Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
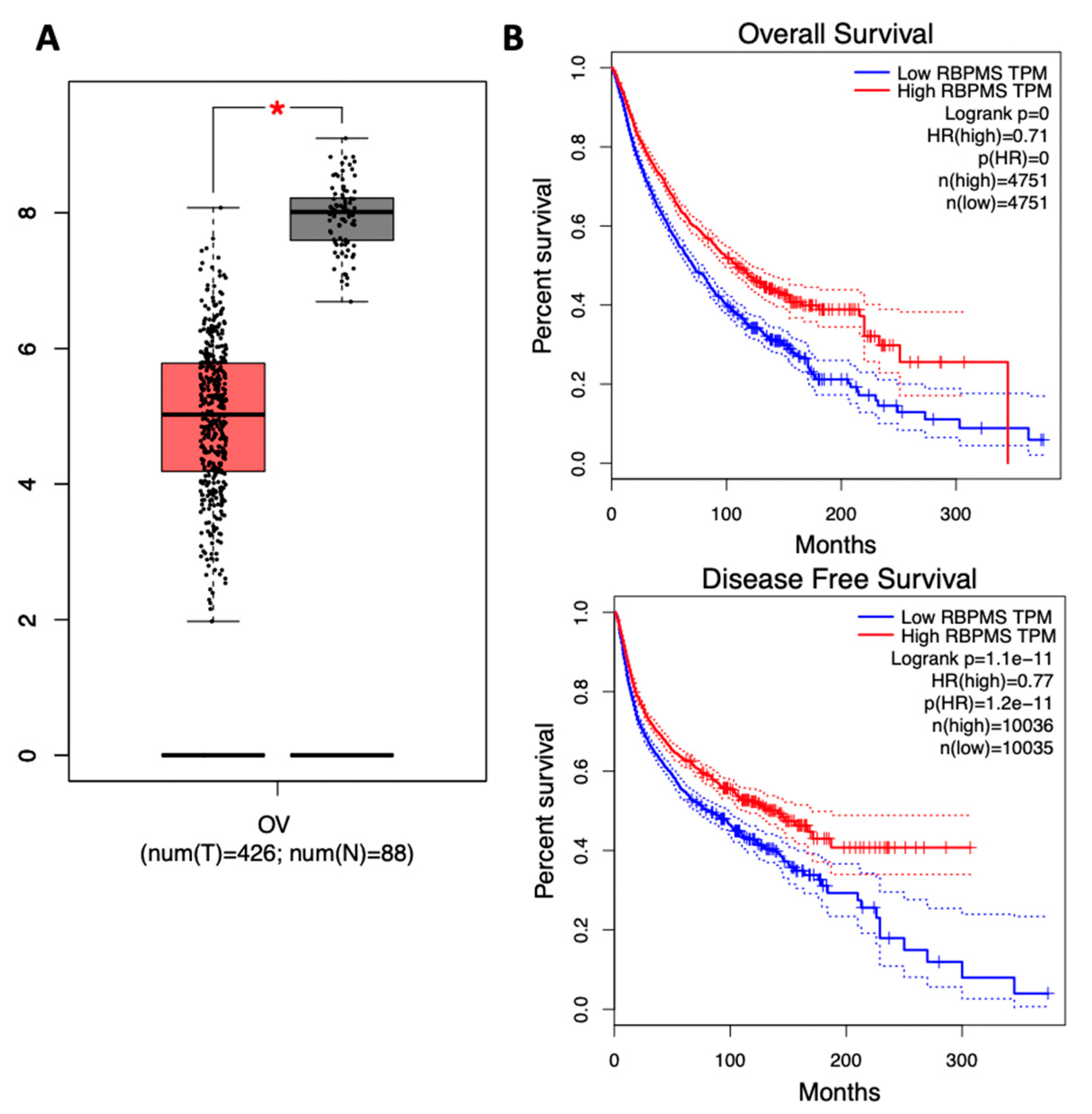
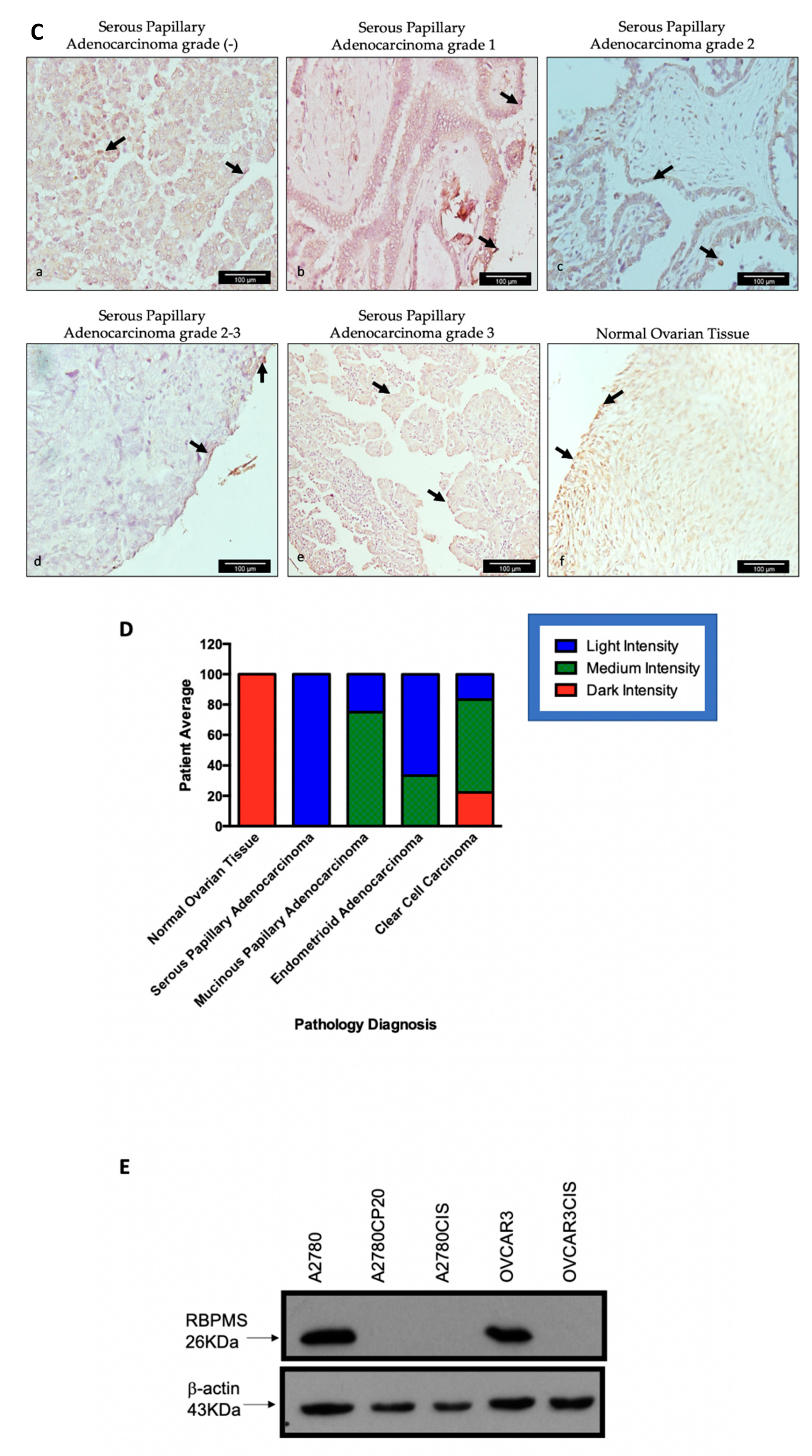
2.1. RBPMS Protein Levels Are Reduced in Ovarian Cancer Tumor Samples and Correlate with Poor Prognosis
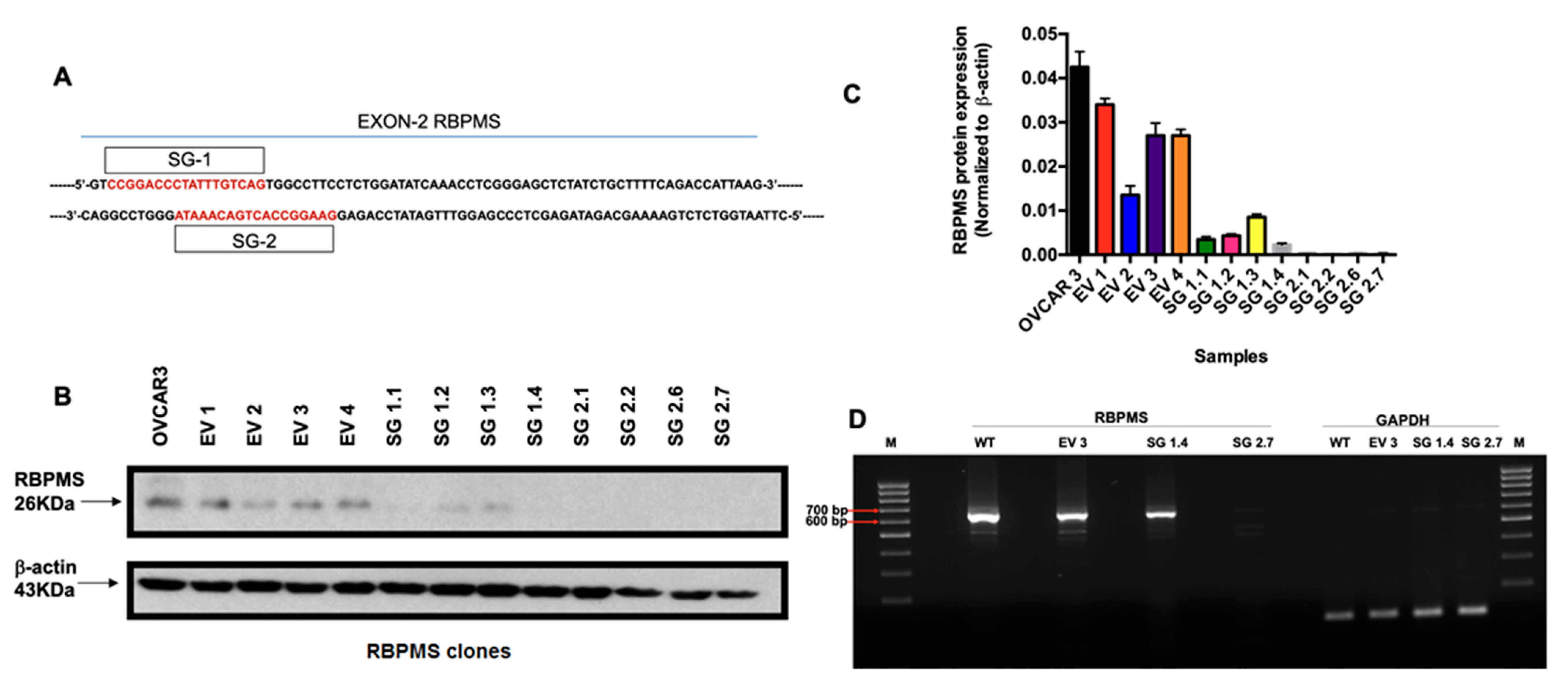
2.2. RBPMS CRISPR/Cas9 Knockout in OVCAR3 Cells
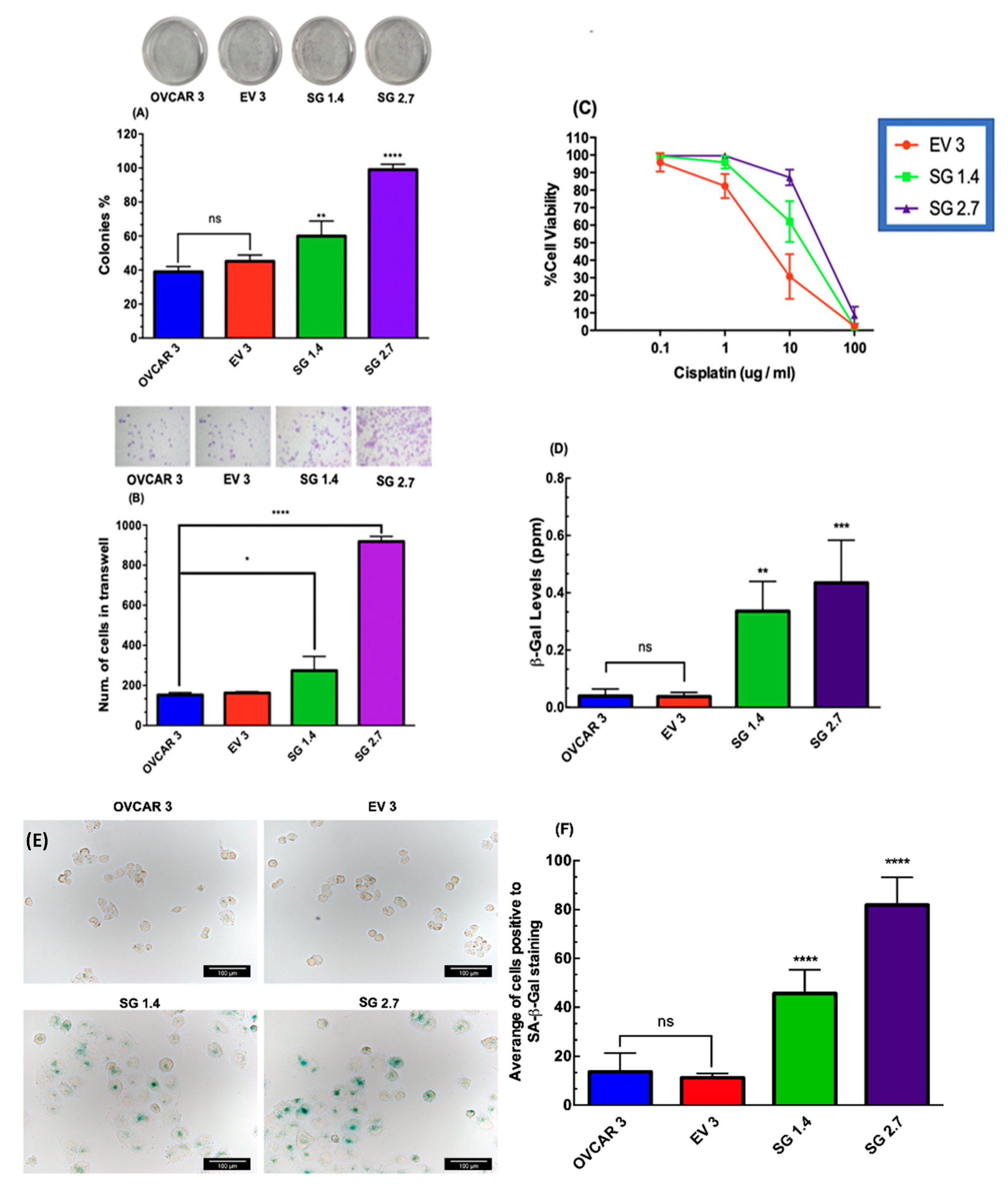
2.3. RBPMS Knockout Increased the Proliferation and Invasion Ability of Ovarian Cancer Cells
2.4. RBPMS Knockout Reduced the Sensitivity of Ovarian Cancer Cells to Cisplatin Treatment
2.5. RBPMS Knockout Increased the Senescence-Associated β-Galactosidase Levels in Ovarian Cancer Cells
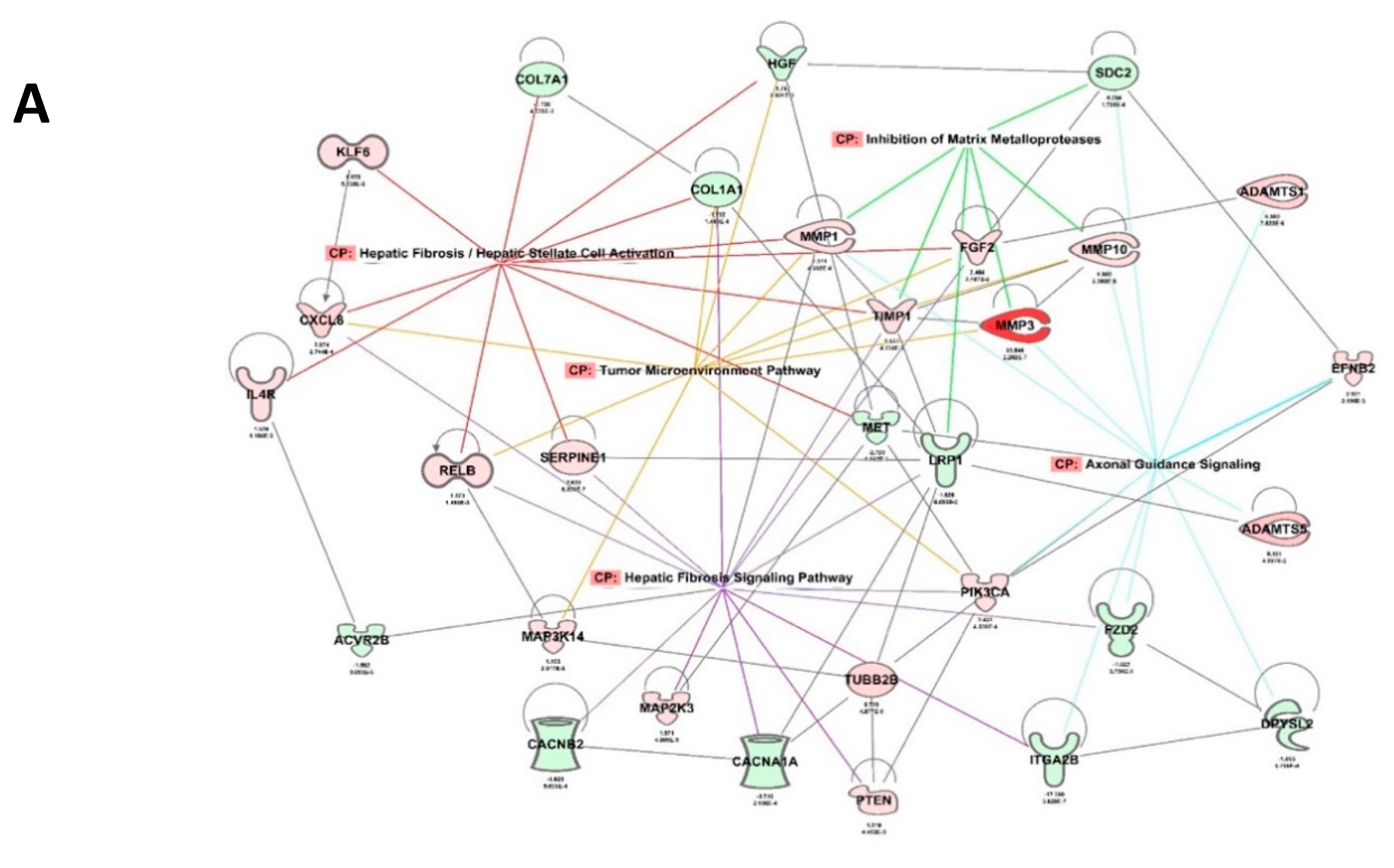
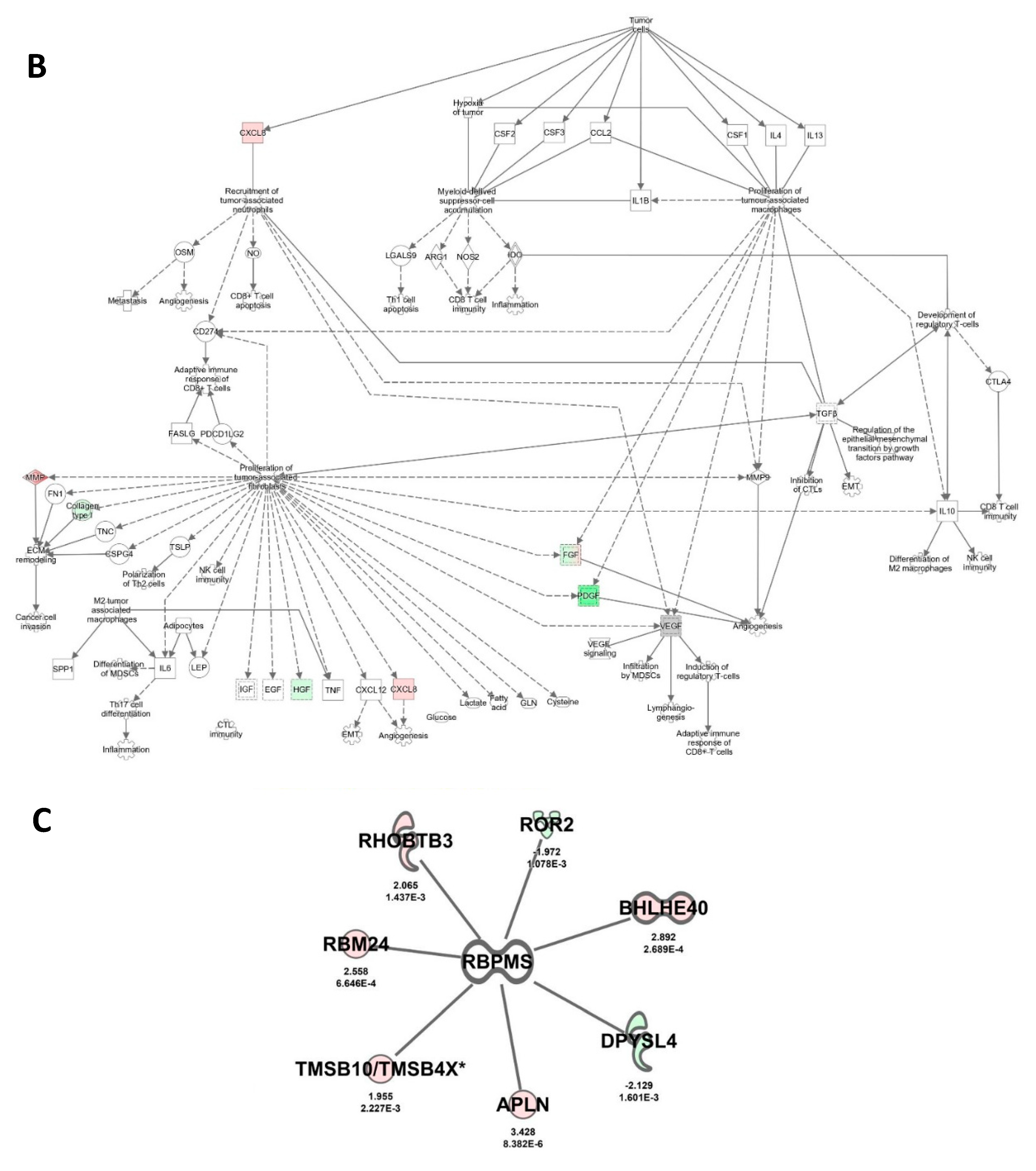
2.6. RBPMS Knockout Altered the Expression of Long-Noncoding RNAs and Protein-Coding Genes Associated with Alteration of the Tumor Microenvironment
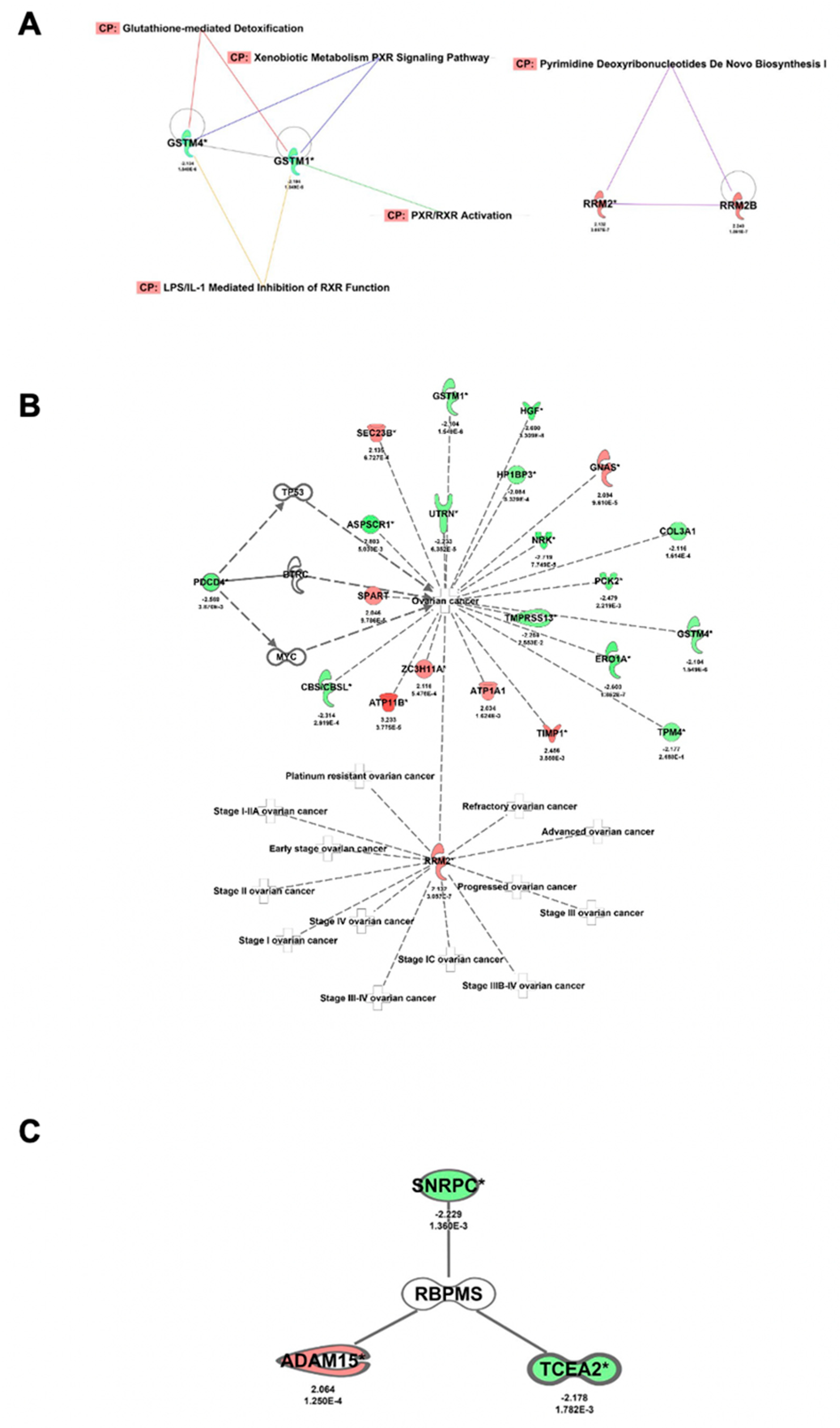
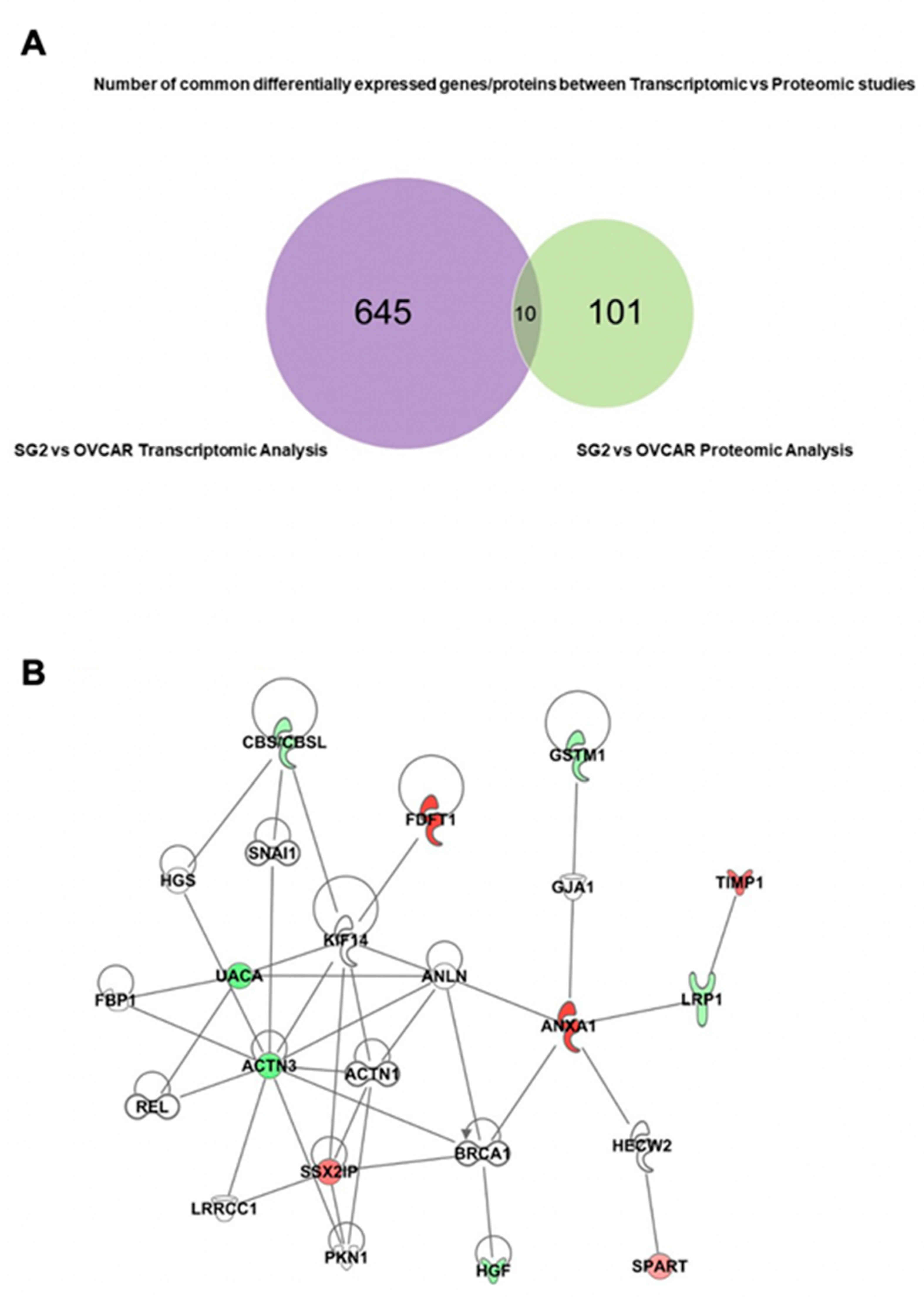
2.7. RBPMS Knockout Altered the Levels of Proteins Associated with Cell Integrity, Cell Detoxification, and RNA Processing
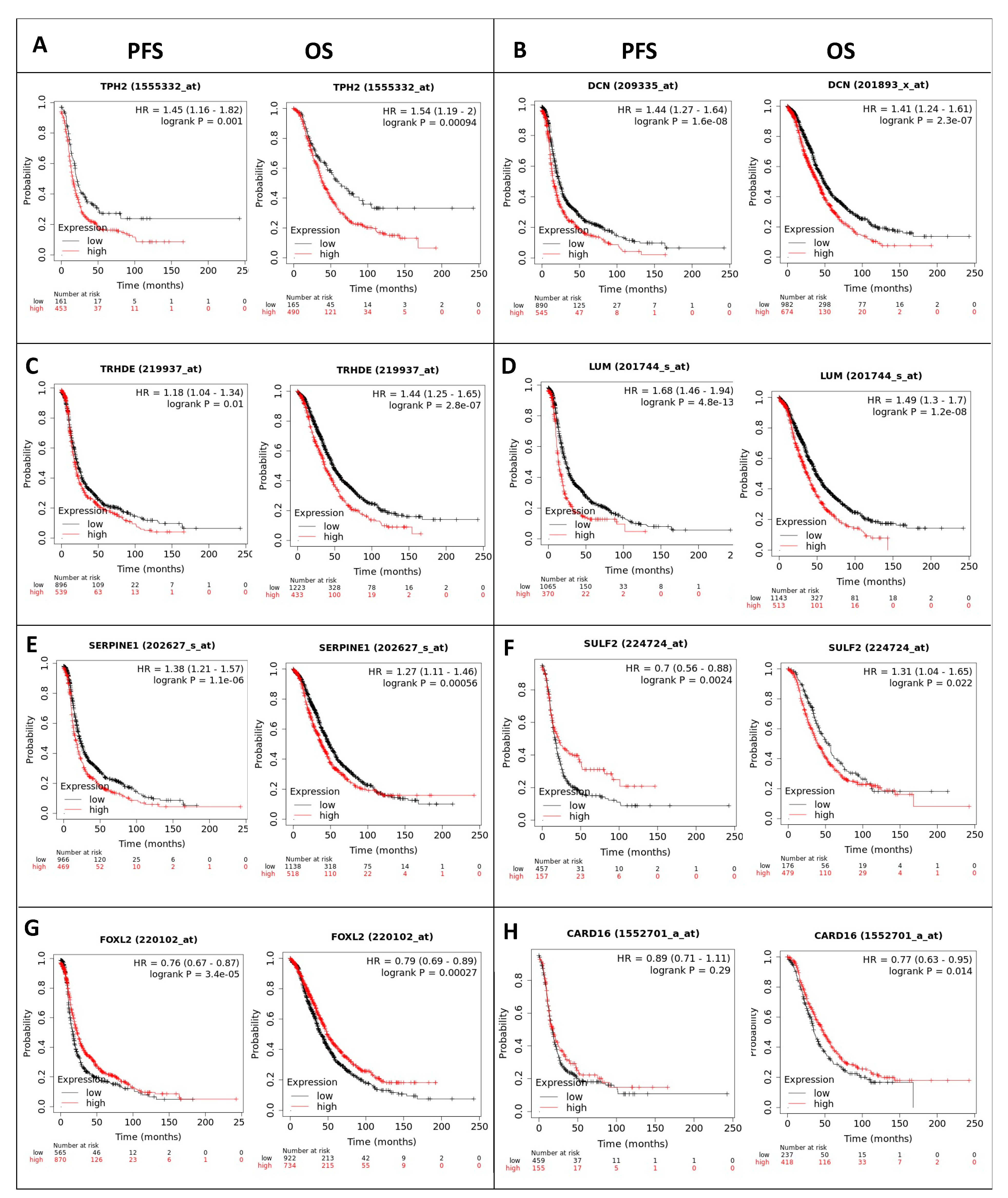
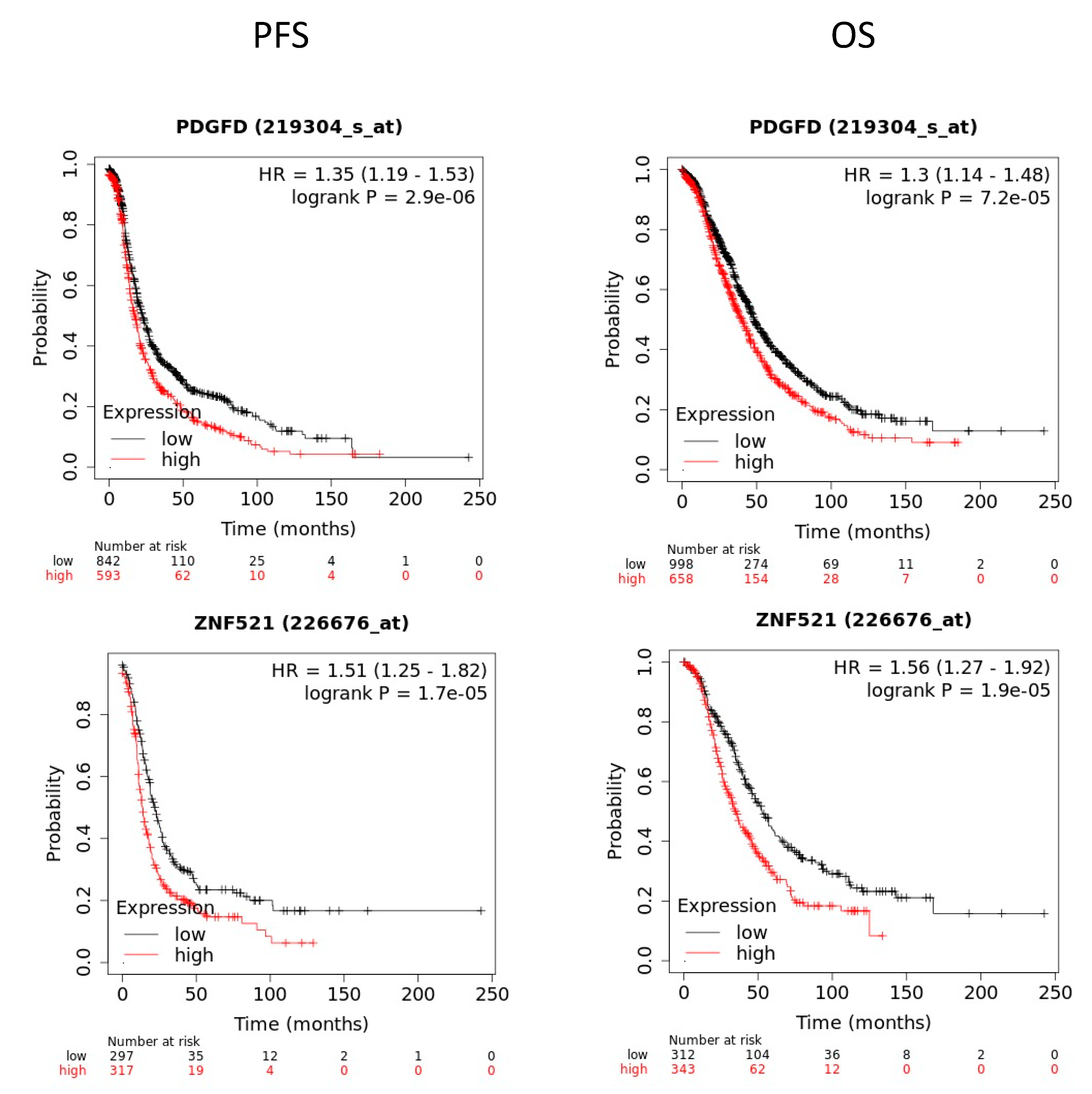
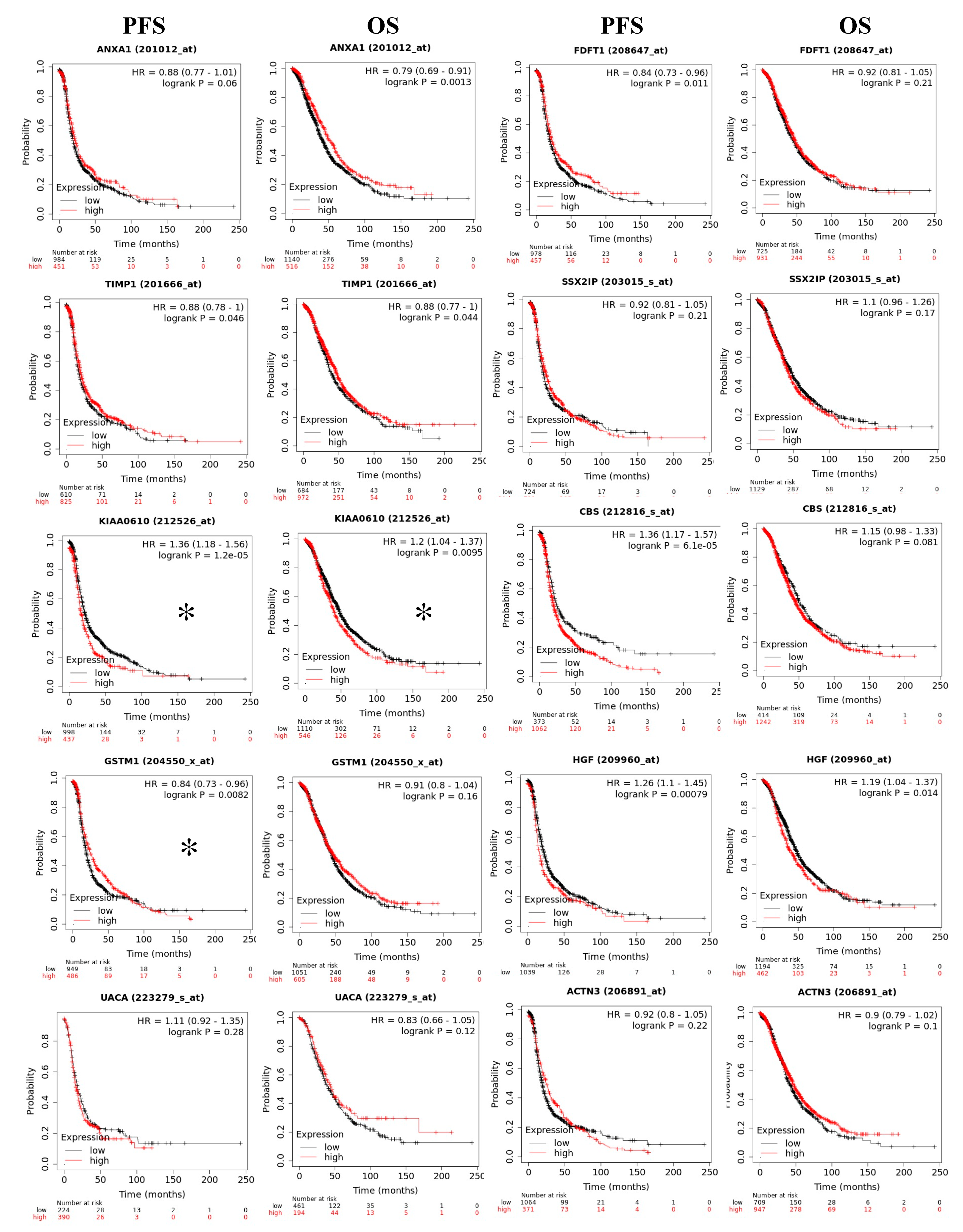
2.8. Prognostic Value of RBPMS Downstream Transcripts
3. Discussion
4. Materials and Methods
4.1. Cancer Databases Examination
4.2. RBPMS Immunohistochemistry in an Ovarian Tissue Array
4.3. Cells and Culture Conditions
4.4. CRISPR Design
4.5. RNA Isolation, cDNA Synthesis, and PCR
4.6. Western Blot Analysis
4.7. Colony Formation and Invasion Assays
4.8. Cell Viability Assays
4.9. Senescence-Associated β-Galactosidase Activity
4.10. RNA Sequencing Library Preparation, Data Processing, and Statistics
4.11. Proteomics, Data Processing, and Statistics
4.12. Clustering and Network Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMullen, M.; Karakasis, K.; Rottapel, R.; Oza, A.M. Advances in ovarian cancer, from biology to treatment. Nat. Cancer 2021, 2, 6–8. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef]
- Mok, S.C.; Kwong, J.; Welch, W.R.; Samimi, G.; Ozbun, L.; Bonome, T.; Birrer, M.J.; Berkowitz, R.S.; Wong, K.K. Etiology and pathogenesis of epithelial ovarian cancer. Dis. Markers 2007, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. New insights into ovarian cancer pathology. Ann. Oncol. 2012, 23 (Suppl. S10), x111–x117. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis 2014, 5, e1257. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Peng, D.J.; Wang, J.; Zhou, J.Y.; Wu, G.S. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2010, 394, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gonzalez, J.M.; Vivas-Mejia, P.E. c-MYC and epithelial ovarian cancer. Front. Oncol. 2021, 11, 601512. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Hu, S.L.; Shen, G.D.; Shen, G. Tumor suppressor genes and their underlying interactions in paclitaxel resistance in cancer therapy. Cancer Cell Int. 2016, 16, 13. [Google Scholar] [CrossRef]
- Wang, F.; Liu, M.; Li, X.; Tang, H. MiR-214 reduces cell survival and enhances cisplatin-induced cytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013, 587, 488–495. [Google Scholar] [CrossRef]
- Baez-Vega, P.M.; Echevarria Vargas, I.M.; Valiyeva, F.; Encarnacion-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martinez, M.J.; Vivas-Mejia, P.E. Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337. [Google Scholar] [CrossRef]
- Gerber, W.V.; Yatskievych, T.A.; Antin, P.B.; Correia, K.M.; Conlon, R.A.; Krieg, P.A. The RNA-binding protein gene, hermes, is expressed at high levels in the developing heart. Mech. Dev. 1999, 80, 77–86. [Google Scholar] [CrossRef]
- Wilmore, H.P.; McClive, P.J.; Smith, C.A.; Sinclair, A.H. Expression profile of the RNA-binding protein gene hermes during chicken embryonic development. Dev. Dyn. 2005, 233, 1045–1051. [Google Scholar] [CrossRef]
- Kwong, J.M.; Caprioli, J.; Piri, N. RNA binding protein with multiple splicing: A new marker for retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki-Silva, E.E.; Gooding, C.; Llorian, M.; Jacob, A.G.; Richards, F.; Buckroyd, A.; Sinha, S.; Smith, C.W. Identification of RBPMS as a mammalian smooth muscle master splicing regulator via proximity of its gene with super-enhancers. eLife 2019, 8, e46327. [Google Scholar] [CrossRef]
- Pereiro, X.; Ruzafa, N.; Urcola, J.H.; Sharma, S.C.; Vecino, E. Differential distribution of RBPMS in pig, rat, and human retina after damage. Int. J. Mol. Sci. 2020, 21, 9330. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, L.; Wang, Y.; Yuan, P.; Xu, X.; Ding, L.; Zhang, H.; Jiang, K.; Song, H.; Chen, Z.; et al. The RNA-binding protein RBPMS1 represses AP-1 signaling and regulates breast cancer cell proliferation and migration. Biochim. Biophys. Acta 2015, 1853, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, M.; Yuan, M.; Chen, R.; Huang, M.J. Identification of a novel RBPMS-ROS1 fusion in an adolescent patient with microsatellite-instable advanced lung adenocarcinoma sensitive to crizotinib: A case report. Clin. Lung Cancer 2020, 21, e78–e83. [Google Scholar] [CrossRef]
- Hornberg, H.; Wollerton-van Horck, F.; Maurus, D.; Zwart, M.; Svoboda, H.; Harris, W.A.; Holt, C.E. RNA-binding protein Hermes/RBPMS inversely affects synapse density and axon arbor formation in retinal ganglion cells in vivo. J. Neurosci. 2013, 33, 10384–10395. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; Smid, M.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; Swiggard, P.A.; Handel, L.M.; Brennan, J.M.; Godwin, A.K.; Ozols, R.F.; Hamilton, T.C. Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and -resistant human ovarian cancer cells. Cancer Res. 1994, 54, 5911–5916. [Google Scholar] [PubMed]
- Lai, G.M.; Ozols, R.F.; Smyth, J.F.; Young, R.C.; Hamilton, T.C. Enhanced DNA repair and resistance to cisplatin in human ovarian cancer. Biochem. Pharmacol. 1988, 37, 4597–4600. [Google Scholar] [CrossRef]
- Santana-Rivera, Y.; Rabelo-Fernandez, R.J.; Quinones-Diaz, B.I.; Grafals-Ruiz, N.; Santiago-Sanchez, G.; Lozada-Delgado, E.L.; Echevarria-Vargas, I.M.; Apiz, J.; Soto, D.; Rosado, A.; et al. Reduced expression of enolase-1 correlates with high intracellular glucose levels and increased senescence in cisplatin-resistant ovarian cancer cells. Am. J. Transl. Res. 2020, 12, 1275–1292. [Google Scholar]
- Gordon, R.R.; Nelson, P.S. Cellular senescence and cancer chemotherapy resistance. Drug Resist. Update 2012, 15, 123–131. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in Cancer. Cell Commun. Signal. 2018, 16, 44. [Google Scholar] [CrossRef]
- Shibata, E.; Dutta, A. A human cancer cell line initiates DNA replication normally in the absence of ORC5 and ORC2 proteins. J. Biol. Chem. 2020, 295, 16949–16959. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, J.; Karamichali, I.; Sangwallek, J.; Deiss, S.; Bar, K.; Coles, M.; Hartmann, M.D.; Lupas, A.N.; Hernandez Alvarez, B. Characterization of MCU-binding proteins MCUR1 and CCDC90B—Representatives of a protein family conserved in prokaryotes and eukaryotic organelles. Structure 2019, 27, 464–475 e6. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Lin, L.; Hu, N.; Yang, Y.; Gao, Y.; Pei, Y.; Chen, K.; Sun, B. KIF2C exerts an oncogenic role in nonsmall cell lung cancer and is negatively regulated by miR-325-3p. Cell Biochem. Funct. 2019, 37, 424–431. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, L.; Cao, J.; Guo, Y.; Wu, Y.; Gao, W. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol. Ther. Oncolytics 2021, 20, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, X.; Liu, T.; Chen, L.; Han, L.; Xu, J.; Jin, G.; Harada, K.; Lin, Z.; Ren, X. Expression of endoplasmic reticulum oxidoreductase 1-α in cholangiocarcinoma tissues and its effects on the proliferation and migration of cholangiocarcinoma cells. Cancer Manag. Res. 2019, 11, 6727–6739. [Google Scholar] [CrossRef]
- Ince, T.A.; Sousa, A.D.; Jones, M.A.; Harrell, J.C.; Agoston, E.S.; Krohn, M.; Selfors, L.M.; Liu, W.; Chen, K.; Yong, M.; et al. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat. Commun. 2015, 6, 7419. [Google Scholar] [CrossRef]
- Quinones-Diaz, B.I.; Reyes-Gonzalez, J.M.; Sanchez-Guzman, V.; Conde-Del Moral, I.; Valiyeva, F.; Santiago-Sanchez, G.S.; Vivas-Mejia, P.E. MicroRNA-18a-5p suppresses tumor growth via targeting matrix metalloproteinase-3 in cisplatin-resistant ovarian cancer. Front. Oncol. 2020, 10, 602670. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Mamouni, K.; Lokeshwar, B.L. The role of beta-arrestins in regulating stem cell phenotypes in normal and tumorigenic cells. Int. J. Mol. Sci. 2020, 21, 9310. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Smith, D.K.; Morera, D.S.; Gao, J.; Hennig, M.J.; Hoy, J.J.; Pearce, R.F.; Dabke, I.R.; Li, J.; Merseburger, A.S.; et al. Beta-arrestins regulate stem cell-like phenotype and response to chemotherapy in bladder cancer. Mol. Cancer Ther. 2019, 18, 801–811. [Google Scholar] [CrossRef]
- Mao, S.; Ling, Q.; Pan, J.; Li, F.; Huang, S.; Ye, W.; Wei, W.; Lin, X.; Qian, Y.; Wang, Y.; et al. Inhibition of CPT1a as a prognostic marker can synergistically enhance the antileukemic activity of ABT199. J. Transl. Med. 2021, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tang, Y.; Xiao, F.; Xiong, J.; Shen, K.; Liu, Y.; Zhang, W.; Zheng, L.; Zhou, J.; Xiao, M. Hemophagocytic Lymphohistocytosis in the Chinese Han Population May Be Associated with an STXBP2 Gene Polymorphism. PLoS ONE 2016, 11, e0159454. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Du, L.; Nuerlan, A.H.; Wang, X.L.; Yang, Y.W.; Guo, R. High expression of RRM2 as an independent predictive factor of poor prognosis in patients with lung adenocarcinoma. Aging 2020, 13, 3518–3535. [Google Scholar] [CrossRef]
- Avdulov, S.; Li, S.; Michalek, V.; Burrichter, D.; Peterson, M.; Perlman, D.M.; Manivel, J.C.; Sonenberg, N.; Yee, D.; Bitterman, P.B.; et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell 2004, 5, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S.H. Glutathione S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) and prostate cancer: A case-control study in Tehran, Iran. Prostate Cancer Prostat. Dis. 2011, 14, 105–113. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Xu, W.; Liu, F.; Huang, S.; He, M. GSTM1 polymorphism contribute to colorectal cancer in Asian populations: A prospective meta-analysis. Sci. Rep. 2015, 5, 12514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, Y.; Yang, H.; Zhao, D.; Li, L.; Liu, X. FLNA promotes chemoresistance of colorectal cancer through inducing epithelial-mesenchymal transition and smad2 signaling pathway. Am. J. Cancer Res. 2020, 10, 403–423. [Google Scholar]
- Ding, C.; He, R.; Zhang, J.; Dong, Z.; Wu, J. Pseudogene HSPA7 is a poor prognostic biomarker in Kidney Renal Clear Cell Carcinoma (KIRC) and correlated with immune infiltrates. Cancer Cell Int. 2021, 21, 435. [Google Scholar] [CrossRef]
- Lesko, E.; Majka, M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front. Biosci. 2008, 13, 1271–1280. [Google Scholar] [CrossRef]
- Goughnour, P.C.; Park, M.C.; Kim, S.B.; Jun, S.; Yang, W.S.; Chae, S.; Cho, S.; Song, C.; Lee, J.H.; Hyun, J.K.; et al. Extracellular vesicles derived from macrophages display glycyl-tRNA synthetase 1 and exhibit anti-cancer activity. J. Extracell. Vesicles 2020, 10, e12029. [Google Scholar] [CrossRef] [PubMed]
- Lankat-Buttgereit, B.; Goke, R. The tumour suppressor Pdcd4: Recent advances in the elucidation of function and regulation. Biol. Cell 2009, 101, 309–317. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Song, X.; Liu, C.; Shi, Y.; Wang, Y.; Afonja, O.; Ma, C.; Chen, Y.H.; Zhang, L. Programmed cell death 4 enhances chemosensitivity of ovarian cancer cells by activating death receptor pathway in vitro and in vivo. Cancer Sci. 2010, 101, 2163–2170. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Halder, J.B.; Meltzer, P.S.; Gonda, T.A.; Mangala, L.S.; Rupaimoole, R.; Lu, C.; Nagaraja, A.S.; Gharpure, K.M.; Kang, Y.; et al. ATP11B mediates platinum resistance in ovarian cancer. J. Clin. Investig. 2013, 123, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J.; Gene, M.; Gomez-Catalan, J.; Galan, M.C.; Fuentes, M.; Ramon, J.M.; Rodamilans, M.; Huguet, E.; Corbella, J. Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) polymorphisms and lung cancer risk among Northwestern Mediterraneans. Carcinogenesis 1997, 18, 1529–1533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gyorffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, N.; Pourabdollah, M.; Abdi, J.; Reece, D.; Chang, H. Dysregulation of EZH2/miR-138 axis contributes to drug resistance in multiple myeloma by downregulating RBPMS. Leukemia 2018, 32, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mou, Z.; Zhang, Z.; Wu, S.; Zhou, Q.; Chen, Y.; Gong, J.; Xu, C.; Ou, Y.; Chen, X.; et al. Circular RNA RBPMS inhibits bladder cancer progression via miR-330-3p/RAI2 regulation. Mol. Ther. Nucleic Acids 2021, 23, 872–886. [Google Scholar] [CrossRef]
- Rohit Sharma, G.S.S.-S.; Rabelo-Fernández, R.J.; Quiñones-Díaz, B.I.; Valiyeva, F.; Vivas-Mejia, P.E. Crispr/cas-9-mediated genome editing reveals that RBPMS acts as a tumor suppressor in ovarian cancer. In Proceedings of the American Association for Cancer Research Annual Meeting, Philadelphia, PA, USA, 17–21 May 2021. [Google Scholar]
- Idoko-Akoh, A.; Taylor, L.; Sang, H.M.; McGrew, M.J. High fidelity CRISPR/Cas9 increases precise monoallelic and biallelic editing events in primordial germ cells. Sci. Rep. 2018, 8, 15126. [Google Scholar] [CrossRef] [PubMed]
- Vleugel, M.M.; Greijer, A.E.; Bos, R.; van der Wall, E.; van Diest, P.J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006, 37, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shen, Q.; DuPre, E.; Kim, H.; Hilsenbeck, S.; Brown, P.H. cFos is critical for MCF-7 breast cancer cell growth. Oncogene 2005, 24, 6516–6524. [Google Scholar] [CrossRef]
- Lukey, M.J.; Greene, K.S.; Erickson, J.W.; Wilson, K.F.; Cerione, R.A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat. Commun. 2016, 7, 11321. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Prince, S. Decorin: A growth factor antagonist for tumor growth inhibition. Biomed. Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef]
- Hu, X.; Villodre, E.S.; Larson, R.; Rahal, O.M.; Wang, X.; Gong, Y.; Song, J.; Krishnamurthy, S.; Ueno, N.T.; Tripathy, D.; et al. Decorin-mediated suppression of tumorigenesis, invasion, and metastasis in inflammatory breast cancer. Commun. Biol. 2021, 4, 72. [Google Scholar] [CrossRef]
- Suhaimi, S.A.; Chan, S.C.; Rosli, R. Matrix metallopeptidase 3 polymorphisms: Emerging genetic markers in human breast cancer metastasis. J. Breast Cancer 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef]
- Yang, J.D.; Ma, L.; Zhu, Z. SERPINE1 as a cancer-promoting gene in gastric adenocarcinoma: Facilitates tumour cell proliferation, migration, and invasion by regulating EMT. J. Chemother. 2019, 31, 408–418. [Google Scholar] [CrossRef]
- Klein, R.M.; Bernstein, D.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. SERPINE1 expression discriminates site-specific metastasis in human melanoma. Exp. Dermatol. 2012, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Meng, Y.H.; Wang, J.L.; Yang, B.B.; Zhang, F.; Tang, S.J. FOXL2 suppresses proliferation, invasion and promotes apoptosis of cervical cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 1534–1543. [Google Scholar] [PubMed]
- Han, Y.; Wu, J.; Yang, W.; Wang, D.; Zhang, T.; Cheng, M. New STAT3-FOXL2 pathway and its function in cancer cells. BMC Mol. Cell Biol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Gialeli, C.; Nikitovic, D.; Kletsas, D.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. PDGF/PDGFR signaling and targeting in cancer growth and progression: Focus on tumor microenvironment and cancer-associated fibroblasts. Curr. Pharm. Des. 2014, 20, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Yongbin, Y.; Jinghua, L.; Zhanxue, Z.; Aimin, Z.; Youchao, J.; Yanhong, S.; Manjing, J. TES was epigenetically silenced and suppressed the epithelial-mesenchymal transition in breast cancer. Tumour Biol. 2014, 35, 11381–11389. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, M.; Bo, Y.; Li, W.; Zhou, R. Ras-ERK1/2 signaling accelerates the progression of colorectal cancer via mediation of H2BK5ac. Life Sci. 2019, 230, 89–96. [Google Scholar] [CrossRef]
- Jiang, M.C.; Ni, J.J.; Cui, W.Y.; Wang, B.Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar] [PubMed]
- Zhuan, B.; Lu, Y.; Chen, Q.; Zhao, X.; Li, P.; Yuan, Q.; Yang, Z. Overexpression of the long noncoding RNA TRHDE-AS1 inhibits the progression of lung cancer via the miRNA-103/KLF4 axis. J. Cell Biochem. 2019, 120, 17616–17624. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, T.; Zhang, N.; Ma, Y.; Shi, S.; Zhang, R.; Zheng, X.; Zhao, L. LncRNA TRHDE-AS1 inhibit the scar fibroblasts proliferation via miR-181a-5p/PTEN axis. J. Mol. Histol. 2021, 52, 419–426. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Wu, G.; Zhang, W.; Xu, S.; Wang, W. Long noncoding RNA PURPL promotes cell proliferation in liver cancer by regulating p53. Mol. Med. Rep. 2019, 19, 4998–5006. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Soussi, T.; Lozano, G. p53 mutation heterogeneity in cancer. Biochem. Biophys. Res. Commun. 2005, 331, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. p53 mutations in cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Masannat, J.; Purayil, H.T.; Zhang, Y.; Russin, M.; Mahmud, I.; Kim, W.; Liao, D.; Daaka, Y. Betaarrestin2 mediates renal cell carcinoma tumor growth. Sci. Rep. 2018, 8, 4879. [Google Scholar] [CrossRef]
- Aye, Y.; Li, M.; Long, M.J.; Weiss, R.S. Ribonucleotide reductase and cancer: Biological mechanisms and targeted therapies. Oncogene 2015, 34, 2011–2021. [Google Scholar] [CrossRef]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- Yang, X.; Pang, Y.; Zhang, J.; Shi, J.; Zhang, X.; Zhang, G.; Yang, S.; Wang, J.; Hu, K.; Wang, J.; et al. High expression levels of ACTN1 and ACTN3 indicate unfavorable prognosis in acute myeloid leukemia. J. Cancer 2019, 10, 4286–4292. [Google Scholar] [CrossRef] [PubMed]
- Konicek, B.W.; Dumstorf, C.A.; Graff, J.R. Targeting the eIF4F translation initiation complex for cancer therapy. Cell Cycle 2008, 7, 2466–2471. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, P.; Fu, Y.; Chen, H.; Zhang, M.; Huang, Q.; Li, D.; Li, B.; Wu, K. Targeting ANXA1 abrogates Treg-mediated immune suppression in triple-negative breast cancer. J. Immunother. Cancer 2020, 8, e000169. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.T.; Lee, C.H. Roles of farnesyl-diphosphate farnesyltransferase 1 in tumour and tumour microenvironments. Cells 2020, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Tuzmen, S.; Hostetter, G.; Watanabe, A.; Ekmekci, C.; Carrigan, P.E.; Shechter, I.; Kallioniemi, O.; Miller, L.J.; Mousses, S. Characterization of farnesyl diphosphate farnesyl transferase 1 (FDFT1) expression in cancer. Per. Med. 2019, 16, 51–65. [Google Scholar] [CrossRef]
- Weng, M.L.; Chen, W.K.; Chen, X.Y.; Lu, H.; Sun, Z.R.; Yu, Q.; Sun, P.F.; Xu, Y.J.; Zhu, M.M.; Jiang, N.; et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1alpha pathway suppression. Nat. Commun. 2020, 11, 1869. [Google Scholar] [CrossRef]
- Casella, C.; Miller, D.H.; Lynch, K.; Brodsky, A.S. Oxysterols synergize with statins by inhibiting SREBP-2 in ovarian cancer cells. Gynecol. Oncol. 2014, 135, 333–341. [Google Scholar] [CrossRef]
- Zheng, L.; Li, L.; Lu, Y.; Jiang, F.; Yang, X.A. SREBP2 contributes to cisplatin resistance in ovarian cancer cells. Exp. Biol. Med. 2018, 243, 655–662. [Google Scholar] [CrossRef]
- Hekmat, O.; Munk, S.; Fogh, L.; Yadav, R.; Francavilla, C.; Horn, H.; Wurtz, S.O.; Schrohl, A.S.; Damsgaard, B.; Romer, M.U.; et al. TIMP-1 increases expression and phosphorylation of proteins associated with drug resistance in breast cancer cells. J. Proteome Res. 2013, 12, 4136–4151. [Google Scholar] [CrossRef]
- Offenberg, H.; Brunner, N.; Mansilla, F.; Orntoft Torben, F.; Birkenkamp-Demtroder, K. TIMP-1 expression in human colorectal cancer is associated with TGF-B1, LOXL2, INHBA1, TNF-AIP6 and TIMP-2 transcript profiles. Mol. Oncol. 2008, 2, 233–240. [Google Scholar] [CrossRef]
- De Bruijn, D.R.; dos Santos, N.R.; Kater-Baats, E.; Thijssen, J.; van den Berk, L.; Stap, J.; Balemans, M.; Schepens, M.; Merkx, G.; van Kessel, A.G. The cancer-related protein SSX2 interacts with the human homologue of a Ras-like GTPase interactor, RAB3IP, and a novel nuclear protein, SSX2IP. Genes Chromosomes Cancer 2002, 34, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, Y.; Zhang, Y.; Zhu, Z.; Huo, K. SSX2IP promotes metastasis and chemotherapeutic resistance of hepatocellular carcinoma. J. Transl. Med. 2013, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kawahara, B.; Gupta, D.; Tsai, R.; Khachatryan, M.; Roy-Chowdhuri, S.; Bose, S.; Yoon, A.; Faull, K.; Farias-Eisner, R.; et al. Role of cystathionine beta-synthase in human breast Cancer. Free Radic. Biol. Med. 2015, 86, 228–238. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L.; et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhu, Z.R.; Wang, H.; Li, W.W.; Cao, C.H.; Liu, L.; Wu, D.H. Knockdown of UACA inhibitsproliferation and invasion and promotes senescence of hepatocellular carcinoma cells. Int. J. Clin. Exp. Pathol. 2018, 11, 4666–4675. [Google Scholar] [PubMed]
- Burikhanov, R.; Shrestha-Bhattarai, T.; Qiu, S.; Shukla, N.; Hebbar, N.; Lele, S.M.; Horbinski, C.; Rangnekar, V.M. Novel mechanism of apoptosis resistance in cancer mediated by extracellular PAR-4. Cancer Res. 2013, 73, 1011–1019. [Google Scholar] [CrossRef]
- Vivas-Mejia, P.; Benito, J.M.; Fernandez, A.; Han, H.D.; Mangala, L.; Rodriguez-Aguayo, C.; Chavez-Reyes, A.; Lin, Y.G.; Carey, M.S.; Nick, A.M.; et al. c-Jun-NH2-kinase-1 inhibition leads to antitumor activity in ovarian cancer. Clin. Cancer Res. 2010, 16, 184–194. [Google Scholar] [CrossRef]
- Vivas-Mejia, P.E.; Rodriguez-Aguayo, C.; Han, H.D.; Shahzad, M.M.; Valiyeva, F.; Shibayama, M.; Chavez-Reyes, A.; Sood, A.K.; Lopez-Berestein, G. Silencing survivin splice variant 2B leads to antitumor activity in taxane--resistant ovarian cancer. Clin. Cancer Res. 2011, 17, 3716–3726. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, J.M.; Quinones-Diaz, B.I.; Santana, Y.; Baez-Vega, P.M.; Soto, D.; Valiyeva, F.; Marcos-Martinez, M.J.; Fernandez-de Thomas, R.J.; Vivas-Mejia, P.E. Downstream effectors of ILK in cisplatin-resistant ovarian cancer. Cancers 2020, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Cong, L.; Zhang, F. Genome engineering using CRISPR-Cas9 system. Methods Mol. Biol. 2015, 1239, 197–217. [Google Scholar] [PubMed]
- Echevarria-Vargas, I.M.; Valiyeva, F.; Vivas-Mejia, P.E. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS ONE 2014, 9, e97094. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 2012, 7, 1534–1550. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. GtRNAdb 2.0: An expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016, 44, D184–D189. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, J.; Domingues, D.; Lopes, F.M. RNA-Seq differential expression analysis: An extended review and a software tool. PLoS ONE 2017, 12, e0190152. [Google Scholar] [CrossRef] [PubMed]
| Type of Ovarian Cancer | Num. of Patient | Num. of Samples | Average Age | Subgroups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Tissue | 9 | 27 | 33 | Grades | |||||||
| (-) | I | I-II | II | II-III | III | ||||||
| 27 | 0 | 0 | 0 | 0 | 0 | ||||||
| Stage | |||||||||||
| I | 1a | 1A | II | IIB | IC | IIIC | IV | ||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Clear Cell Carcinoma | 5 | 18 | 49 | Grades | |||||||
| (-) | I | I-II | II | II-III | III | ||||||
| 18 | |||||||||||
| Stage | |||||||||||
| I | 1a | 1A | II | IIB | IC | IIIC | IV | ||||
| 15 | 3 | ||||||||||
| Serous Papillary Adenocarcinoma | 47 | 138 | 48 | Grades | |||||||
| (-) | I | I-II | II | II-III | III | ||||||
| 2 | 22 | 0 | 33 | 4 | 77 | ||||||
| Stage | |||||||||||
| I | 1a | 1A | II | IIB | IC | IIIC | IV | ||||
| 39 | 3 | 6 | 33 | 3 | 9 | 36 | 9 | ||||
| Endometrioid Adenocarcinoma | 4 | 12 | 51 | Grades | |||||||
| (-) | I | I-II | II | II-III | III | ||||||
| 0 | 0 | 0 | 11 | 1 | 0 | ||||||
| Stage | |||||||||||
| I | 1a | 1A | II | IIB | IC | IIIC | IV | ||||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Mucinous Adenocarcinoma | 4 | 12 | 50 | Grades | |||||||
| (-) | I | I-II | II | II-III | III | ||||||
| 2 | 4 | 2 | 4 | 0 | 0 | ||||||
| Stage | |||||||||||
| I | 1a | 1A | II | IIB | IC | IIIC | IV | ||||
| 3 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | ||||
| Ingenuity Canonical Pathways | log10 (p-Value) | Ratio | Number of Genes | Genes |
|---|---|---|---|---|
| Hepatic Fibrosis/Hepatic Stellate Cell Activation | 7.54 | 0.111 | 21 | ACTA2, CCR5, COL11A2, COL12A1, COL1A1, COL6A1, COL7A1, CXCL8, FGF2, HGF, IFNGR1, IL4R, IL6R, KLF6, MET, MMP1, MYL7, PDGFD, RELB, SERPINE1, TIMP1 |
| Axonal Guidance Signaling | 5.26 | 0.0635 | 31 | ADAM19, ADAMTS1, ADAMTS10, ADAMTS5, BMP5, DPYSL2, EFNB2, EPHA7, FZD2, GNG5, HHIP, ITGA2B, ITGB8, LRRC4C, MET, MMP1, MMP10, MMP16, MMP3, MYL7, NRP2, PAK3, PDGFD, PIK3CA, PLCD4, PLCE1, ROBO3, SDC2, SEMA4A, TUBB2B, UNC5B |
| Hepatic Fibrosis Signaling Pathway | 4.45 | 0.0631 | 26 | ACTA2, ACVR2B, CACNA1A, CACNB2, CACNG7, COL11A2, COL1A1, CXCL8, FGF2, FZD2, ITGA2B, ITGB8, LRP1, MAP2K3, MMP1, MYL7, MYLK, PDGFD, PIK3CA, PTEN, RELB, RHOH, RND3, SERPINE1, TCF7L1, TIMP1 |
| Inhibition of Matrix Metalloproteases | 4.3 | 0.184 | 7 | LRP1, MMP1, MMP10, MMP16, MMP3, SDC2, TIMP1 |
| Tumor Microenvironment Pathway | 4.15 | 0.0843 | 15 | COL1A1, CXCL8, FGF2, FGF20, FGF5, HGF, IL6R, MAP3K14, MMP1, MMP10, MMP16, MMP3, PDGFD, PIK3CA, RELB |
| ID | Symbol | Gene Name | Fold Change | p Value |
|---|---|---|---|---|
| ENSG00000236333 | TRHDE-AS1 | TRHDE antisense RNA 1 | 102.584 | 1.55 × 10−9 |
| ENSG00000139287 | TPH2 | Tryptophan hydroxylase 2 | 35.23 | 1.01 × 10−7 |
| ENSG00000011465 | DCN | Decorin | 32.901 | 1.38 × 10−8 |
| ENSG00000149968 | MMP3 | Matrix metallopeptidase 3 | 30.545 | 2.2 × 10−7 |
| ENSG00000072657 | TRHDE | Thyrotropin releasing hormone degrading enzyme | 20.316 | 9.97 × 10−8 |
| ENSG00000165092 | ALDH1A1 | Aldehyde dehydrogenase 1 family member A1 | 17.445 | 1.11 × 10−9 |
| ENSG00000139329 | LUM | Lumican | 15.076 | 3 × 10−10 |
| ENSG00000135046 | ANXA1 | Annexin A1 | 11.889 | 2.24 × 10−8 |
| ENSG00000230426 | LINC01036 | Long intergenic non-protein coding RNA 1036 | 4.594 | 5.06 × 10−10 |
| ENSG00000106366 | SERPINE1 | Serpin family E member 1 | 2.62 | 6.33 × 10−7 |
| ENSG00000196562 | SULF2 | Sulfatase 2 | −70.19 | 6.09 × 10−20 |
| ENSG00000006047 | YBX2 | Y-box binding protein 2 | −113.464 | 7.9 × 10−23 |
| ENSG00000173727 | LOC101927789 | FAU, ubiquitin like and ribosomal protein S30 fusion pseudogene | −230.342 | 5.94 × 10−20 |
| ENSG00000183770 | FOXL2 | Forkhead box L2 | −262.198 | 4.99 × 10−26 |
| ENSG00000170962 | PDGFD | Platelet derived growth factor D | −597.324 | 3.21 × 10−20 |
| ENSG00000135269 | TES | Testin LIM domain protein | −701.647 | 5.83 × 10−28 |
| ENSG00000204397 | CARD16 | Caspase recruitment domain family member 16 | −1361.887 | 1.48 × 10−32 |
| ENSG00000251095 | LOC105377329 | Uncharacterized LOC105377329 | −1382.487 | 3.83 × 10−28 |
| ENSG00000198795 | ZNF521 | Zinc finger protein 521 | −1412.411 | 2.15 × 10−27 |
| ENSG00000250337 | PURPL | p53 upregulated regulator of p53 levels | −1852.805 | 3.08 × 10−26 |
| Symbol | Biological Role | Reference |
|---|---|---|
| ARRB1 | A scaffold protein that participates in the agonist-mediated desensitization of G-protein-coupled receptors. Depending on the cancer type, it has been reported as an oncogene or tumor suppressor gene. | [39] |
| ARRB2 | A scaffold protein that participates in the agonist-mediated desensitization of G-protein-coupled receptor. Increased in colorectal cancer, renal cell carcinoma, and glioblatoma. | [40] |
| CPT1A | Plays a critical role in increasing the fatty acid oxidation required for the cellular fuel demands in radioresistant and chemoresistant cancer cells. | [41] |
| STXBP2 | Significantly expressed in hemophagocytic lymphohistiocytosis (HLH), a disease featuring severe hyperinflammation caused by the uncontrolled proliferation of activated lymphocytes and macrophages. | [42] |
| RRM2B | Plays a crucial role in DNA repair, DNA damage response, oxygen sensing, and apoptosis pathways. Highly amplified in multiple tumor types. | [43] |
| RAB27A | Belongs to a small GTPase superfamily (Rab family). Increased in many cancers. Governs a variety of oncogenic functions, including cell proliferation, motility, metastasis, and chemosensitivity. | [31] |
| ORC3 | Highly conserved six-subunit protein complex essential for the initiation of DNA replication in eukaryotic cells. | [32] |
| CCDC90B | Presumably a mitochondrial protein characterized by the presence of a domain of unknown function DUF1640. | [33] |
| RRM2 | A reductase that catalyzes the formation of deoxyribonucleotides from ribonucleotides. Its increased levels have been associated with cell proliferation, invasion, and migration; its downregulation induces apoptosis and G1 arrest. | [43] |
| KIF2C | Functions as a microtubule-dependent molecular motor. Acts like an oncogene in many cancer types, where it promotes cell proliferation, migration, invasion, and metastasis. | [34] |
| FSCN1 | An actin-bundling protein that cross links F-actin microfilaments into tight, parallel bundles. Elevated FSCN1 levels have been correlated with aggressive clinical progression, poor prognosis, and poor survival outcomes in many cancer types. | [35] |
| PABPC4L | Possesses a critical role in RNA processing. It travels from the nucleus to the cytoplasm with mRNAs, increases eIF4F assembly at caps, forms closed-looped RNA, aids in the recruitment of ribosomal subunits to 5′ UTRs, and increases the reuse of translational machinery after polypeptide synthesis. | [44] |
| GSTM1 | Plays a role in the detoxification of metabolites of environmental carcinogens and protecting hosts against cancer. | [45] |
| GSTM4 | Similar GSTM1, it functions in the detoxification of electrophilic compounds, including carcinogens, therapeutic drugs, environmental toxins, and products of oxidative stress, via conjugation with glutathione. | [46] |
| FLNA | An actin-binding protein that crosslinks actin filaments and links actin filaments to membrane glycoprotein, which contributes to stabilizing the cytoskeleton network and supports cell integrity. | [47] |
| HSPA7 | Transcribed in response to stress and plays a causal role in cancer initiation. HSPA7 is a poor prognostic biomarker in kidney and hepatocellular cancers. | [48] |
| HGF | A receptor of MET, which plays a role in cancer growth and metastasis. Activation of MET activates multiple cellular responses involved in cell survival, morphogenesis, adhesion, migration, breakdown of the extracellular matrix (ECM), and angiogenesis. | [49] |
| ERO1A | A hypoxia-induced endoplasmic reticulum oxidase that regulates the translation and folding of oxidized proteins. Its high expression is associated with poor prognosis in patients due to its promoting the cell proliferation and migration of cancer cells. | [36] |
| GARS1 | Aminoacyl-tRNA synthetases that charge tRNAs with their cognate amino acids. | [50] |
| Ingenuity Canonical Pathways | −log10 (p-Value) | Ratio | Number of Proteins | Proteins |
|---|---|---|---|---|
| LPS/IL-1 Mediated Inhibition of RXR Function | 3.9 | 0.0332 | 7 | ACSL1, ALDH2, CPT1A, GSTM1, GSTM4, HS2ST1, MGST1 |
| Pyrimidine Deoxyribonucleotides De Novo Biosynthesis I | 3.7 | 0.136 | 3 | AK4, RRM2, RRM2B |
| Glutathione-mediated Detoxification | 3.53 | 0.12 | 3 | GSTM1, GSTM4, MGST1 |
| Xenobiotic Metabolism PXR Signaling Pathway | 3.47 | 0.0341 | 6 | ALDH2, GSTM1, GSTM4, HS2ST1, MGST1, PRKAR1A |
| Accession | Symbol | Gene Name | Fold Change | p Value |
|---|---|---|---|---|
| P49407-2 | ARRB1 | Arrestin beta 1 | 3.586 | 1.67 × 10−7 |
| P32121-5 | ARRB2 | Arrestin beta 2 | 3.586 | 1.67 × 10−7 |
| P50416-2 | CPT1A | Carnitine palmitoyltransferase 1A | 2.864 | 1.93 × 10−8 |
| M0R376 | STXBP2 | Syntaxin binding protein 2 | 2.43 | 1.55 × 10−8 |
| Q7LG56-3 | RRM2B | Ribonucleotide reductase regulatory TP53 inducible subunit M2B | 2.24 | 1.09 × 10−7 |
| P51159-2 | RAB27A | RAB27A, member RAS oncogene family | 2.201 | 1.59 × 10−6 |
| Q9UBD5-3 | ORC3 | Origin recognition complex subunit 3 | 2.173 | 2.47 × 10−7 |
| E9PKQ5 | CCDC90B | Coiled-coil domain containing 90B | 2.15 | 1.15 × 10−7 |
| A0A286YFD6 | RRM2 | Ribonucleotide reductase regulatory subunit M2 | 2.132 | 3.06 × 10−7 |
| Q5JR91 | KIF2C | Kinesin family member 2C | 2.097 | 1.83 × 10−6 |
| Q16658 | FSCN1 | Fascin actin-bundling protein 1 | −2.037 | 3.76 × 10−6 |
| P0CB38 | PABPC4L | Poly(A) binding protein cytoplasmic 4 like | −2.086 | 3.33 × 10−6 |
| P09488-2 | GSTM1 | Glutathione S-transferase mu 1 | −2.104 | 1.55 × 10−6 |
| A0A0A0MR85 | GSTM4 | Glutathione S-transferase mu 4 | −2.104 | 1.55 × 10−6 |
| H0Y5F3 | FLNA | Filamin A | −2.47 | 3.25 × 10−8 |
| P48741 | HSPA7 | Heat shock protein family A (Hsp70) member 7 | −2.491 | 1.41 × 10−6 |
| P14210-6 | HGF | Hepatocyte growth factor | −2.6 | 3.31 × 10−8 |
| G3V2H0 | ERO1A | Endoplasmic reticulum oxidoreductase 1 alpha | −2.603 | 1.85 × 10−7 |
| P41250 | GARS1 | Glycyl-tRNA synthetase 1 | −2.73 | 3.21 × 10−7 |
| F5GYB7 | RECQL | RecQ like helicase | −3.468 | 5.25 × 10−8 |
| Symbol | Biological Role | Reference |
|---|---|---|
| ARRB1 | Scaffold protein that participates in the agonist-mediated desensitization of G-protein-coupled receptors. Depending on the cancer type, it has been reported as an oncogene or tumor suppressor gene. | [39] |
| ARRB2 | Scaffold protein that participates in the agonist-mediated desensitization of G-protein-coupled receptor. Increased in colorectal cancer, renal cell carcinoma, and glioblastoma. | [40] |
| CPT1A | Plays a critical role in increasing the fatty acid oxidation required for the cellular fuel demands in radioresistant and chemoresistant cancer cells. | [41] |
| STXBP2 | Significantly expressed in hemophagocytic lymphohistiocytosis (HLH), a disease of severe hyperinflammation caused by the uncontrolled proliferation of activated lymphocytes and macrophages. | [42] |
| RRM2B | Plays a crucial role in DNA repair, DNA damage response, oxygen sensing, and apoptosis pathways. It is highly amplified in multiple tumor types. | [43] |
| RAB27A | Belongs to a small GTPase superfamily (Rab family). Increased in many cancers. Governs a variety of oncogenic functions, including cell proliferation, cell motility, metastasis, and chemosensitivity. | [31] |
| ORC3 | Highly conserved six-subunit protein complex essential for the initiation of DNA replication in eukaryotic cells. | [32] |
| CCDC90B | Presumably, a mitochondrial protein is characterized by the presence of a domain of unknown function DUF1640. | [33] |
| RRM2 | This reductase catalyzes the formation of deoxyribonucleotides from ribonucleotides. Its increased levels have been associated with cell proliferation, invasion, and migration. Its downregulation induces apoptosis and G1 arrest. | [43] |
| KIF2C | Functions as a microtubule-dependent molecular motor. Acts like an oncogene in many cancer types, where it promotes cell proliferation, migration, invasion, and metastasis. | [34] |
| FSCN1 | An actin-bundling protein that cross-links F-actin microfilaments into tight, parallel bundles. Elevated FSCN1 levels have been correlated with aggressive clinical progression, poor prognosis, and poor survival outcomes in many cancer types. | [35] |
| PABPC4L | Has a critical role in RNA processing. It travels from the nucleus to the cytoplasm with mRNAs, increases eIF4F assembly at caps, forms closed-looped RNA, aids in the recruitment of ribosomal subunits to 5′ UTRs, and increases the reuse of translational machinery after polypeptide synthesis. | [44] |
| GSTM1 | Plays a role in the detoxification of metabolites of environmental carcinogens and protects hosts against cancer. | [54] |
| GSTM4 | Similar to GSTM1, functions in the detoxification of electrophilic compounds, including carcinogens, therapeutic drugs, environmental toxins, and products of oxidative stress, via conjugation with glutathione. | [46] |
| FLNA | An actin-binding protein that crosslinks actin filaments and links actin filaments to membrane glycoproteins, contributing to stabilizing the cytoskeleton network and supporting cell integrity. | [47] |
| HSPA7 | Transcribed in response to stress and plays a causal role in cancer initiation. HSPA7 is a poor prognostic biomarker in kidney and hepatocellular cancers. | [48] |
| HGF | A receptor of MET, which play a role in cancer growth and metastasis. The activation of MET activates multiple cellular responses involved in cell survival, morphogenesis, adhesion, migration, breakdown of the extracellular matrix (ECM), and angiogenesis. | [49] |
| ERO1A | Is a hypoxia-induced endoplasmic reticulum oxidase that regulates the translation and folding of oxidized proteins. The high expression of ERO1A is associated with poor prognosis in patients by its promoting cell proliferation and the migration of cancer cells. | [36] |
| GARS1 | Aminoacyl-tRNA synthetases that charge tRNAs with their cognate amino acids. | [50] |
| Gene Symbol | Uniprot | Gene Name | Fold Change RNAseq | p-Value RNAseq | Fold Change Proteomics | p-Values Proteomics |
|---|---|---|---|---|---|---|
| ANXA1 | Q5T3N1 | Annexin A1 | 11.889 | 2.24 × 10−08 | 3.047 | 4.59 × 10−5 |
| FDFT1 | E9PJG4 | Farnesyl-diphosphate farnesyltransferase 1 | 6.7 | 2.03 × 10−06 | 2.587 | 3.30 × 10−6 |
| TIMP1 | Q5H9A7 | TIMP metallopeptidase inhibitor 1 | 2.637 | 4.83 × 10−3 | 2.456 | 3.55 × 10−3 |
| SSX2IP | S4R403 | SSX family member 2 interacting protein | 2.406 | 5.83 × 10−4 | 2.009 | 9.71 × 10−6 |
| SPART | Q8N0X7 | Spartin | 1.738 | 7.7 × 10−4 | 2.046 | 9.79 × 10−5 |
| CBS/CBSL | H7C2W0 | Cystathionine beta-synthase | −1.517 | 8.02 × 10−3 | −2.314 | 2.92 × 10−4 |
| GSTM1 | P09488-2 | Glutathione S-transferase mu 1 | −1.643 | 6.72 × 10−4 | −2.104 | 1.55 × 10−6 |
| HGF | P14210-6 | Hepatocyte growth factor | −1.767 | 2.93 × 10−3 | −2.6 | 3.31 × 10−8 |
| UACA | F5H2B9 | Uveal autoantigen with coiled-coil domains and ankyrin repeats | −2.451 | 2.08 × 10−4 | −2.212 | 2.44 × 10−3 |
| ACTN3 | Q08043 | Actinin alpha 3 | −2.455 | 4.93 × 10−4 | −2.091 | 1.92 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo-Fernández, R.J.; Santiago-Sánchez, G.S.; Sharma, R.K.; Roche-Lima, A.; Carrion, K.C.; Rivera, R.A.N.; Quiñones-Díaz, B.I.; Rajasekaran, S.; Siddiqui, J.; Miles, W.; et al. Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells. Int. J. Mol. Sci. 2022, 23, 535. https://doi.org/10.3390/ijms23010535
Rabelo-Fernández RJ, Santiago-Sánchez GS, Sharma RK, Roche-Lima A, Carrion KC, Rivera RAN, Quiñones-Díaz BI, Rajasekaran S, Siddiqui J, Miles W, et al. Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells. International Journal of Molecular Sciences. 2022; 23(1):535. https://doi.org/10.3390/ijms23010535
Chicago/Turabian StyleRabelo-Fernández, Robert J., Ginette S. Santiago-Sánchez, Rohit K. Sharma, Abiel Roche-Lima, Kelvin Carrasquillo Carrion, Ricardo A. Noriega Rivera, Blanca I. Quiñones-Díaz, Swetha Rajasekaran, Jalal Siddiqui, Wayne Miles, and et al. 2022. "Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells" International Journal of Molecular Sciences 23, no. 1: 535. https://doi.org/10.3390/ijms23010535
APA StyleRabelo-Fernández, R. J., Santiago-Sánchez, G. S., Sharma, R. K., Roche-Lima, A., Carrion, K. C., Rivera, R. A. N., Quiñones-Díaz, B. I., Rajasekaran, S., Siddiqui, J., Miles, W., Rivera, Y. S., Valiyeva, F., & Vivas-Mejia, P. E. (2022). Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells. International Journal of Molecular Sciences, 23(1), 535. https://doi.org/10.3390/ijms23010535









