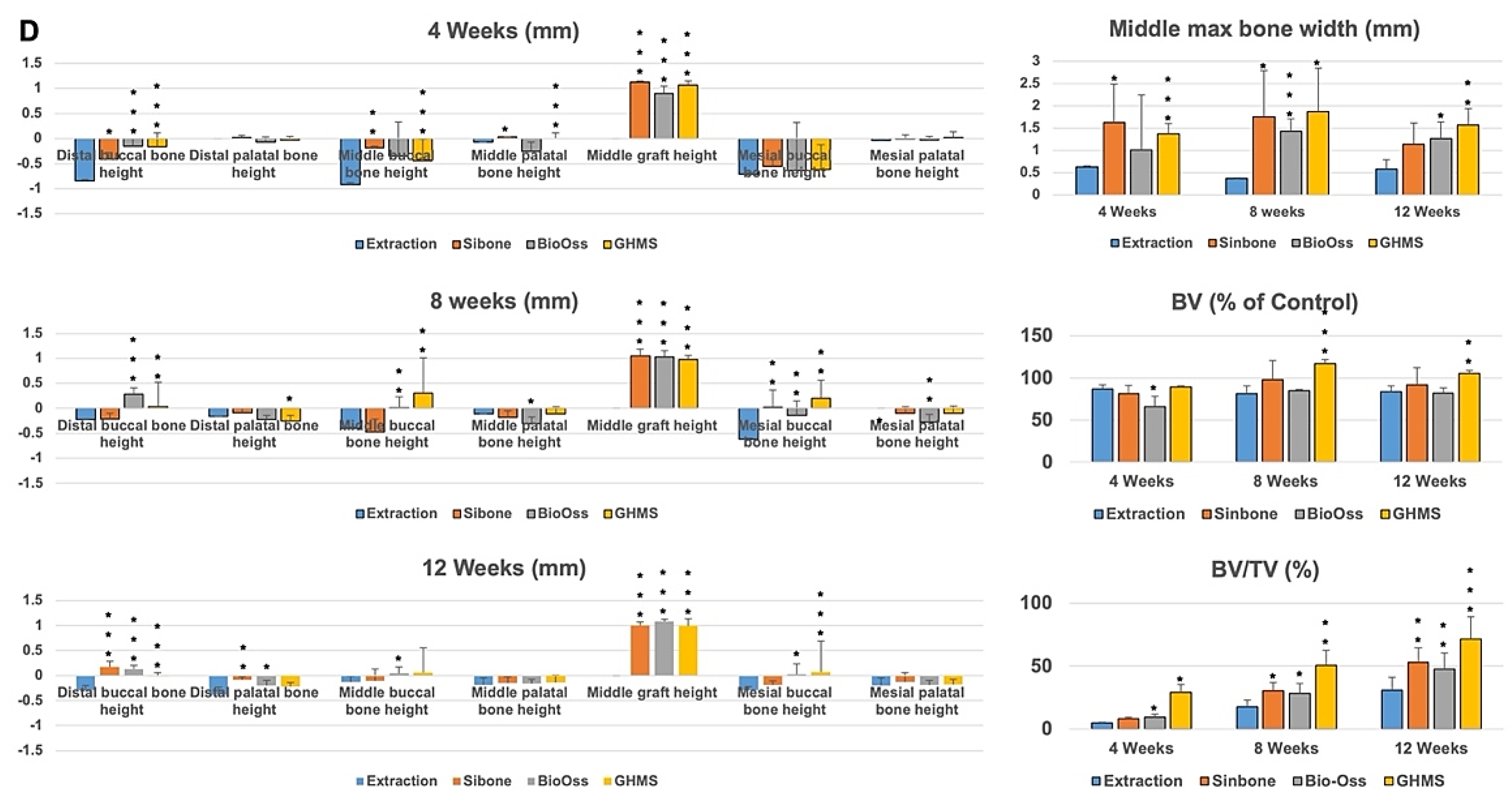
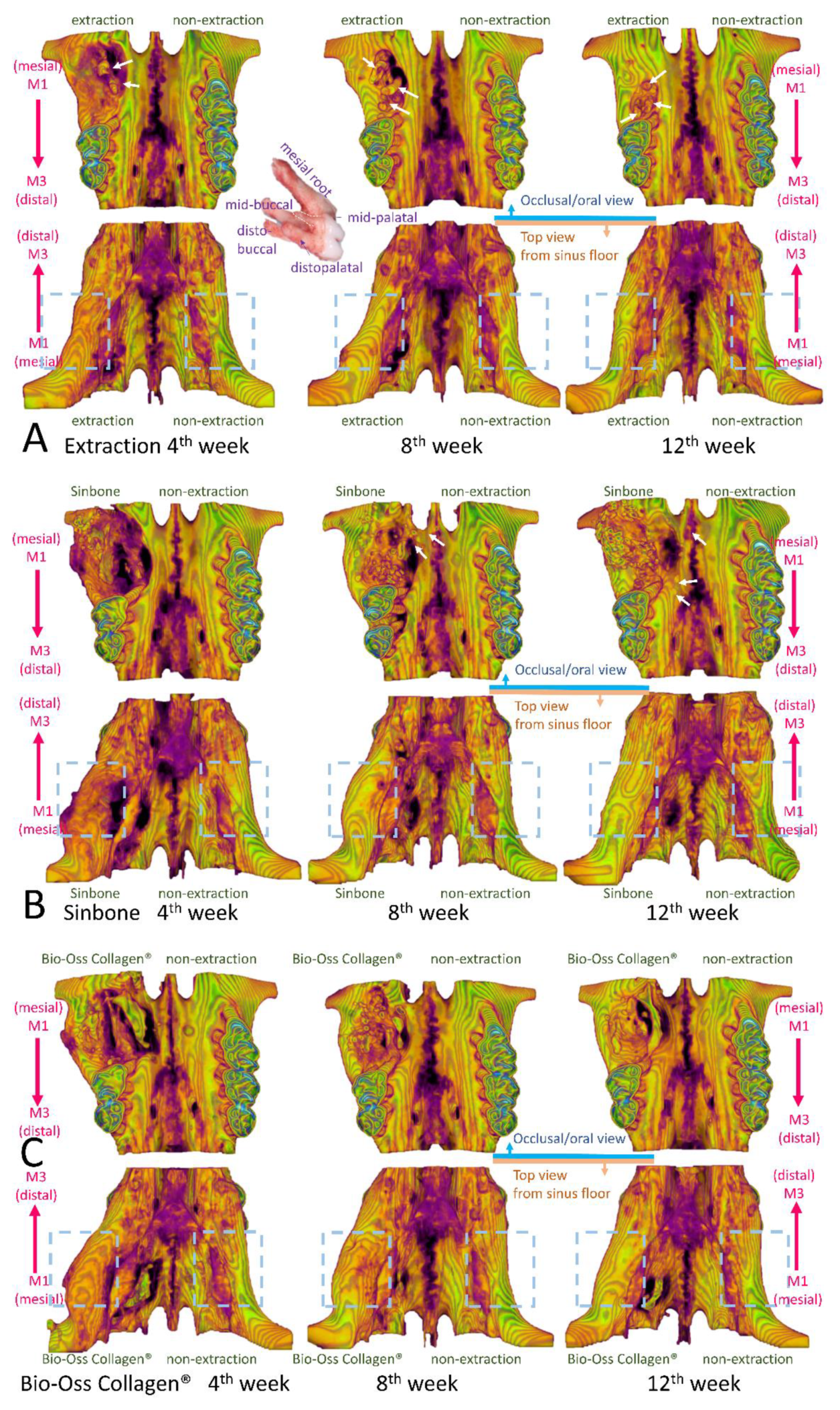
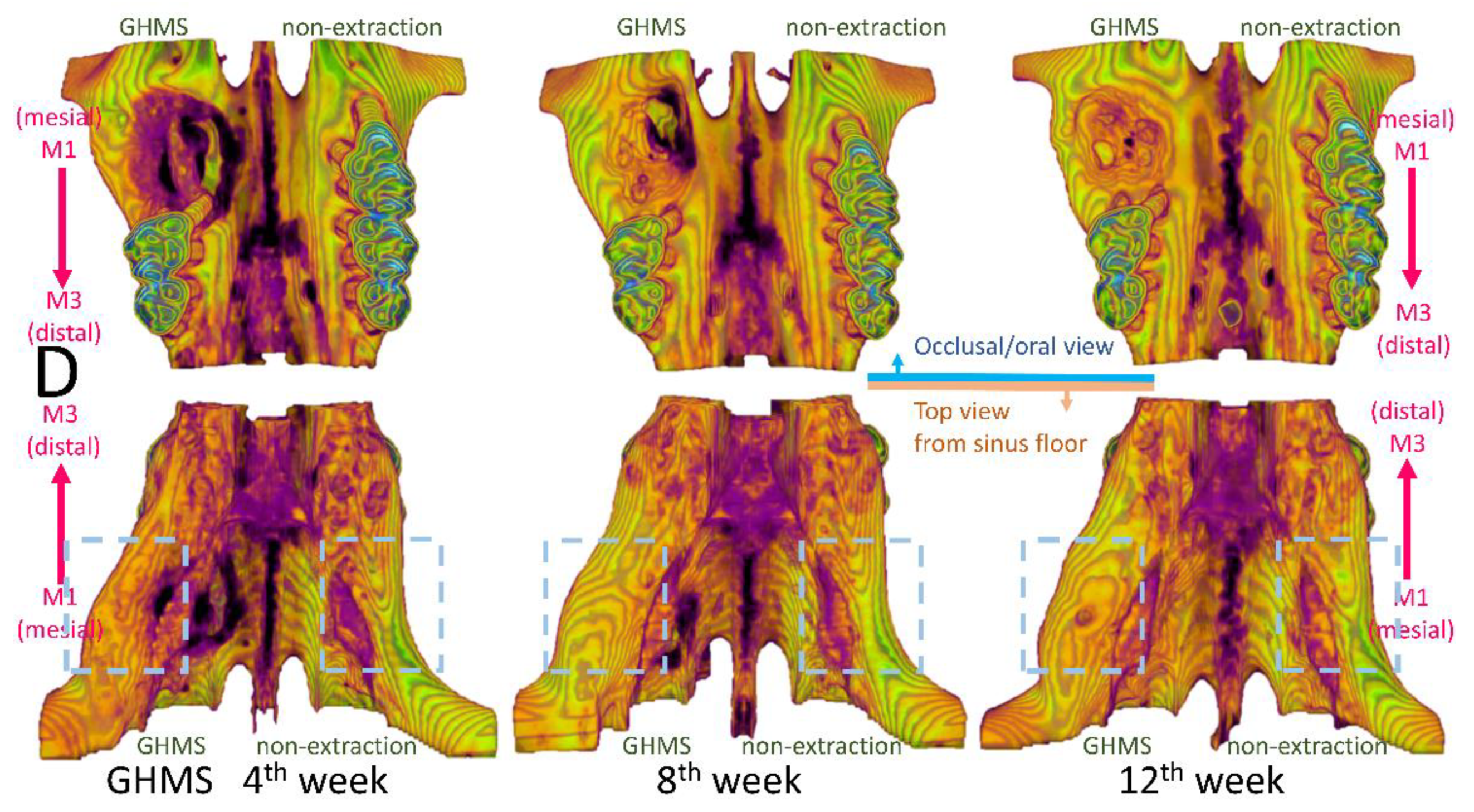
2.4. Micro-Computed Tomography (Micro-CT) Analysis
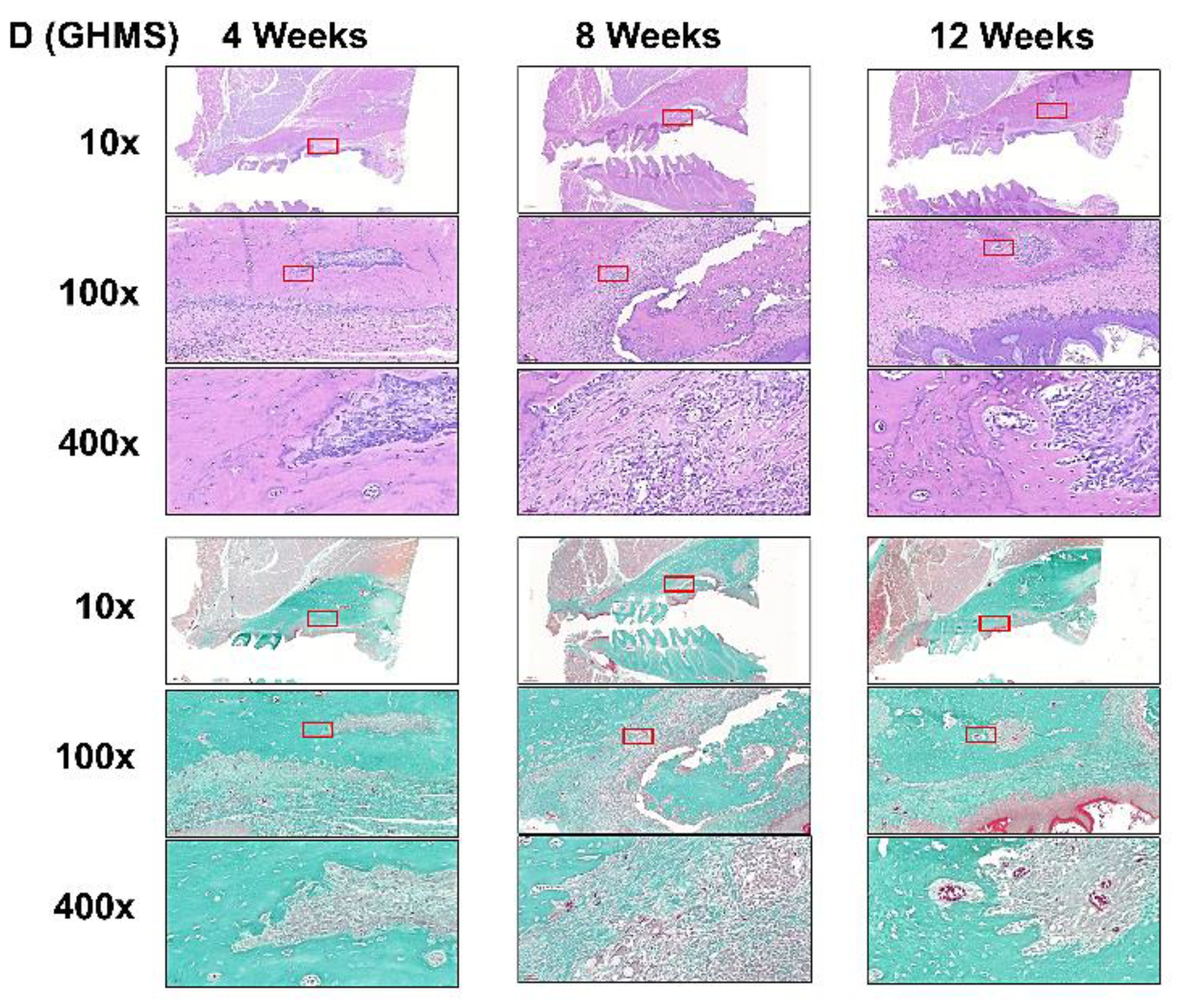
2.5. Histological Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Gelatin/Nano-Hydroxyapatite/Metformin Scaffold (GHMS)
4.3. Characterization of Gelatin/Nano-Hydroxyapatite/Metformin Scaffold (GHMS)
4.4. In Vitro Study
4.4.1. Isolation and Culture of Human Mesenchymal Stem Cells (hMSCs)
4.4.2. Cell Viability of Gelatin/Nano-Hydroxyapatite/Metformin Scaffold (GHMS)
4.4.3. Cytotoxicity
4.4.4. Gene Expression
4.4.5. Protein Expression
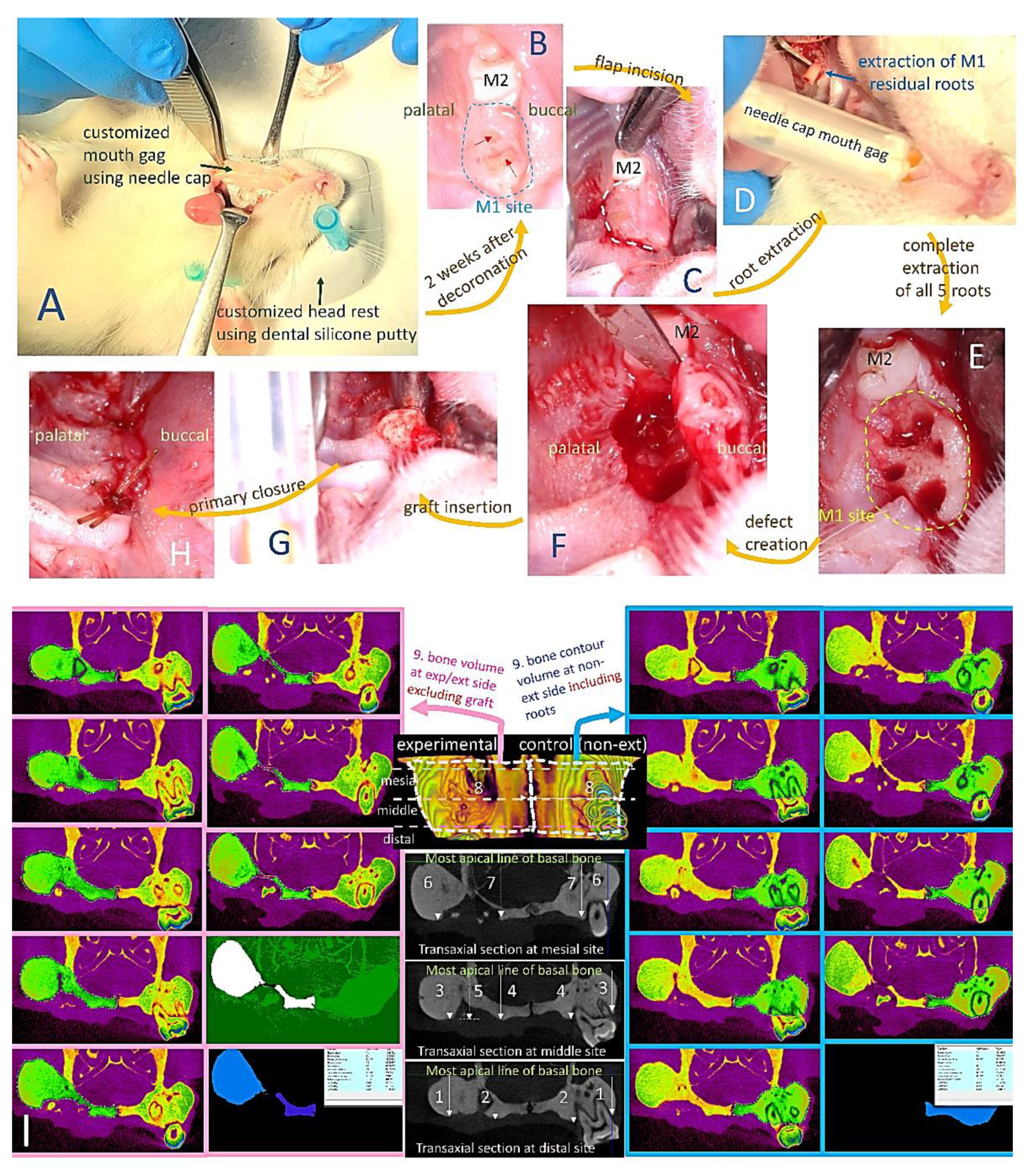
4.5. In Vivo Study
4.5.1. Micro-Computed Tomography (Micro-CT) Analysis
4.5.2. Histological Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef]
- Mardas, N.; Trullenque-Eriksson, A.; Macbeth, N.; Petrie, A.; Donos, N. Does ridge preservation following tooth extraction improve implant treatment outcomes: A systematic review. Clin. Oral Implant. Res. 2015, 26, 180–201. [Google Scholar] [CrossRef]
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2015, 18, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Oberbek, P.; Bolek, T.; Chlanda, A.; Hirano, S.; Kusnieruk, S.; Rogowska-Tylman, J.; Nechyporenko, G.; Zinchenko, V.; Swieszkowski, W.; Puzyn, T. Characterization and influence of hydroxyapatite nanopowders on living cells. Beilstein J. Nanotechnol. 2018, 9, 3079–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iafisco, M.; Varoni, E.; Battistella, E.; Pietronave, S.; Prat, M.; Roveri, N.; Rimondini, L. The Cooperative Effect of Size and Crystallinity Degree on the Resorption of Biomimetic Hydroxyapatite for Soft Tissue Augmentation. Int. J. Artif. Organs 2010, 33, 765–774. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Fan, Y. Adverse Biological Effect of TiO2 and Hydroxyapatite Nanoparticles Used in Bone Repair and Replacement. Int. J. Mol. Sci. 2016, 17, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- López-Martínez, F.; Moreno, G.G.; Olivares-Ponce, P.; Jaramillo, D.E.; De Val, J.E.M.S.; Calvo-Guirado, J.L. Implants failures related to endodontic treatment. An observational retrospective study. Clin. Oral Implant. Res. 2014, 26, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Susin, C.; Min, J.-H.; Suh, H.-Y.; Sang, E.-J.; Ku, Y.; Wikesjö, U.M.E.; Koo, K.-T. Extraction sockets: Erratic healing impeding factors. J. Clin. Periodontol. 2013, 41, 80–85. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Castellanos-Cosano, L.; Machuca, G.; Lopez-Lopez, J.; Martin-Gonzalez, J.; Velasco-Ortega, E.; Sánchez-Domínguez, B.; López-Frías, F.J. Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e356–e361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugan, M.; Wojcicki, A.D.; d’Hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Salazar, G.; Patrushev, N.; Xiong, S.; Hilenski, L.; Fei, B.; Alexander, R.W. Metformin beyond diabetes: Pleiotropic benefits of metformin in attenuation of atherosclerosis. J. Am. Heart Assoc. 2014, 3, e001202. [Google Scholar] [CrossRef]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Cortizo, A.M.; Sedlinsky, C. Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J. Diabetes 2016, 7, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; D’Onofrio, L.; Eastell, R.; Schwartz, A.V.; Pozzilli, P.; Napoli, N. Oral anti-diabetic drugs and fracture risk, cut to the bone: Safe or dangerous? A narrative review. Osteoporos. Int. 2015, 26, 2073–2089. [Google Scholar] [CrossRef]
- Zhen, D.; Chen, Y.; Tang, X. Metformin reverses the deleterious effects of high glucose on osteoblast function. J. Diabetes Its Complicat. 2010, 24, 334–344. [Google Scholar] [CrossRef]
- Schurman, L.; McCarthy, A.D.; Sedlinsky, C.; Gangoiti, M.V.; Arnol, V.; Bruzzone, L.; Cortizo, A.M. Metformin Reverts Deleterious Effects of Advanced Glycation End-Products (AGEs) on Osteoblastic Cells. Exp. Clin. Endocrinol. Diabetes 2008, 116, 333–340. [Google Scholar] [CrossRef]
- Bak, E.J.; Park, H.G.; Kim, M.; Kim, S.W.; Kim, S.; Choi, S.-H.; Cha, J.-H.; Yoo, Y.-J. The Effect of Metformin on Alveolar Bone in Ligature-Induced Periodontitis in Rats: A Pilot Study. J. Periodontol. 2010, 81, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.; Rao, N.S.; Naik, S.B.; Kumari, M. Efficacy of Varying Concentrations of Subgingivally Delivered Metformin in the Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.S.; Pradeep, A.; Kumari, M.; Naik, S.B. Locally Delivered 1% Metformin Gel in the Treatment of Smokers with Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 1165–1171. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Hu, Y.; Peng, B. Protective Effect of Metformin on Periapical Lesions in Rats by Decreasing the Ratio of Receptor Activator of Nuclear Factor Kappa B Ligand/Osteoprotegerin. J. Endod. 2012, 38, 943–947. [Google Scholar] [CrossRef]
- Lai, E.H.-H.; Yang, C.-N.; Lin, S.-K.; Wang, H.-W.; Kok, S.-H.; Hong, C.-Y.; Su, I.-H.; Yang, H.; Chang, J.Z.-C. Metformin Ameliorates Periapical Lesions through Suppression of Hypoxia-induced Apoptosis of Osteoblasts. J. Endod. 2018, 44, 1817–1825. [Google Scholar] [CrossRef]
- Wang, H.-W.; Lai, E.H.-H.; Yang, C.-N.; Lin, S.-K.; Hong, C.-Y.; Yang, H.; Chang, J.Z.-C.; Kok, S.-H. Intracanal Metformin Promotes Healing of Apical Periodontitis via Suppressing Inducible Nitric Oxide Synthase Expression and Monocyte Recruitment. J. Endod. 2020, 46, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, X.; Ling-Ling, E.; Wang, D.-S.; Liu, H.-C. The transmembrane transport of metformin by osteoblasts from rat mandible. Arch. Oral Biol. 2009, 54, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of human extraction sockets filled with Bio-OssR. Clin. Oral Implant. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Canullo, L.; Dellavia, C.; Pellegrini, G.; Simion, M. Socket grafting in the posterior maxilla reduces the need for sinus augmentation. Int. J. Periodontics Restor. Dent. 2010, 30, 265–273. [Google Scholar]
- Zhao, L.; Xu, T.; Hu, W.; Chung, K.H. Preservation and augmentation of molar extraction sites affected by severe bone defect due to advanced periodontitis: A prospective clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Monje, A.; Lin, G.H.; Gargallo-Albiol, J.; Chan, H.L.; Wang, H.L.; Hernández-Alfaro, F. Alveolar ridge split on horizontal bone augmentation: A systematic review. Int. J. Oral Maxillofac. Implants. 2015, 30, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, Z.T.; Wainwright, M.; Troedhan, A. Flapless Piezotome Crest Split Achieves Comparable Outcomes to Autologous Onlay Grafts with Significant Less Patient Morbidity and Complications-A Randomized Clinical Study. J. Oral Maxillofac. Surg. 2020, 78, 1953–1964. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin. Oral Implants Res. 2009, 20, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Linder, E.; Lindhe, J. Effect of a xenograft on early bone formation in extraction sockets: An experimental study in dog. Clin. Oral Implants Res. 2009, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2017, 11, 2935–2949. [Google Scholar] [CrossRef]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020, 239, 119833. [Google Scholar] [CrossRef]
- Singh, V.; Nayak, D.; Uppoor, A.; Shah, D. Clinical and radiographic evaluation of Nano-crystalline hydroxyapatite bone graft (Sybograf®) in combination with bioresorbable collagen membrane (Periocol®) in periodontal intrabony defects. Dent. Res. J. 2012, 9, 60–67. [Google Scholar] [CrossRef]
- Gorojod, R.M.; Porte Alcon, S.; Dittler, M.L.; Gonzalez, M.C.; Kotler, M.L. Nanohydroxyapatite Exerts Cytotoxic Effects and Prevents Cellular Proliferation and Migration in Glioma Cells. Toxicol. Sci. 2019, 169, 34–42. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, H.-E.; Salih, V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin–hydroxyapatite for tissue engineering scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Hokugo, A.; Ozeki, M.; Kawakami, O.; Sugimoto, K.; Mushimoto, K.; Morita, S.; Tabata, Y. Augmented Bone Regeneration Activity of Platelet-Rich Plasma by Biodegradable Gelatin Hydrogel. Tissue Eng. 2005, 11, 1224–1233. [Google Scholar] [CrossRef]
- Fang, C.-H.; Lin, Y.-W.; Lin, F.-H.; Sun, J.-S.; Chao, Y.-H.; Lin, H.-Y.; Chang, Z.-C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrambeigi, S.; Yousefi, B.; Rahimi, M.; Shafiei-Irannejad, V. Metformin; An old antidiabetic drug with new potentials in bone disorders. Biomed. Pharmacother. 2018, 109, 1593–1601. [Google Scholar] [CrossRef]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 437–446. [Google Scholar] [CrossRef]
- Sun, C.-K.; Weng, P.-W.; Chang, Z.-C.; Lin, Y.-W.; Tsuang, F.-Y.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Metformin-Incorporated Gelatin/Hydroxyapatite Nano-Fibers Scaffold for Bone Regeneration. Tissue Eng. Part A 2021. [Google Scholar] [CrossRef]


| Peak Position on FTIR Spectra (cm−1) | Assignment of Bonds | Mode of Vibration |
|---|---|---|
| 3420 | O-H | stretching |
| 3369 | N-H | primary stretching vibration |
| 3294 | N-H | primary stretching vibration |
| 3155 | N-H | secondary stretching |
| 2800–2950 | C-H | stretching |
| 1626 | C-N | stretching |
| 1567 | C-N | stretching |
| 1063 | (PO4)3- | stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-H.; Sun, C.-K.; Lin, Y.-W.; Hung, M.-C.; Lin, H.-Y.; Li, C.-H.; Lin, I.-P.; Chang, H.-C.; Sun, J.-S.; Chang, J.Z.-C. Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model. Int. J. Mol. Sci. 2022, 23, 558. https://doi.org/10.3390/ijms23010558
Fang C-H, Sun C-K, Lin Y-W, Hung M-C, Lin H-Y, Li C-H, Lin I-P, Chang H-C, Sun J-S, Chang JZ-C. Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model. International Journal of Molecular Sciences. 2022; 23(1):558. https://doi.org/10.3390/ijms23010558
Chicago/Turabian StyleFang, Chih-Hsiang, Chung-Kai Sun, Yi-Wen Lin, Min-Chih Hung, Hung-Ying Lin, Ching-Hung Li, I-Ping Lin, Hung-Chen Chang, Jui-Sheng Sun, and Jenny Zwei-Chieng Chang. 2022. "Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model" International Journal of Molecular Sciences 23, no. 1: 558. https://doi.org/10.3390/ijms23010558
APA StyleFang, C.-H., Sun, C.-K., Lin, Y.-W., Hung, M.-C., Lin, H.-Y., Li, C.-H., Lin, I.-P., Chang, H.-C., Sun, J.-S., & Chang, J. Z.-C. (2022). Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model. International Journal of Molecular Sciences, 23(1), 558. https://doi.org/10.3390/ijms23010558







