The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice
Abstract
1. Introduction
2. Results
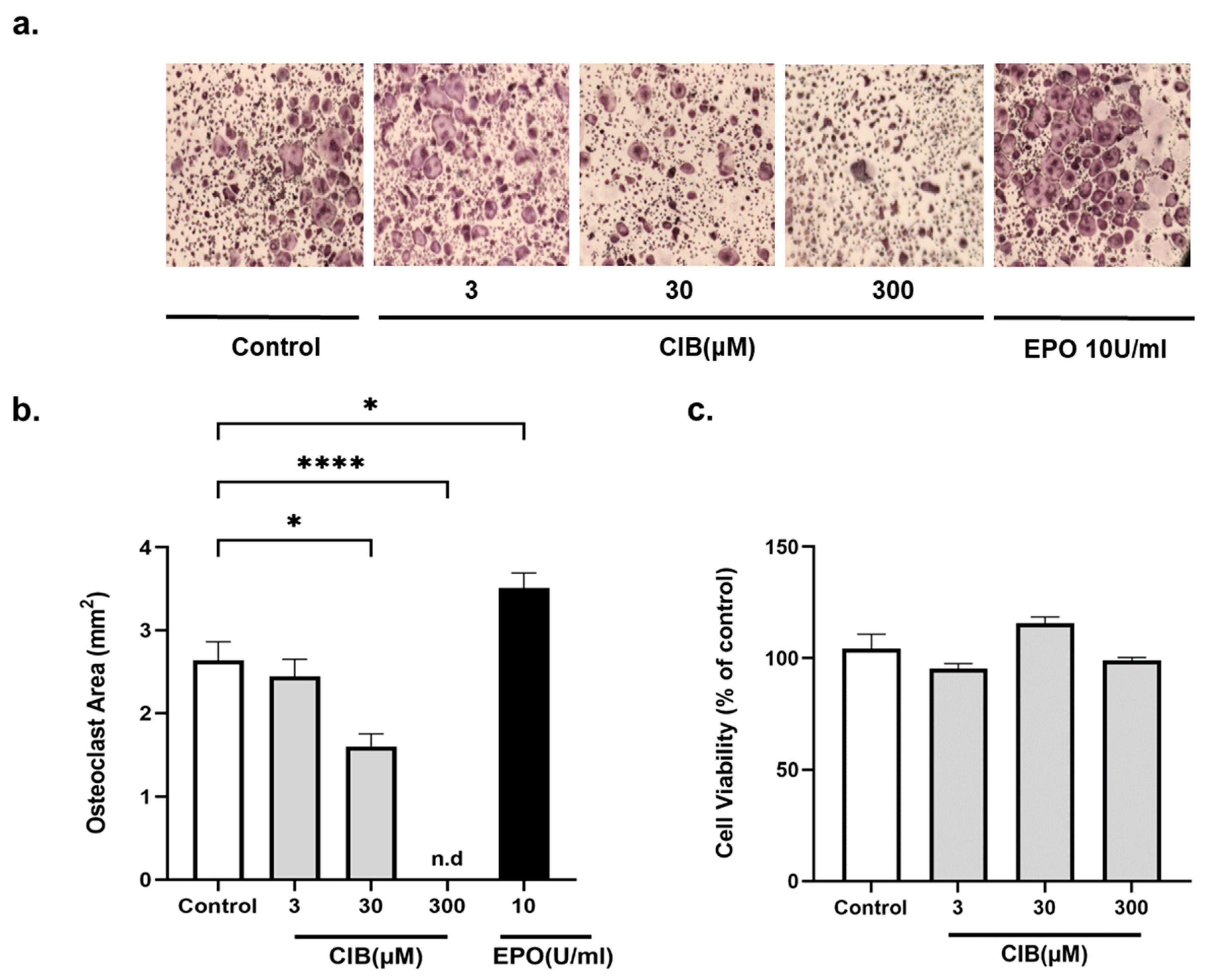
2.1. Cibinetide (CIB) Inhibits Osteoclastogenesis In Vitro, in a Dose Dependent Manner, without Cytotoxic Effects
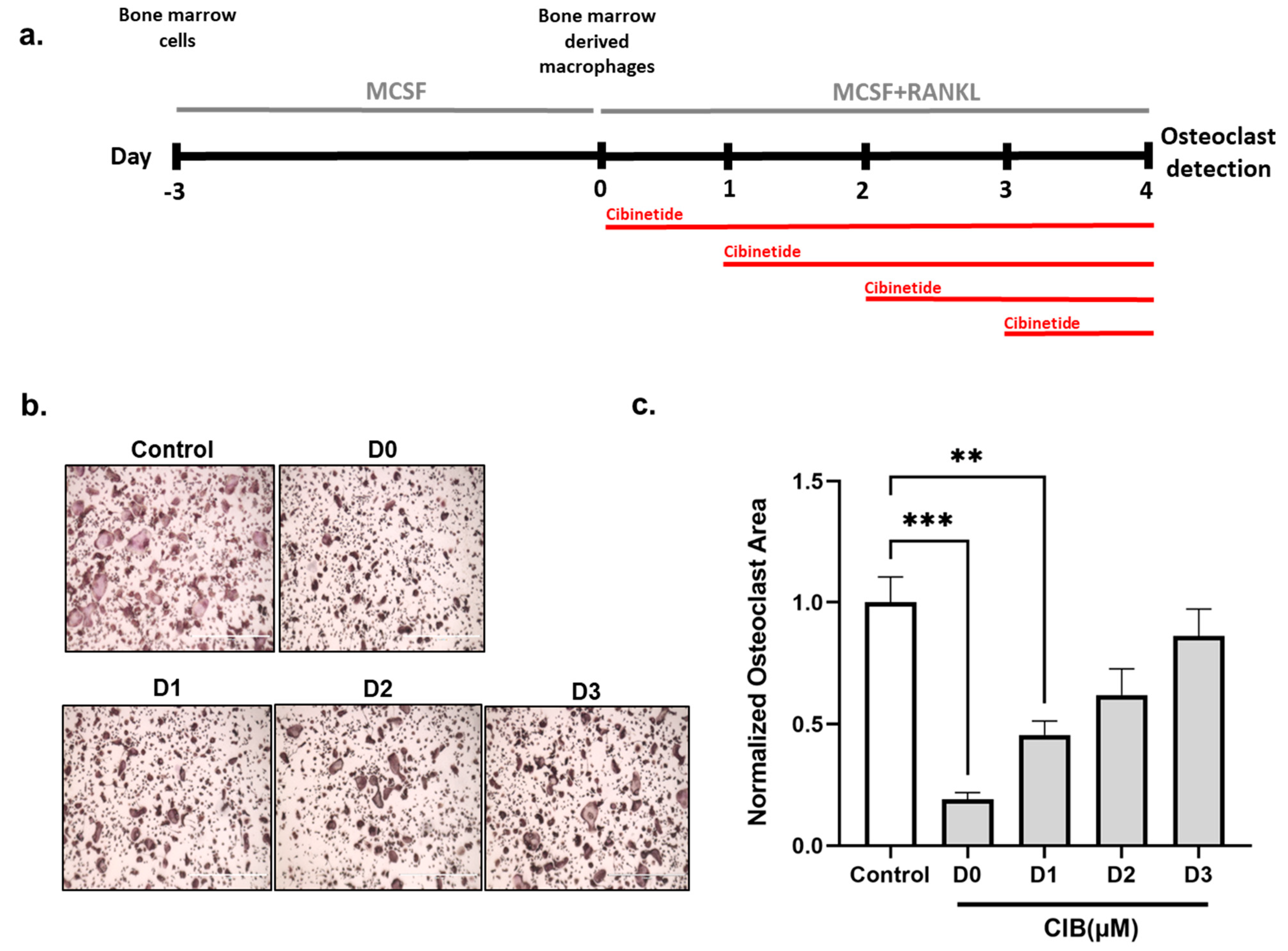
2.2. Cibinetide (CIB) Exerts Its Inhibitory Effects in the Early Stages of Osteoclastogenesis
2.3. Cibinetide (CIB) Suppresses the Expression of Osteoclast-Related Genes
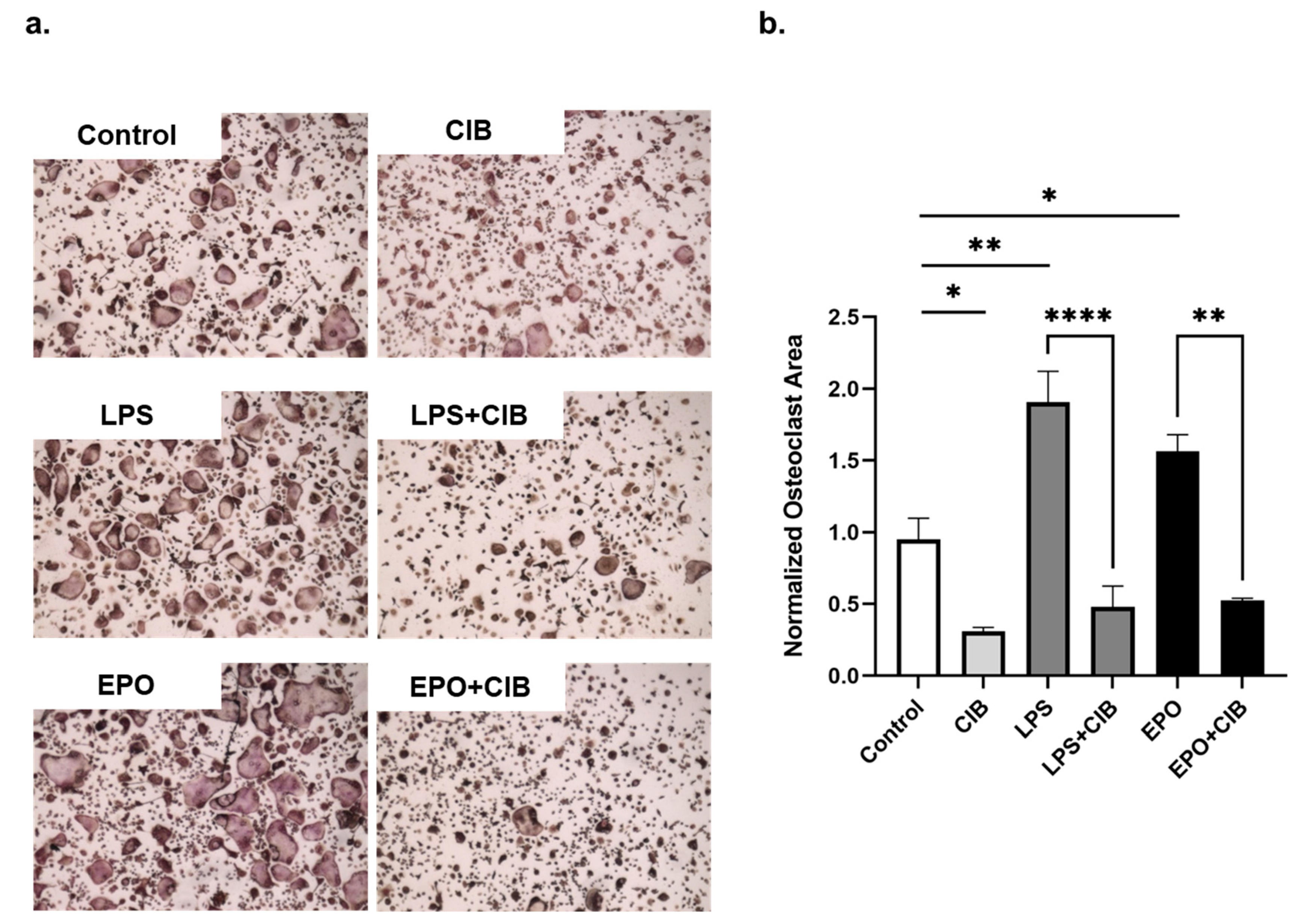
2.4. Cibinetide (CIB) Overrides the Pro-Osteoclastogenic Effects of LPS and EPO
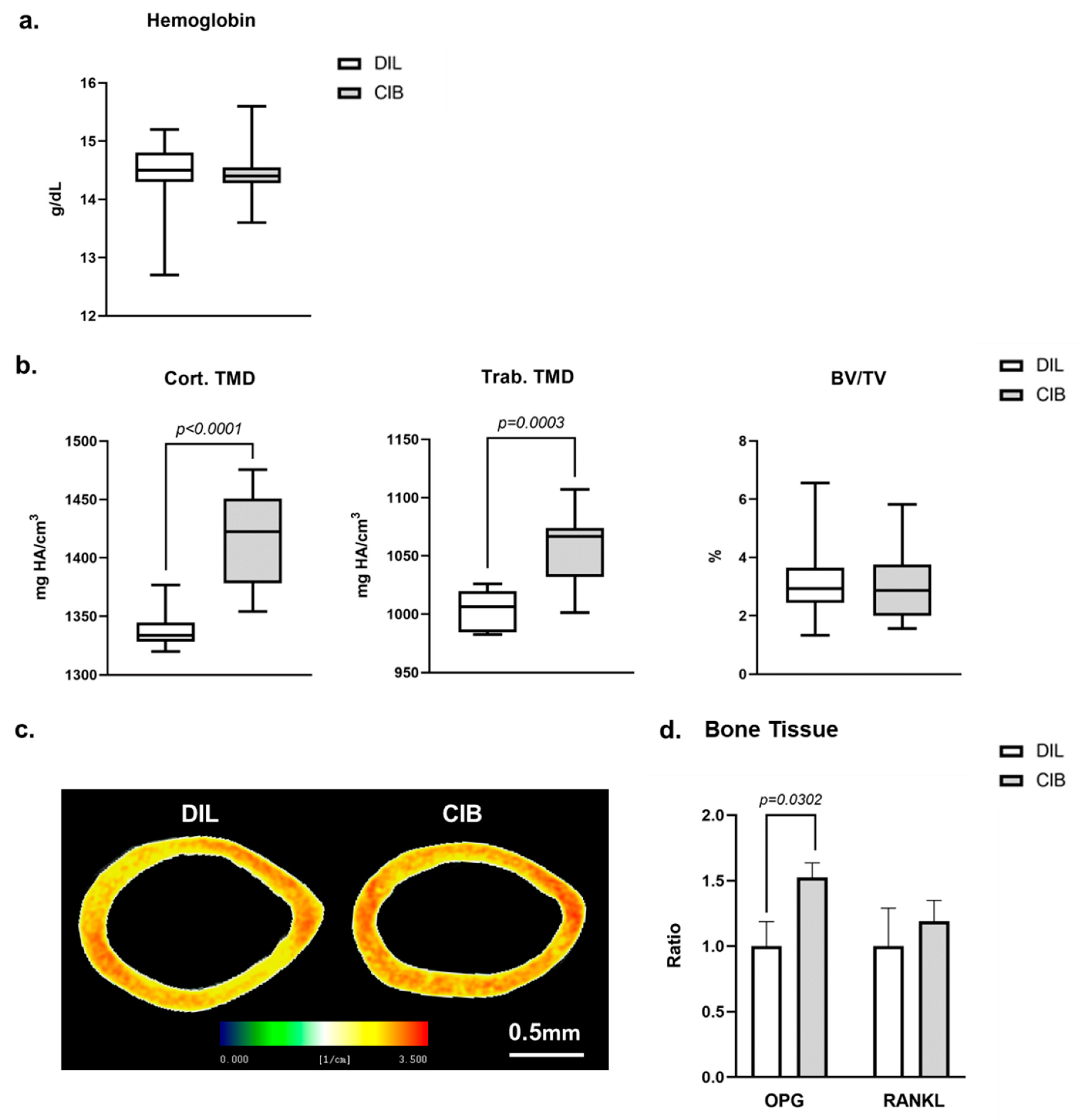
2.5. Cibinetide (CIB) Increases Tissue Mineral Density (TMD) in Both Cortical and Trabecular Bone
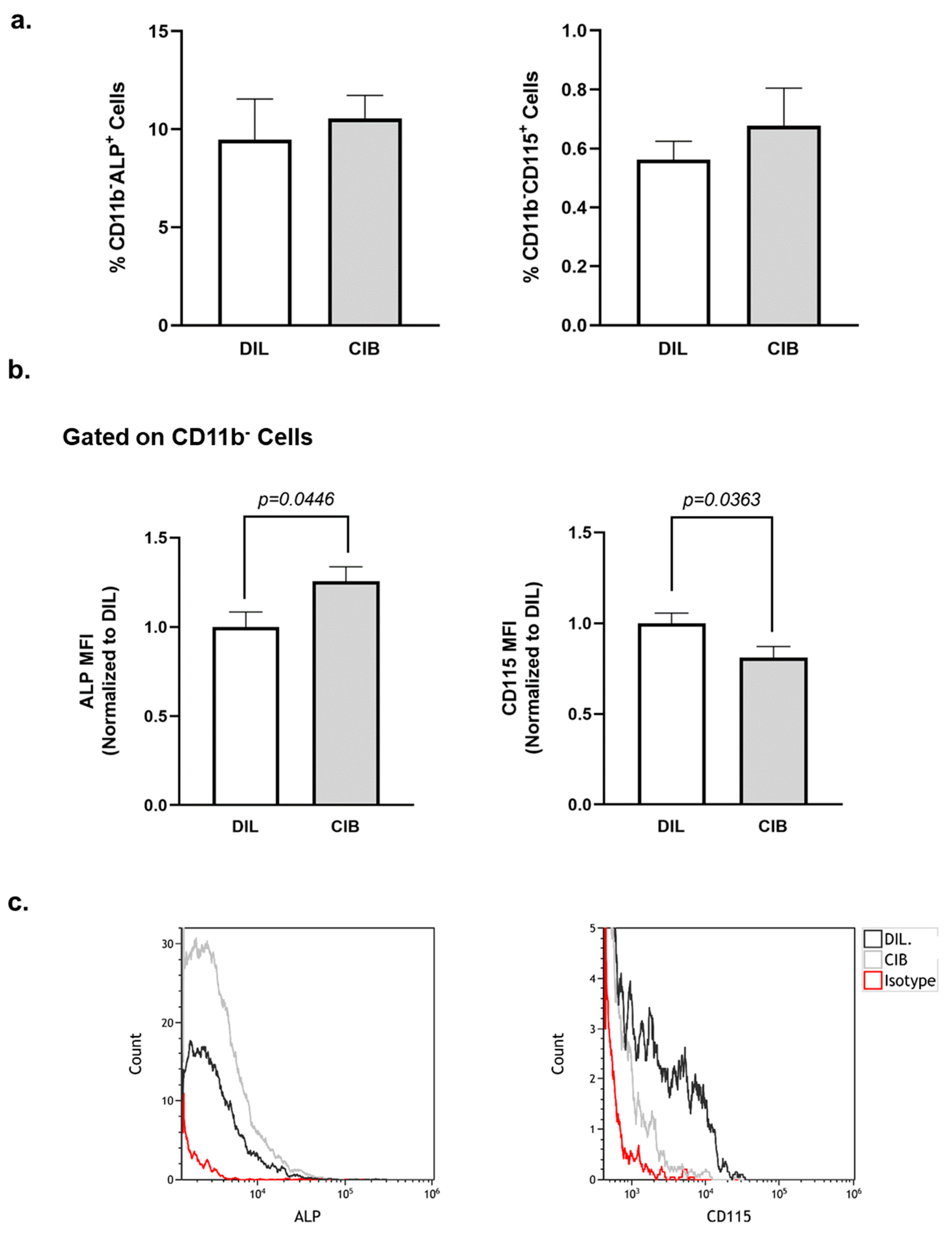
2.6. Cibinetide (CIB) Modulates Alkaline Phosphatase (ALP) and CD115 Expression on CD11b− Bone Marrow Cells
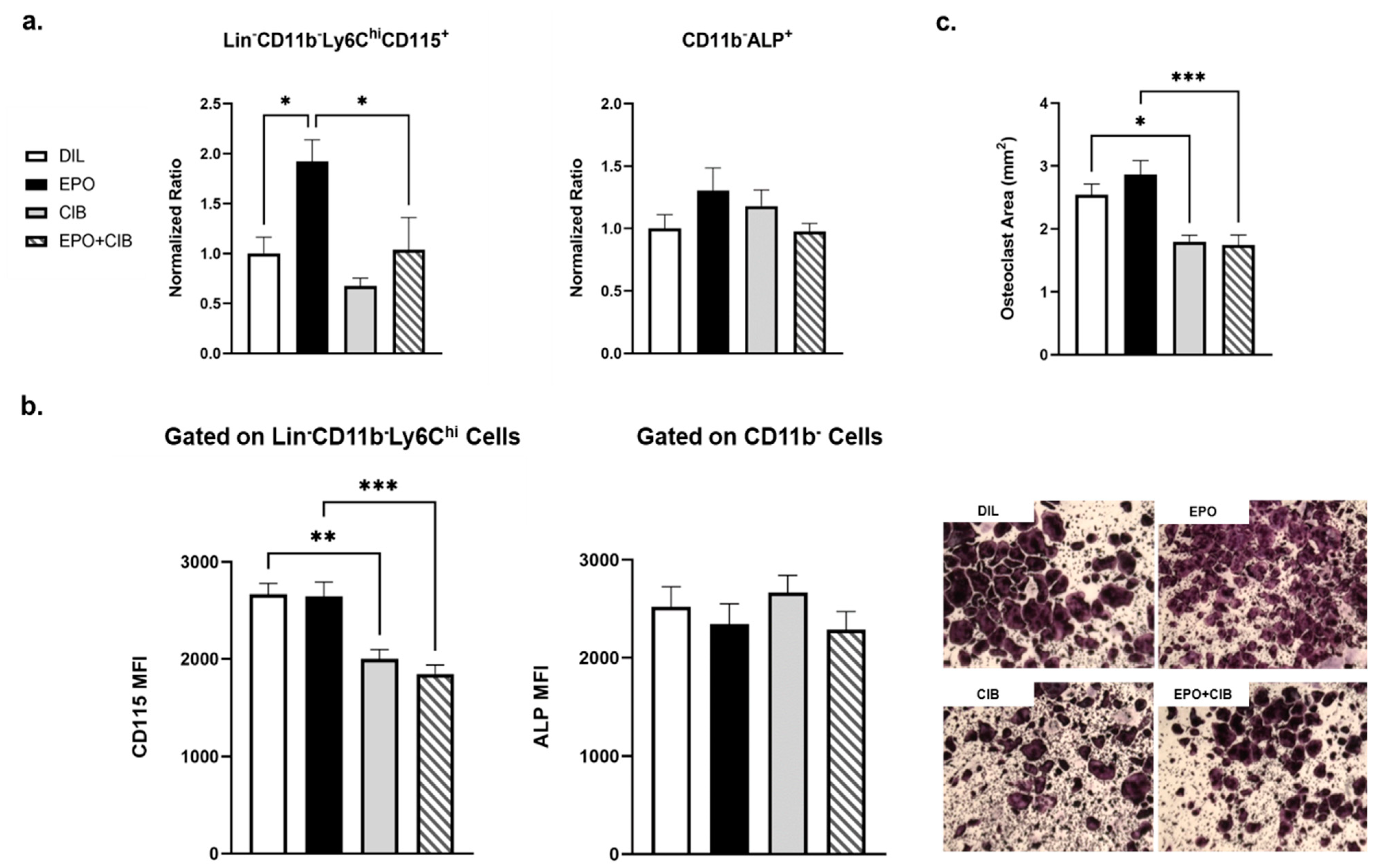
2.7. Cibinetide (CIB) Overrides the Pro-Osteoclastogenic Effects of EPO In Vivo
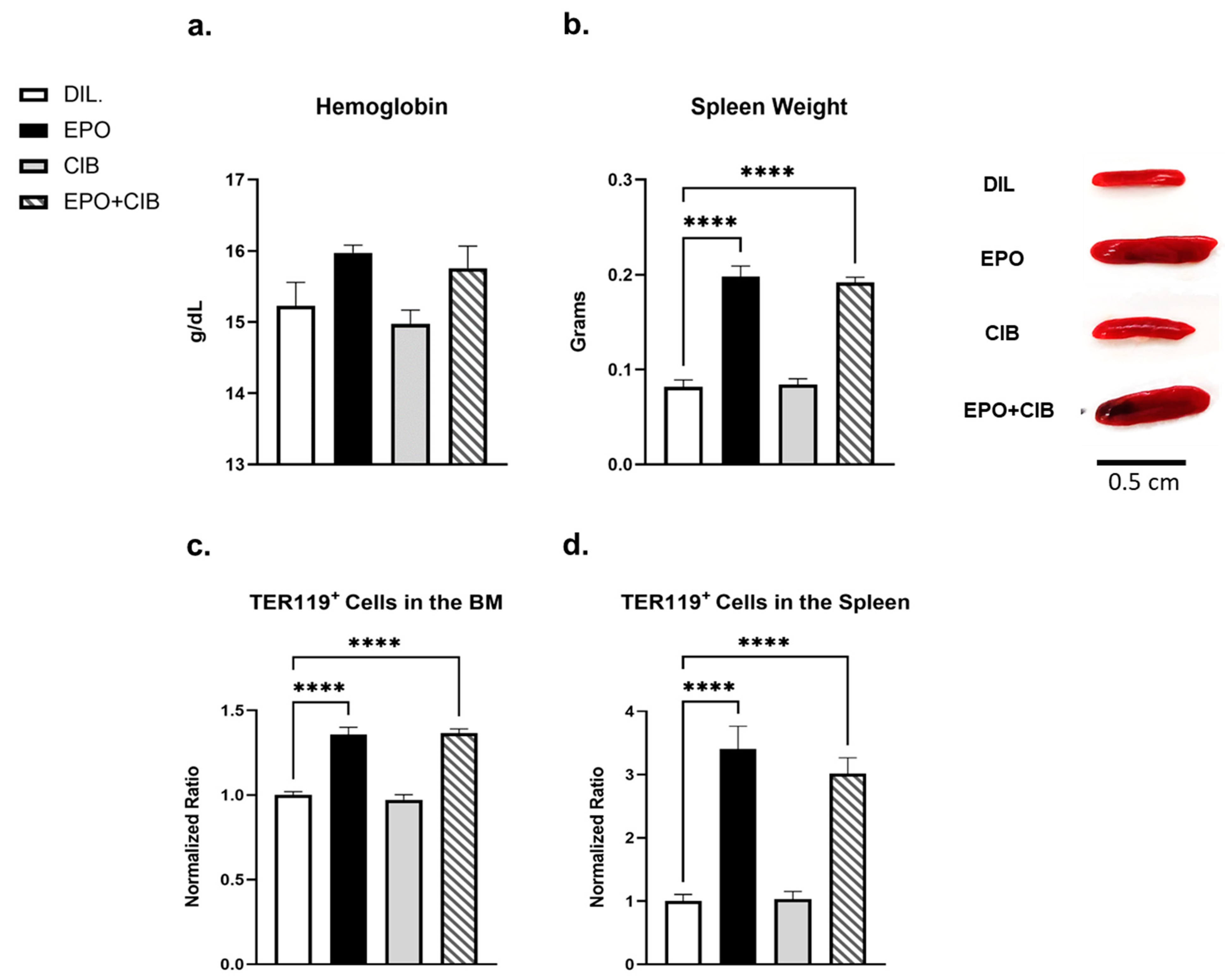
2.8. EPO Maintains Erythropoietic Activity in the Presence of Cibinetide (CIB)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cell Culture
4.4. Microcomputed Tomography (microCT)
4.5. Hemoglobin Levels
4.6. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Assay
4.7. Flow Cytometry
4.8. Real-Time RT PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef]
- Sasaki, R.; Masuda, S.; Nagao, M. Erythropoietin: Multiple physiological functions and regulation of biosynthesis. Biosci. Biotechnol. Biochem. 2000, 64, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L.; Pham, T.; Isaacs, M.; Hankins, W.D. Erythropoietin is both a mitogen and a survival factor. Blood 1991, 77, 1228–1233. [Google Scholar] [CrossRef]
- Ramanath, V.; Gupta, D.; Jain, J.; Chaudhary, K.; Nistala, R. Anemia and chronic kidney disease: Making sense of the recent trials. Rev. Recent Clin. Trials 2012, 7, 187–196. [Google Scholar] [CrossRef]
- Wish, J.B. Past, present, and future of chronic kidney disease anemia management in the United States. Adv. Chronic Kidney Dis. 2009, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Uemura, Y.; Fujisaka, Y.; Sugiyama, T.; Ohmatsu, H.; Katsumata, N.; Okamoto, R.; Saijo, N.; Hotta, T. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: Individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci. 2013, 104, 481–485. [Google Scholar] [CrossRef]
- Westenbrink, B.D.; Lipsic, E.; van der Meer, P.; van der Harst, P.; Oeseburg, H.; Du Marchie Sarvaas, G.J.; Koster, J.; Voors, A.A.; van Veldhuisen, D.J.; van Gilst, W.H.; et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur. Heart J. 2007, 28, 2018–2027. [Google Scholar] [CrossRef]
- Van der Meer, P.; Lipsic, E. Erythropoietin: Repair of the failing heart. J. Am. Coll. Cardiol. 2006, 48, 185–186. [Google Scholar] [CrossRef][Green Version]
- Pankratova, S.; Kiryushko, D.; Sonn, K.; Soroka, V.; Kohler, L.B.; Rathje, M.; Gu, B.; Gotfryd, K.; Clausen, O.; Zharkovsky, A.; et al. Neuroprotective properties of a novel, non-haematopoietic agonist of the erythropoietin receptor. Brain 2010, 133, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Satoh, K.; Fukumoto, Y.; Ito, Y.; Kagaya, Y.; Ishii, N.; Sugamura, K.; Shimokawa, H. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ. Res. 2007, 100, 662–669. [Google Scholar] [CrossRef]
- Tsai, P.T.; Ohab, J.J.; Kertesz, N.; Groszer, M.; Matter, C.; Gao, J.; Liu, X.; Wu, H.; Carmichael, S.T. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J. Neurosci. 2006, 26, 1269–1274. [Google Scholar] [CrossRef]
- Lifshitz, L.; Tabak, G.; Gassmann, M.; Mittelman, M.; Neumann, D. Macrophages as novel target cells for erythropoietin. Haematologica 2010, 95, 1823–1831. [Google Scholar] [CrossRef]
- Brines, M.; Grasso, G.; Fiordaliso, F.; Sfacteria, A.; Ghezzi, P.; Fratelli, M.; Latini, R.; Xie, Q.W.; Smart, J.; Su-Rick, C.J.; et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. USA 2004, 101, 14907–14912. [Google Scholar] [CrossRef]
- Brines, M.; Cerami, A. The receptor that tames the innate immune response. Mol. Med. 2012, 18, 486–496. [Google Scholar] [CrossRef]
- Blake, T.J.; Jenkins, B.J.; D’Andrea, R.J.; Gonda, T.J. Functional cross-talk between cytokine receptors revealed by activating mutations in the extracellular domain of the beta-subunit of the GM-CSF receptor. J. Leukoc. Biol. 2002, 72, 1246–1255. [Google Scholar]
- Jubinsky, P.T.; Krijanovski, O.I.; Nathan, D.G.; Tavernier, J.; Sieff, C.A. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood 1997, 90, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Hercus, T.R.; Dhagat, U.; Kan, W.L.; Broughton, S.E.; Nero, T.L.; Perugini, M.; Sandow, J.J.; D’Andrea, R.J.; Ekert, P.G.; Hughes, T.; et al. Signalling by the betac family of cytokines. Cytokine Growth Factor Rev. 2013, 24, 189–201. [Google Scholar] [CrossRef]
- Leist, M.; Ghezzi, P.; Grasso, G.; Bianchi, R.; Villa, P.; Fratelli, M.; Savino, C.; Bianchi, M.; Nielsen, J.; Gerwien, J.; et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 2004, 305, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Patel, N.S.; Villa, P.; Brines, C.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C.; et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, B.; Han, F.; Li, X.; Xiong, J.; Jiang, M.; Yang, X.; Wu, Y.; Zhang, Z. Erythropoietin-derived nonerythropoietic peptide ameliorates experimental autoimmune neuritis by inflammation suppression and tissue protection. PLoS ONE 2014, 9, e90942. [Google Scholar] [CrossRef]
- Pulman, K.G.; Smith, M.; Mengozzi, M.; Ghezzi, P.; Dilley, A. The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model. Neuroscience 2013, 233, 174–183. [Google Scholar] [CrossRef]
- Dahan, A.; Dunne, A.; Swartjes, M.; Proto, P.L.; Heij, L.; Vogels, O.; van Velzen, M.; Sarton, E.; Niesters, M.; Tannemaat, M.R.; et al. ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol. Med. 2013, 19, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Dunne, A.N.; van Velzen, M.; Proto, P.L.; Ostenson, C.G.; Kirk, R.I.; Petropoulos, I.N.; Javed, S.; Malik, R.A.; Cerami, A.; et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol. Med. 2015, 20, 658–666. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, J.; Luo, B.; Zhu, W.; Liu, Y.; Wang, Z.; Zhang, Z. Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus. J. Cell. Mol. Med. 2018, 22, 3330–3339. [Google Scholar] [CrossRef]
- Yao, M.; Watanabe, M.; Sun, S.; Tokodai, K.; Cerami, A.; Brines, M.; Ostenson, C.G.; Ericzon, B.G.; Lundgren, T.; Kumagai-Braesch, M. Improvement of islet allograft function using cibinetide, an innate repair receptor ligand. Transplantation 2020, 104, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell. Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Lappin, K.M.; Mills, K.I.; Lappin, T.R. Erythropoietin in bone homeostasis-Implications for efficacious anemia therapy. Stem Cells Transl. Med. 2021, 10, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Erythropoietin in bone—Controversies and consensus. Cytokine 2017, 89, 155–159. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Context-dependent skeletal effects of erythropoietin. Vitam. Horm. 2017, 105, 161–179. [Google Scholar]
- Bulycheva, E.; Rauner, M.; Medyouf, H.; Theurl, I.; Bornhauser, M.; Hofbauer, L.C.; Platzbecker, U. Myelodysplasia is in the niche: Novel concepts and emerging therapies. Leukemia 2015, 29, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Singbrant, S.; Russell, M.R.; Jovic, T.; Liddicoat, B.; Izon, D.J.; Purton, L.E.; Sims, N.A.; Martin, T.J.; Sankaran, V.G.; Walkley, C.R. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 2011, 117, 5631–5642. [Google Scholar] [CrossRef] [PubMed]
- Hiram-Bab, S.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Gassmann, M.; Rauner, M.; Franke, K.; Wielockx, B.; Neumann, D.; Gabet, Y. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015, 29, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 21. [Google Scholar] [CrossRef]
- Suresh, S.; Alvarez, J.C.; Dey, S.; Noguchi, C.T. Erythropoietin-induced changes in bone and bone marrow in mouse models of diet-induced obesity. Int. J. Mol. Sci. 2020, 21, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kolomansky, A.; Hiram-Bab, S.; Ben-Califa, N.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Oster, H.S.; Rauner, M.; Wielockx, B.; Neumann, D.; et al. Erythropoietin mediated bone loss in mice is dose-dependent and mostly irreversible. Int. J. Mol. Sci. 2020, 21, 3817. [Google Scholar] [CrossRef] [PubMed]
- Rauner, M.; Murray, M.; Thiele, S.; Watts, D.; Neumann, D.; Gabet, Y.; Hofbauer, L.C.; Wielockx, B. Epo/EpoR signaling in osteoprogenitor cells is essential for bone homeostasis and Epo-induced bone loss. Bone Res. 2021, 9, 42. [Google Scholar] [CrossRef]
- Kristjansdottir, H.L.; Lewerin, C.; Lerner, U.H.; Herlitz, H.; Johansson, P.; Johansson, H.; Karlsson, M.; Lorentzon, M.; Ohlsson, C.; Ljunggren, O.; et al. High plasma erythropoietin predicts incident fractures in elderly men with normal renal function: The MrOS Sweden cohort. J. Bone Miner. Res. 2020, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Wright, E.C.; Wright, D.G.; Abbott, K.C.; Noguchi, C.T. Erythropoietin treatment and the risk of hip fractures in hemodialysis patients. J. Bone Miner. Res. 2021, 36, 1211–1219. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131, jcs216267. [Google Scholar] [CrossRef]
- Pippenger, B.E.; Duhr, R.; Muraro, M.G.; Pagenstert, G.I.; Hugle, T.; Geurts, J. Multicolor flow cytometry-based cellular phenotyping identifies osteoprogenitors and inflammatory cells in the osteoarthritic subchondral bone marrow compartment. Osteoarthr. Cartil. 2015, 23, 1865–1869. [Google Scholar] [CrossRef]
- Das, A.; Wang, X.; Kang, J.; Coulter, A.; Shetty, A.C.; Bachu, M.; Brooks, S.R.; Dell’Orso, S.; Foster, B.L.; Fan, X.; et al. Monocyte subsets with high osteoclastogenic potential and their epigenetic regulation orchestrated by IRF8. J. Bone Miner. Res. 2021, 36, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.F.; Hsu, L.Y.; Niemi, E.C.; Weiss, A.; Aliprantis, A.O.; Nakamura, M.C. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Investig. 2012, 122, 4592–4605. [Google Scholar] [CrossRef]
- Park-Min, K.H.; Lee, E.Y.; Moskowitz, N.K.; Lim, E.; Lee, S.K.; Lorenzo, J.A.; Huang, C.; Melnick, A.M.; Purdue, P.E.; Goldring, S.R.; et al. Negative regulation of osteoclast precursor differentiation by CD11b and beta2 integrin-B-cell lymphoma 6 signaling. J. Bone Miner. Res. 2013, 28, 135–149. [Google Scholar] [CrossRef]
- Omar, I.; Guterman-Ram, G.; Rahat, D.; Tabach, Y.; Berger, M.; Levaot, N. Schlafen2 mutation in mice causes an osteopetrotic phenotype due to a decrease in the number of osteoclast progenitors. Sci. Rep. 2018, 8, 13005. [Google Scholar] [CrossRef]
- Collino, M.; Thiemermann, C.; Cerami, A.; Brines, M. Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol. Ther. 2015, 151, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Lee, C.H.; Sud, S.; Pienta, K.J.; et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef] [PubMed]
- Sadvakassova, G.; Tiedemann, K.; Steer, K.J.D.; Mikolajewicz, N.; Stavnichuk, M.; In-Kyung Lee, I.; Sabirova, Z.; Schranzhofer, M.; Komarova, S.V. Active hematopoiesis triggers exosomal release of PRDX2 that promotes osteoclast formation. Physiol. Rep. 2021, 9, e14745. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Haschka, D.; Dichtl, S.; Sonnweber, T.; Schroll, A.; Asshoff, M.; Mindur, J.E.; Moser, P.L.; Wolf, D.; Swirski, F.K.; et al. Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis. Sci. Rep. 2017, 7, 13012. [Google Scholar] [CrossRef]
- Watanabe, M.; Lundgren, T.; Saito, Y.; Cerami, A.; Brines, M.; Ostenson, C.G.; Kumagai-Braesch, M. A nonhematopoietic erythropoietin analogue, ARA 290, inhibits macrophage activation and prevents damage to transplanted islets. Transplantation 2016, 100, 554–562. [Google Scholar] [CrossRef]
- Inada, M.; Matsumoto, C.; Uematsu, S.; Akira, S.; Miyaura, C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol. 2006, 177, 1879–1885. [Google Scholar] [CrossRef]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef]
- Eger, M.; Hiram-Bab, S.; Liron, T.; Sterer, N.; Carmi, Y.; Kohavi, D.; Gabet, Y. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front. Immunol. 2018, 9, 2963. [Google Scholar] [CrossRef]
- Eger, M.; Liron, T.; Hiram-Bab, S.; Awida, Z.; Giladi, E.; Dangoor, D.; Fridkin, M.; Kohavi, D.; Gozes, I.; Gabet, Y. Therapeutic potential of vasoactive intestinal peptide and its derivative stearyl-norleucine-vip in inflammation-induced osteolysis. Front. Pharmacol. 2021, 12, 638128. [Google Scholar] [CrossRef]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Mancheno, P.E.; Larriera, A.I.; Doty, S.B.; Cardoso, L.; Fritton, S.P. 3D assessment of cortical bone porosity and tissue mineral density using high-resolution microCT: Effects of resolution and threshold method. J. Bone Miner. Res. 2014, 29, 142–150. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.T.; Chang, A.; Mariano, M.M.; Stein, E.A.; Wang, Q.; Lindstrom, T.M.; Sharpe, O.; Roscow, C.; Ho, P.P.; Lee, D.M.; et al. C-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis Res. Ther. 2010, 12, R32. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, Y.J.; Li, T.Y.; Guo, J.X.; Lv, F.; Li, C.L.; Ge, X.T. Colony-stimulating factor 1 receptor inhibition prevents against lipopolysaccharide -induced osteoporosis by inhibiting osteoclast formation. Biomed. Pharmacother. 2019, 115, 108916. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, B.H.; Nevius, E.; Kaplan, J.; Pereira, J.; Insogna, K. Selective deletion of the receptor for CSF1, c-fms, in osteoclasts results in a high bone mass phenotype, smaller osteoclasts in vivo and an impaired response to an anabolic PTH regimen. PLoS ONE 2021, 16, e0247199. [Google Scholar] [CrossRef]
- Lee, K.; Kim, M.Y.; Ahn, H.; Kim, H.S.; Shin, H.I.; Jeong, D. Blocking of the ubiquitin-proteasome system prevents inflammation-induced bone loss by accelerating M-CSF receptor c-Fms degradation in osteoclast differentiation. Int. J. Mol. Sci. 2017, 18, 2054. [Google Scholar] [CrossRef]
- Ji, J.D.; Park-Min, K.H.; Shen, Z.; Fajardo, R.J.; Goldring, S.R.; McHugh, K.P.; Ivashkiv, L.B. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J. Immunol. 2009, 183, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Gliniak, B.C.; Rohrschneider, L.R. Expression of the M-CSF receptor is controlled posttranscriptionally by the dominant actions of GM-CSF or multi-CSF. Cell 1990, 63, 1073–1083. [Google Scholar] [CrossRef]
- Gupta, N.; Barhanpurkar, A.P.; Tomar, G.B.; Srivastava, R.K.; Kour, S.; Pote, S.T.; Mishra, G.C.; Wani, M.R. IL-3 inhibits human osteoclastogenesis and bone resorption through downregulation of c-Fms and diverts the cells to dendritic cell lineage. J. Immunol. 2010, 185, 2261–2272. [Google Scholar] [CrossRef]
- Robb, L.; Drinkwater, C.C.; Metcalf, D.; Li, R.; Kontgen, F.; Nicola, N.A.; Begley, C.G. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc. Natl. Acad. Sci. USA 1995, 92, 9565–9569. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.L.; Lanzinger, M.; Hartmann, F.J.; Schreiner, B.; Mair, F.; Pelczar, P.; Clausen, B.E.; Jung, S.; Greter, M.; Becher, B. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 2015, 43, 502–514. [Google Scholar] [CrossRef]
- Van Velzen, M.; Heij, L.; Niesters, M.; Cerami, A.; Dunne, A.; Dahan, A.; Brines, M. ARA 290 for treatment of small fiber neuropathy in sarcoidosis. Expert Opin. Investig. Drugs 2014, 23, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Therapeutic implications of suppressing osteoclast formation versus function. Rheumatology 2016, 55, ii61–ii63. [Google Scholar] [CrossRef]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Logan, J.G.; Mellis, D.; Capulli, M. Generation and culture of osteoclasts. Bonekey Rep. 2014, 3, 570. [Google Scholar] [CrossRef]
- Hata, K.; Kukita, T.; Akamine, A.; Kukita, A.; Kurisu, K. Trypsinized osteoclast-like multinucleated cells formed in rat bone marrow cultures efficiently form resorption lacunae on dentine. Bone 1992, 13, 139–146. [Google Scholar] [CrossRef]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.; Gabet, Y.; Cogan, J.; Shi, Y.; Tank, A.; Sasaki, T.; Criswell, B.; Dixon, A.; Lee, C.; Tam, J.; et al. Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS ONE 2009, 4, e5438. [Google Scholar] [CrossRef]
- Gabet, Y.; Baniwal, S.K.; Leclerc, N.; Shi, Y.; Kohn-Gabet, A.E.; Cogan, J.; Dixon, A.; Bachar, M.; Guo, L.; Turman, J.E., Jr.; et al. Krox20/EGR2 deficiency accelerates cell growth and differentiation in the monocytic lineage and decreases bone mass. Blood 2010, 116, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, P.; Koller, B.; Muller, R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif. Tissue Int. 1996, 58, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Antibody | Source | Identifier |
|---|---|---|
| TER-119-APC | BioLegend | Cat#: 116211 |
| CD71-PE | BioLegend | Cat#: 113807 |
| CD11b-APC | BioLegend | Cat#:101211 |
| CD115-PE | Miltenyi Biotec | Cat#:130112828 |
| LY6G-FITC | BioLegend | Cat#: 127605 |
| TER119-FITC | BioLegend | Cat#: 116205 |
| CD3ε-FITC | BioLegend | Cat#: 100305 |
| B220-FITC | BioLegend | Cat#:103205 |
| LY6C- PerCP/Cy5.5 | BioLegend | Cat#: 128011 |
| Alkaline Phosphatase (ALPL) | R&D systems | Cat#: AF2910 |
| CD115-APC | eBioscience | Cat#: 14115282 |
| Goat IgG (H+L)-PE | R&D systems | Cat#: F0107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awida, Z.; Bachar, A.; Saed, H.; Gorodov, A.; Ben-Califa, N.; Ibrahim, M.; Kolomansky, A.; Iden, J.A.; Graniewitz Visacovsky, L.; Liron, T.; et al. The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice. Int. J. Mol. Sci. 2022, 23, 55. https://doi.org/10.3390/ijms23010055
Awida Z, Bachar A, Saed H, Gorodov A, Ben-Califa N, Ibrahim M, Kolomansky A, Iden JA, Graniewitz Visacovsky L, Liron T, et al. The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice. International Journal of Molecular Sciences. 2022; 23(1):55. https://doi.org/10.3390/ijms23010055
Chicago/Turabian StyleAwida, Zamzam, Almog Bachar, Hussam Saed, Anton Gorodov, Nathalie Ben-Califa, Maria Ibrahim, Albert Kolomansky, Jennifer Ana Iden, Liad Graniewitz Visacovsky, Tamar Liron, and et al. 2022. "The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice" International Journal of Molecular Sciences 23, no. 1: 55. https://doi.org/10.3390/ijms23010055
APA StyleAwida, Z., Bachar, A., Saed, H., Gorodov, A., Ben-Califa, N., Ibrahim, M., Kolomansky, A., Iden, J. A., Graniewitz Visacovsky, L., Liron, T., Hiram-Bab, S., Brines, M., Gabet, Y., & Neumann, D. (2022). The Non-Erythropoietic EPO Analogue Cibinetide Inhibits Osteoclastogenesis In Vitro and Increases Bone Mineral Density in Mice. International Journal of Molecular Sciences, 23(1), 55. https://doi.org/10.3390/ijms23010055






