Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Prediction of Amyloidogenicity of Ribosomal S1 Protein from S. aureus
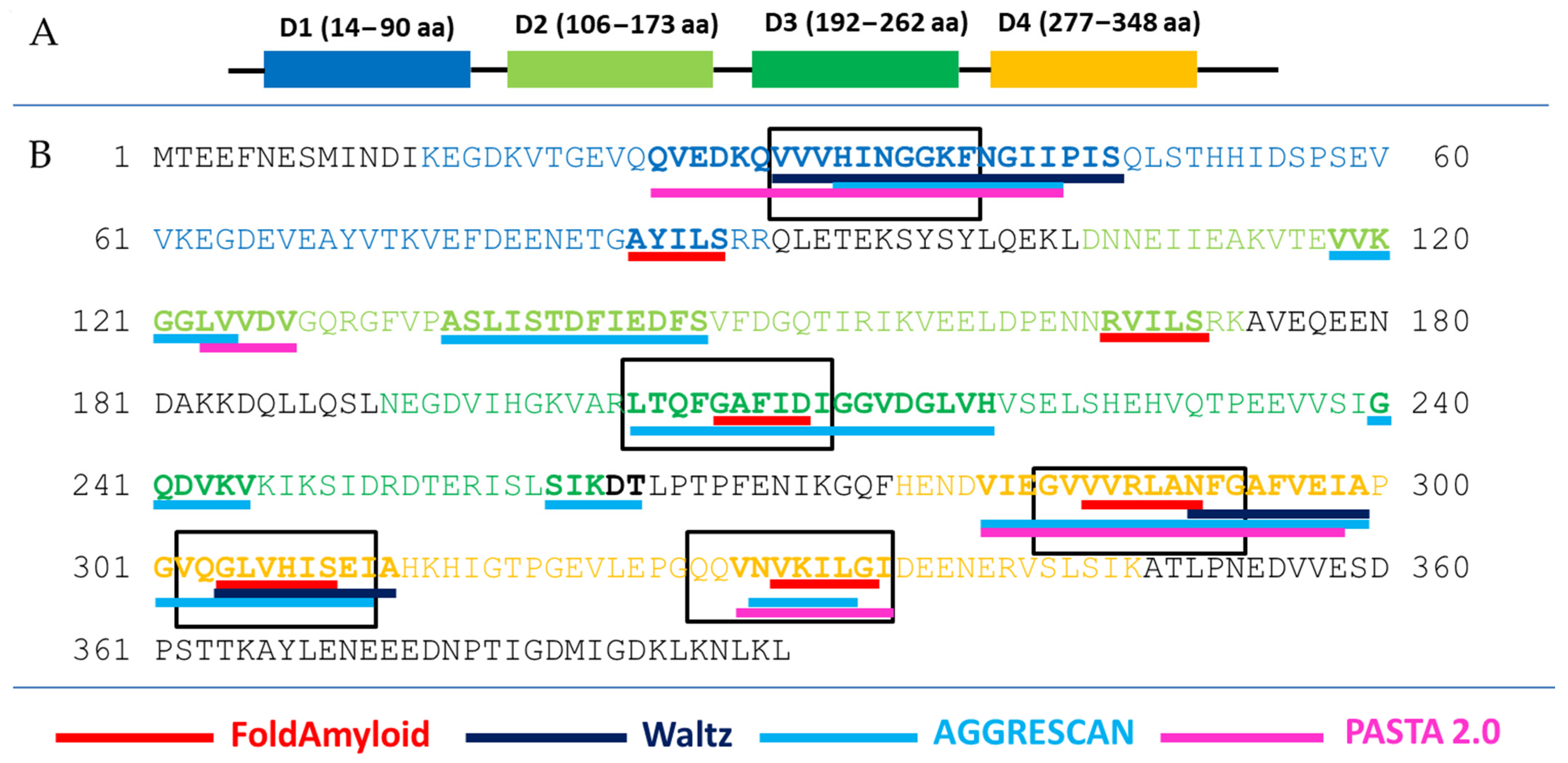
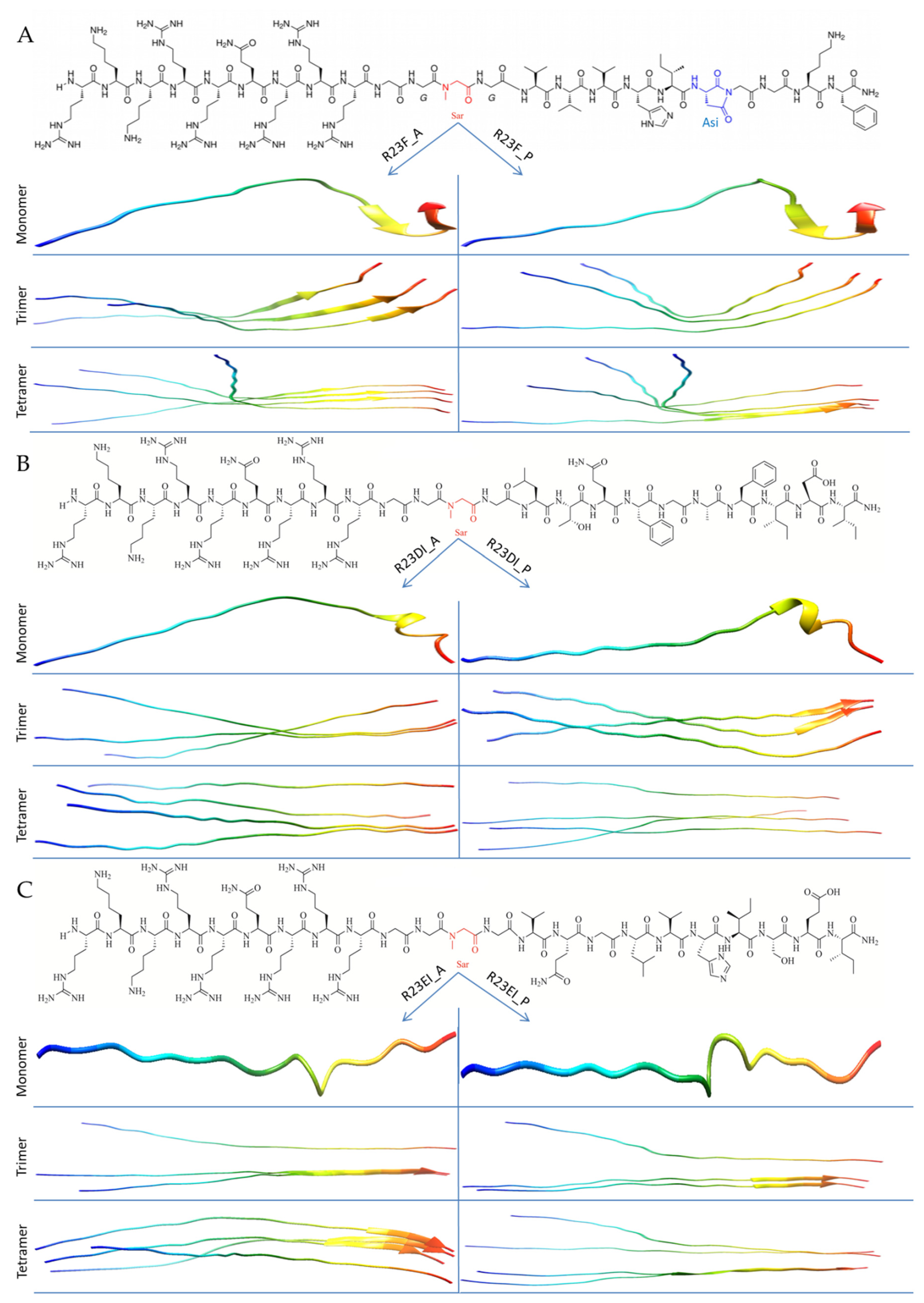
2.2. Construction of Hybrid Peptides
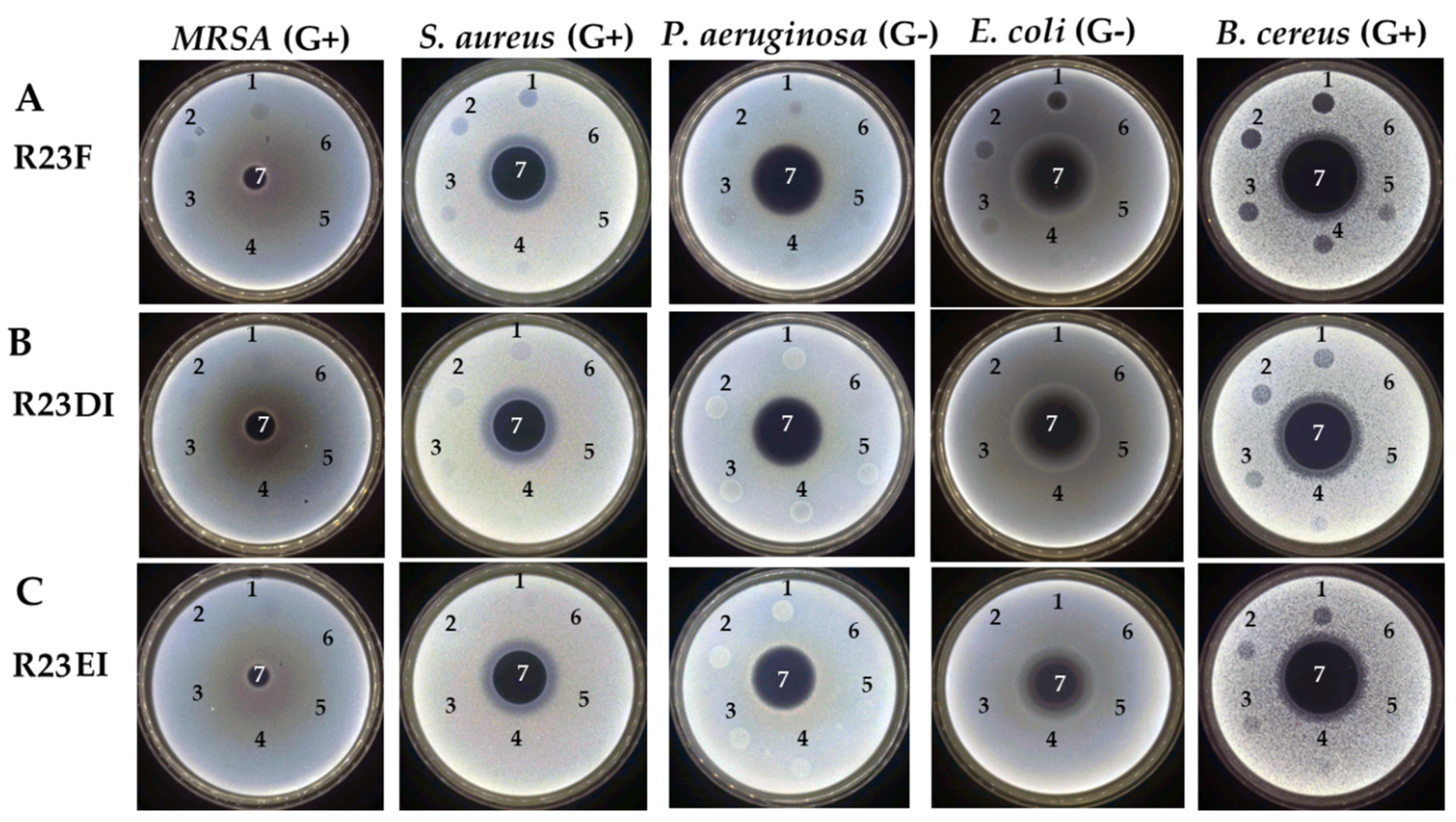
2.3. Determination of the Antibacterial Activity of the R23F, R23DI, R23EI Peptides by Agar Diffusion Assay
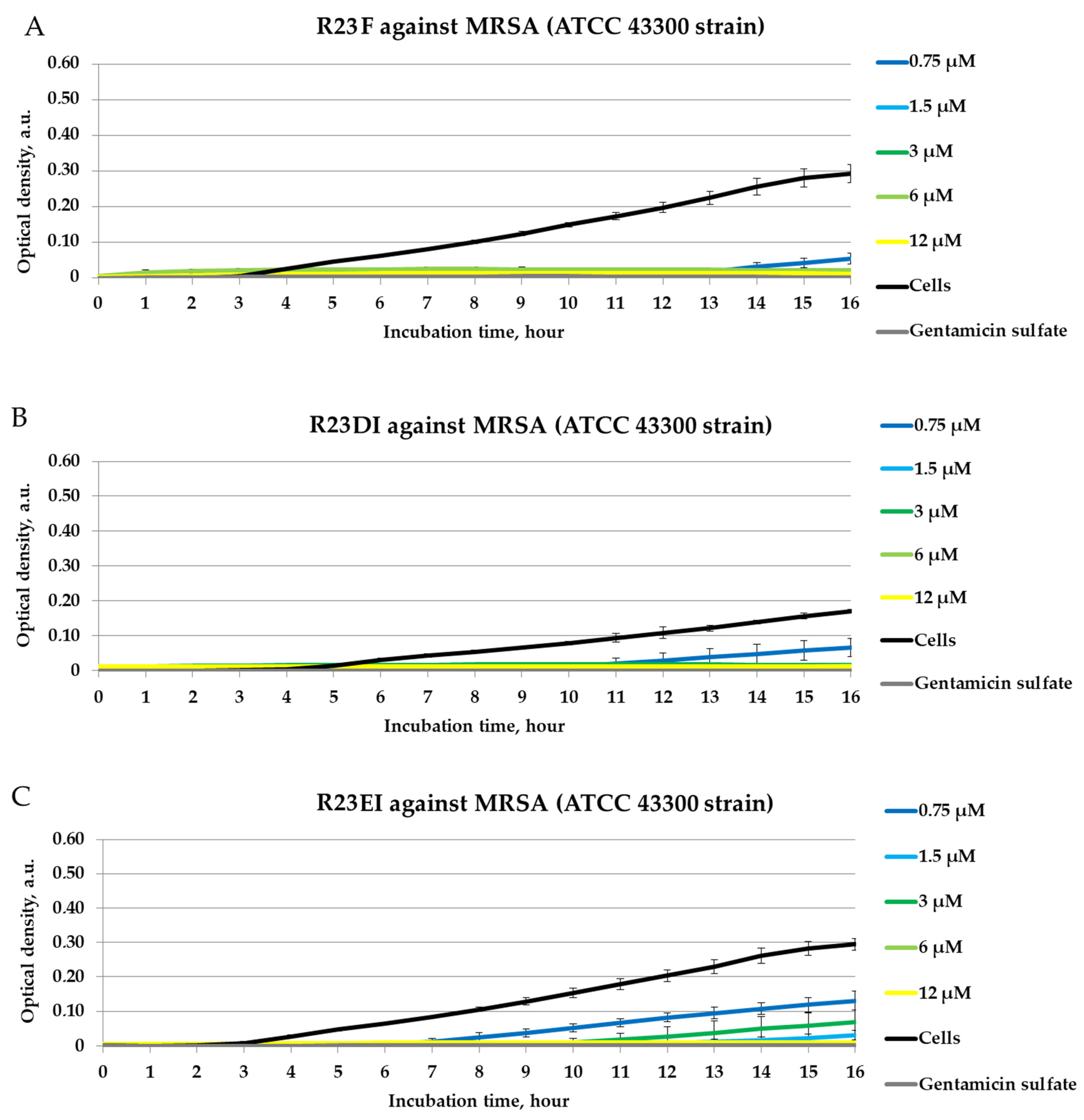
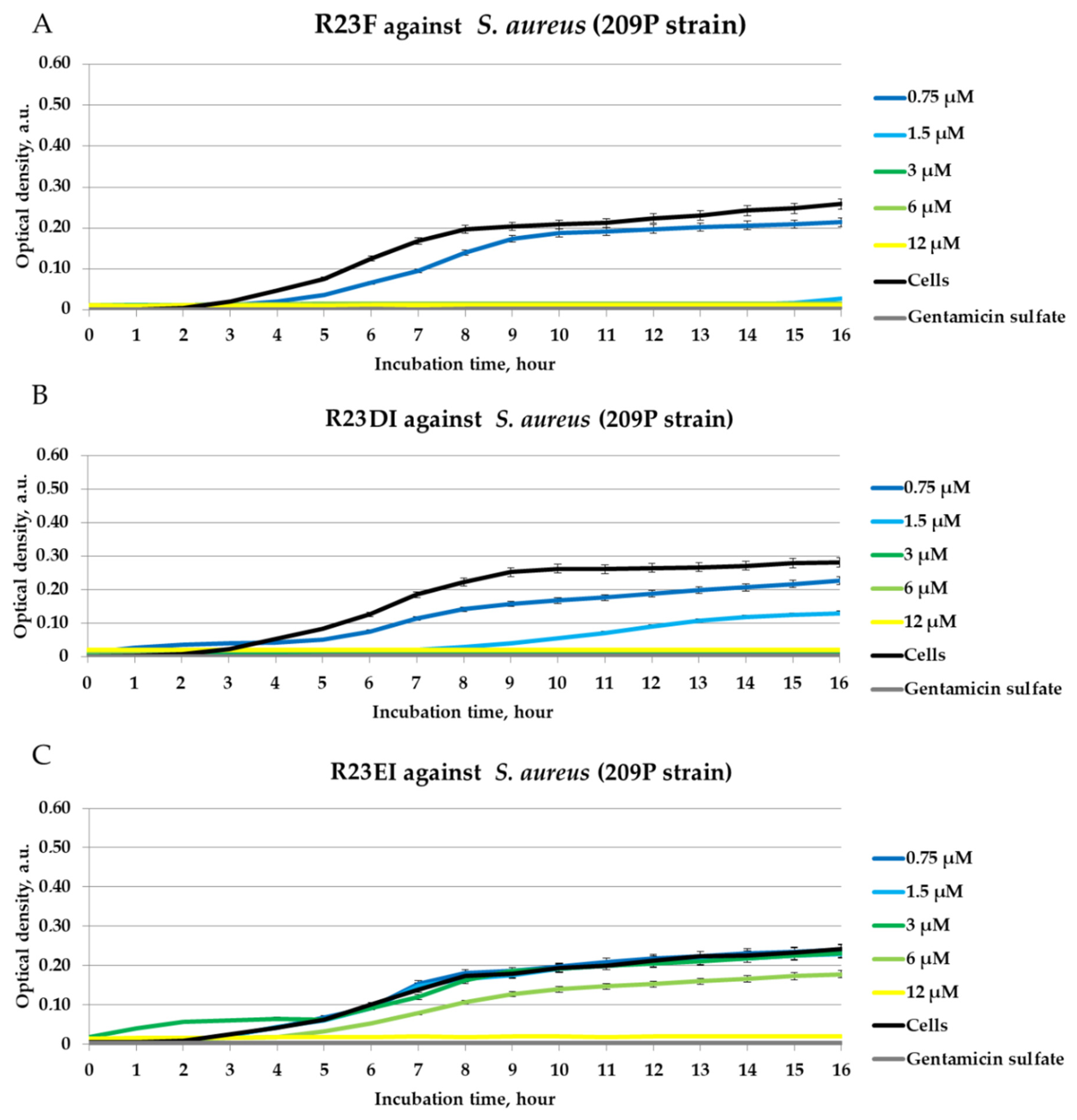
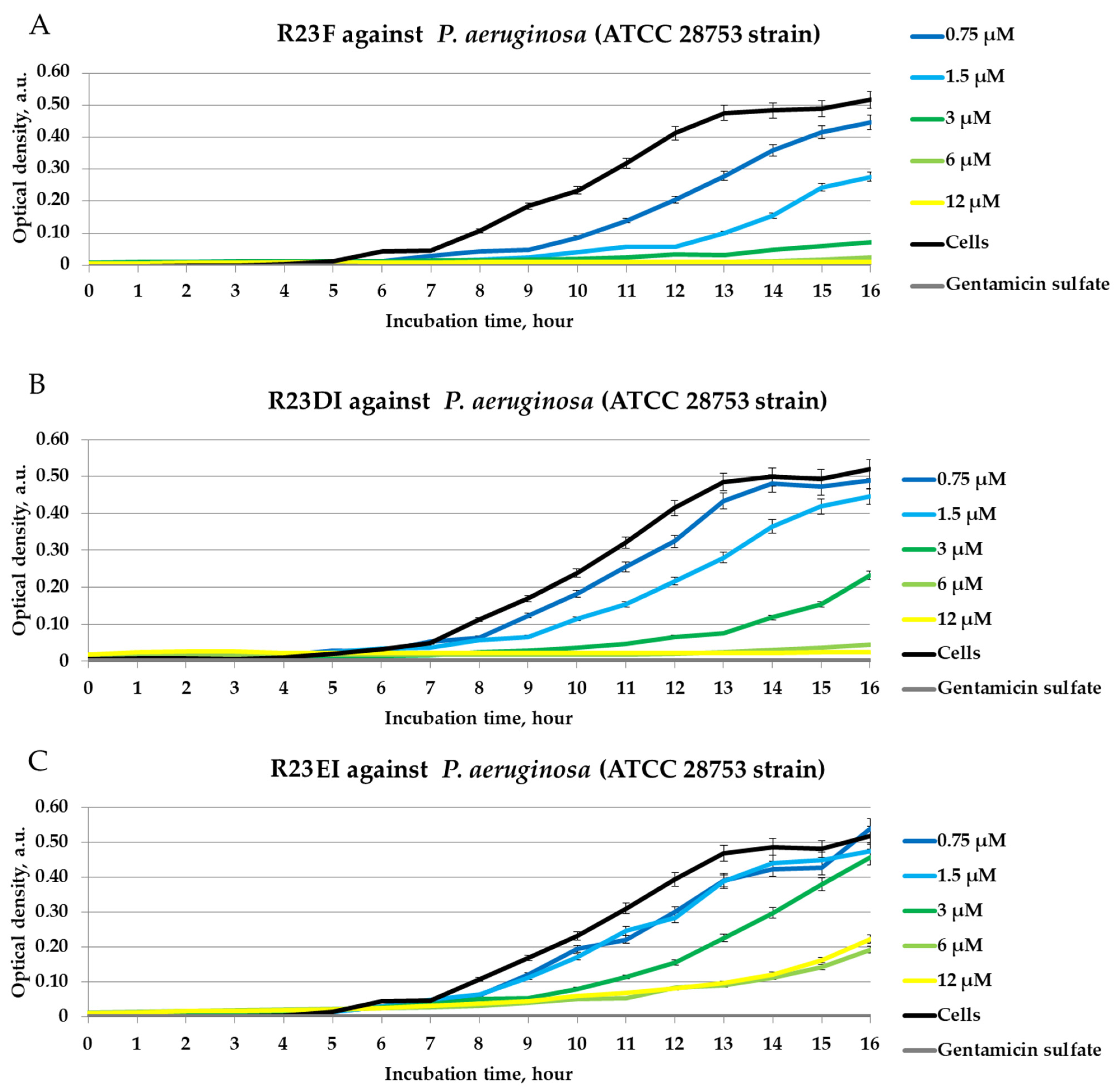
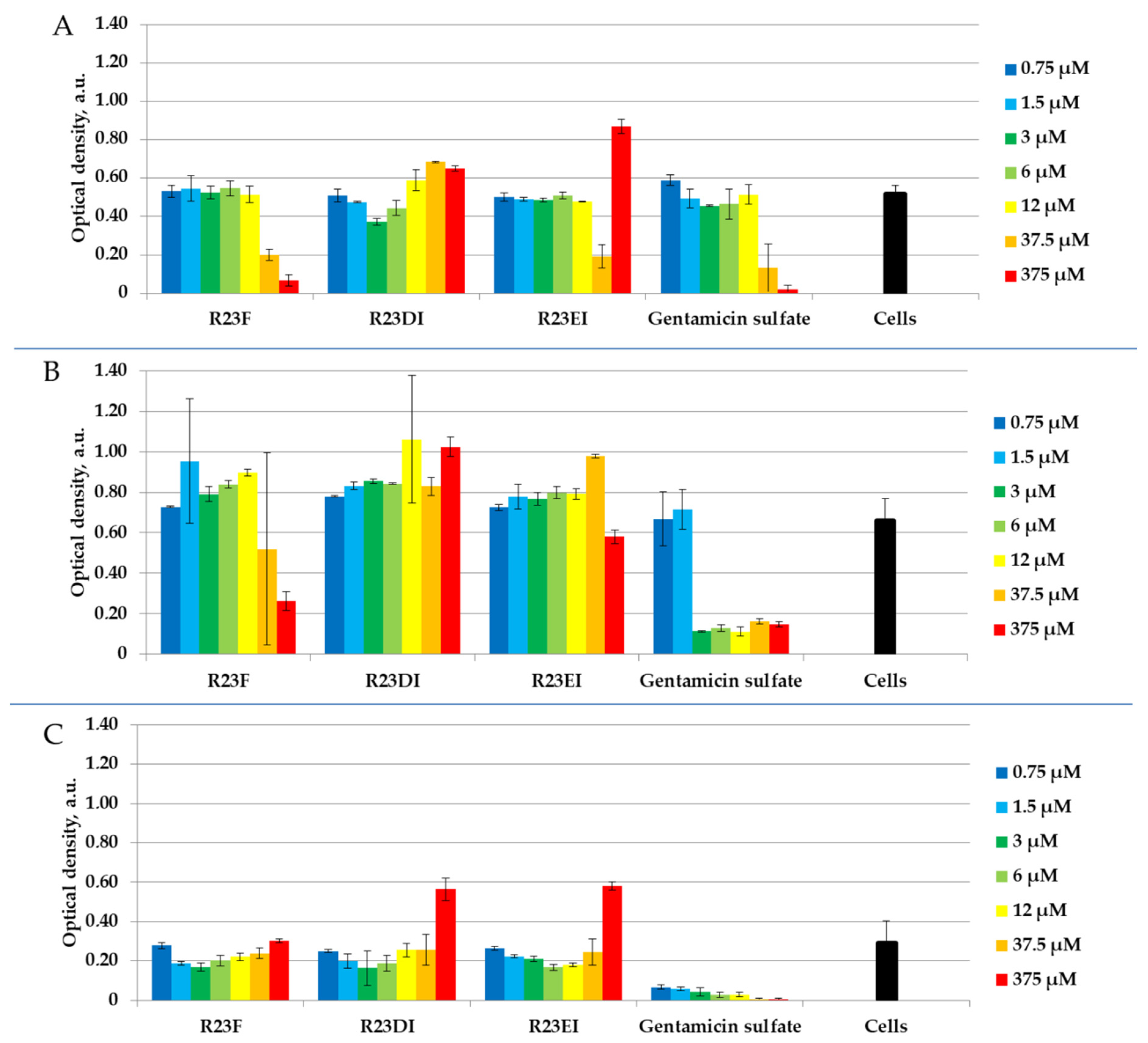
2.4. Antibacterial Activity of Peptides against MRSA, S. aureus, and P. aeruginosa in Liquid Medium
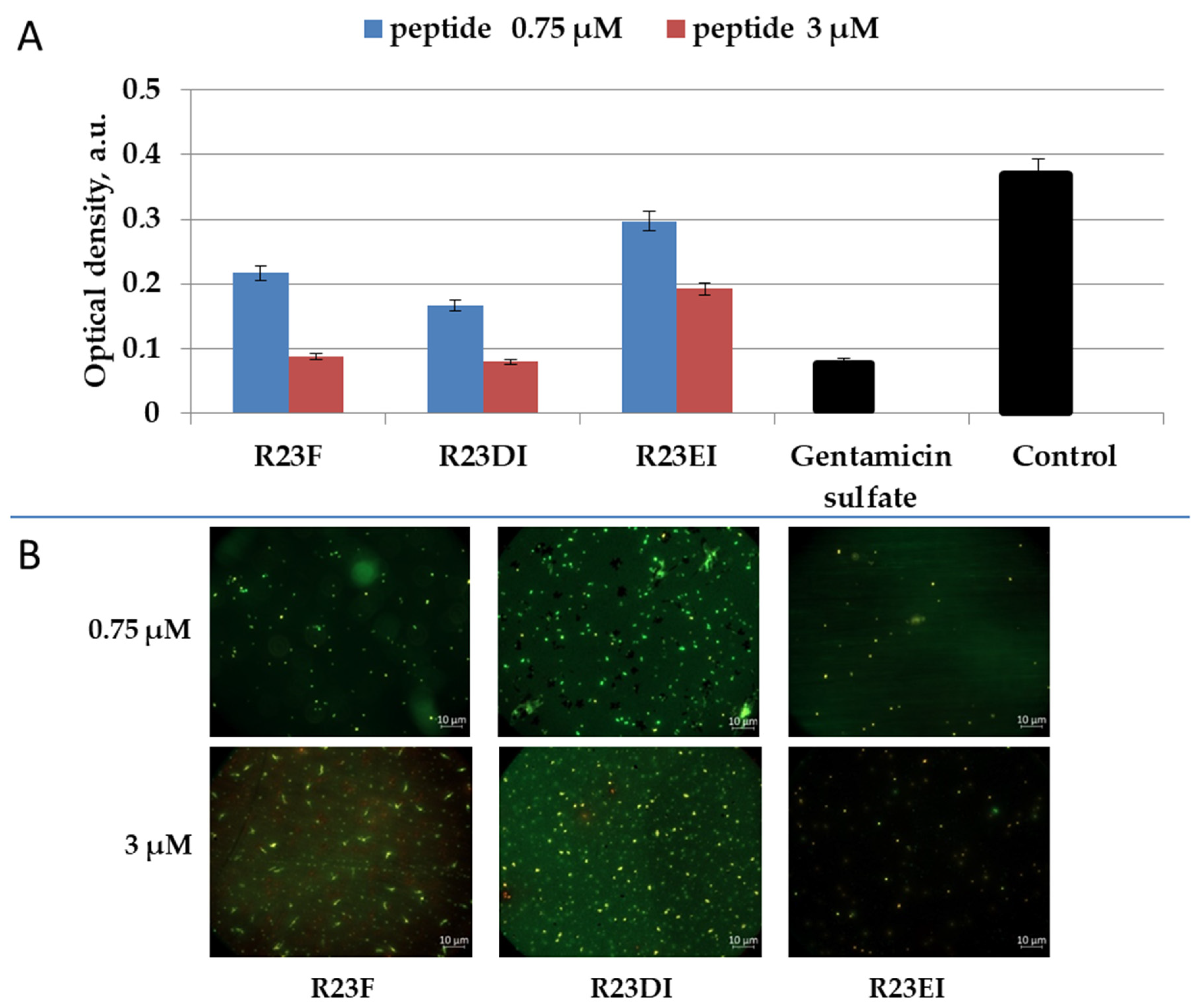
2.5. Viability of MRSA and S. aureus Cells after Peptide Treatment
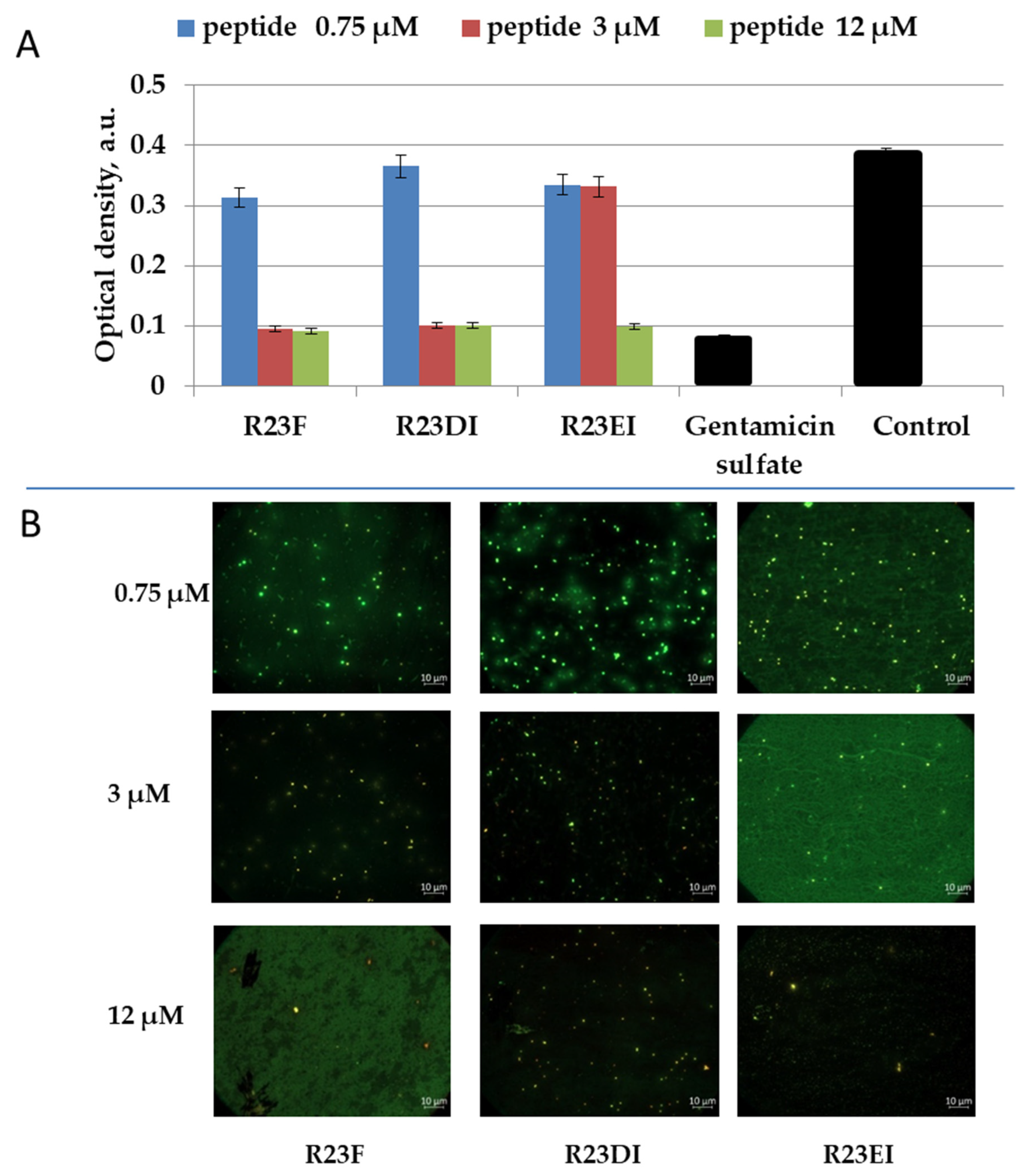
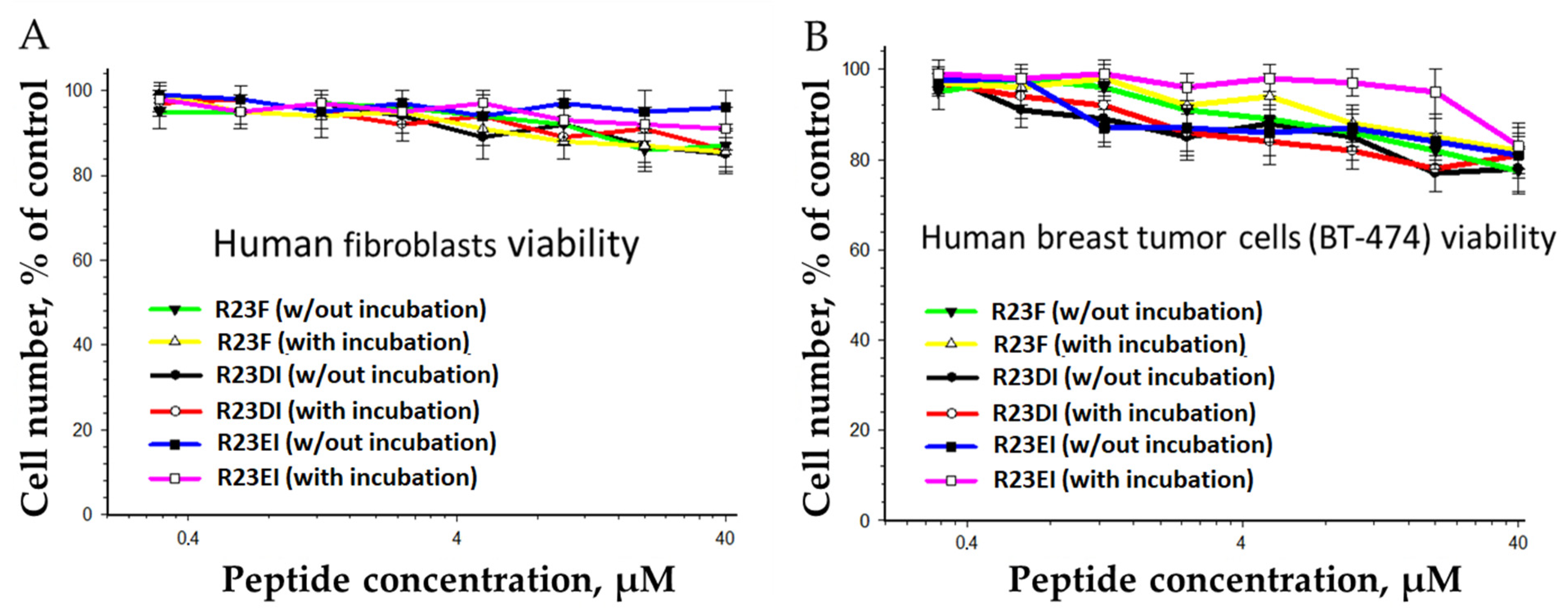
2.6. Toxicity of R23F, R23DI, and R23EI against Eukaryotic Cells
3. Discussions
4. Conclusions
5. Materials and Methods
5.1. Bioinformatics Analysis of Peptides
5.2. Synthesis and Characterization of Peptides
5.2.1. Peptide Synthesis
5.2.2. Prediction of Secondary Structures of Peptides by AlphaFold 2
5.3. Microorganisms
5.4. Determination of the Antibacterial Activity of Peptides by Agar Diffusion Assay
5.5. Measurement of the Antibacterial Activity of Peptides against MRSA, S. aureus, and P. aeruginosa in Liquid Medium
5.6. Assessment Bacterial Viability
5.7. Determination of Toxicity of Peptides against Eukaryotic Cells
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial peptide |
| CPP | Cell-penetrating peptide |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MIC | Minimum inhibitory concentration |
| PAD | Potential amyloidogenic determinant |
References
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453. [Google Scholar] [CrossRef]
- Wang, G.; Zietz, C.M.; Mudgapalli, A.; Wang, S.; Wang, Z. The evolution of the antimicrobial peptide database over 18 years: Milestones and new features. Protein Sci. 2021, 2, 4185. [Google Scholar] [CrossRef] [PubMed]
- Aronica, P.G.A.; Reid, L.M.; Desai, N.; Li, J.; Fox, S.J.; Yadahalli, S.; Essex, J.W.; Verma, C.S. Computational Methods and Tools in Antimicrobial Peptide Research. J. Chem. Inf. Model. 2021, 61, 3172–3196. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aristizábal, I.; Ocampo-Ibáñez, I.D. Antimicrobial Peptides with Antibacterial Activity against Vancomycin-Resistant Staphylococcus aureus Strains: Classification, Structures, and Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 7927. [Google Scholar] [CrossRef] [PubMed]
- Ciołek, L.; Biernat, M.; Jaegermann, Z.; Zaczyńska, E.; Czarny, A.; Jastrzębska, A.; Olszyna, A. The studies of cytotoxicity and antibacterial activity of composites with ZnO-doped bioglass. Int. J. Appl. Ceram. Technol. 2019, 16, 541–551. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Araújo, P.M. Antimicrobial Polymer−Based Assemblies: A Review. Int. J. Mol. Sci. 2021, 22, 5424. [Google Scholar] [CrossRef] [PubMed]
- Moridi, K.; Hemmaty, M.; Akbari Eidgahi, M.R.; Fathi Najafi, M.; Zare, H.; Ghazvini, K.; Neshani, A. Construction, cloning, and expression of Melittin antimicrobial peptide using Pichia pastoris expression system. Gene Rep. 2020, 21, 100900. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Kurpe, S.R.; Grishin, S.Y.; Surin, A.K.; Panfilov, A.V.; Slizen, M.V.; Chowdhury, S.D.; Galzitskaya, O.V. Antimicrobial and Amyloidogenic Activity of Peptides. Can Antimicrobial Peptides Be Used against SARS-CoV-2? Int. J. Mol. Sci. 2020, 21, 9552. [Google Scholar] [CrossRef] [PubMed]
- Venko, K.; Novič, M.; Stoka, V.; Žerovnik, E. Prediction of Transmembrane Regions, Cholesterol, and Ganglioside Binding Sites in Amyloid-Forming Proteins Indicate Potential for Amyloid Pore Formation. Front. Mol. Neurosci. 2021, 14, 9496. [Google Scholar] [CrossRef]
- Chiou, S.-J.; Ko, H.-J.; Hwang, C.-C.; Hong, Y.-R. The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 6330. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Raimondi, F.; Rajendran, L.; Temussi, P.A. Why does the Aβ peptide of Alzheimer share structural similarity with antimicrobial peptides? Commun. Biol. 2020, 3, 135. [Google Scholar] [CrossRef]
- Garbuzynskiy, S.O.; Lobanov, M.Y.; Galzitskaya, O.V. FoldAmyloid: A method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2010, 26, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Conchillo-Solé, O.; de Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinform. 2007, 8, 65. [Google Scholar] [CrossRef]
- Walsh, I.; Seno, F.; Tosatto, S.C.E.; Trovato, A. PASTA 2.0: An improved server for protein aggregation prediction. Nucleic Acids Res. 2014, 42, W301–W307. [Google Scholar] [CrossRef] [PubMed]
- Oliveberg, M. Waltz, an exciting new move in amyloid prediction. Nat. Methods 2010, 7, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Akter, Z.; Haque, A.; Hossain, M.S.; Ahmed, F.; Islam, M.A. Aggregation Prone Regions in Antibody Sequences Raised against Vibrio cholerae: A Bioinformatic Approach. Curr. Bioinform. 2021, 15, 988–1009. [Google Scholar] [CrossRef]
- Kalebina, T.S.; Plotnikova, T.A.; Gorkovskii, A.A.; Selyakh, I.O.; Galzitskaya, O.V.; Bezsonov, E.E.; Gellissen, G.; Kulaev, I.S. Amyloid-like properties of Saccharomyces cerevisiae cell wall glucantransferase Bgl2p. Prion 2008, 2, 91–96. [Google Scholar] [CrossRef]
- Louros, N.; Orlando, G.; De Vleeschouwer, M.; Rousseau, F.; Schymkowitz, J. Structure-based machine-guided mapping of amyloid sequence space reveals uncharted sequence clusters with higher solubilities. Nat. Commun. 2020, 11, 3314. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, J.W.; Kotulska, M. PATH—Prediction of Amyloidogenicity by Threading and Machine Learning. Sci. Rep. 2020, 10, 7721. [Google Scholar] [CrossRef]
- Szulc, N.; Burdukiewicz, M.; Gąsior-Głogowska, M.; Wojciechowski, J.W.; Chilimoniuk, J.; Mackiewicz, P.; Šneideris, T.; Smirnovas, V.; Kotulska, M. Bioinformatics methods for identification of amyloidogenic peptides show robustness to misannotated training data. Sci. Rep. 2021, 11, 8934. [Google Scholar] [CrossRef] [PubMed]
- Milošević, J.; Prodanović, R.; Polović, N. On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy. Molecules 2021, 26, 970. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.V.; Dovidchenko, N.V.; Galzitskaya, O.V. Anomalous Kinetics of Amyloidogenesis Suggest a Competition between Oligomers and Fibrils. Mol. Biol. 2018, 52, 62–68. [Google Scholar] [CrossRef]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.-J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The structure of the antimicrobial human cathelicidin LL-37 shows oligomerization and channel formation in the presence of membrane mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef] [PubMed]
- Kabelka, I.; Vácha, R. Advances in Molecular Understanding of α-Helical Membrane-Active Peptides. Acc. Chem. Res. 2021, 54, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Kurpe, S.; Grishin, S.; Surin, A.; Selivanova, O.; Fadeev, R.; Dzhus, U.; Gorbunova, E.; Mustaeva, L.; Azev, V.; Galzitskaya, O. Antimicrobial and Amyloidogenic Activity of Peptides Synthesized on the Basis of the Ribosomal S1 Protein from Thermus Thermophilus. Int. J. Mol. Sci. 2020, 21, 6382. [Google Scholar] [CrossRef]
- Mink, C.; Strandberg, E.; Wadhwani, P.; Melo, M.N.; Reichert, J.; Wacker, I.; Castanho, M.A.R.B.; Ulrich, A.S. Overlapping Properties of the Short Membrane-Active Peptide BP100 With (i) Polycationic TAT and (ii) α-helical Magainin Family Peptides. Front. Cell. Infect. Microbiol. 2021, 11, 9542. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Domnin, P.A.; Kravchenko, S.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Makarova, M.A.; Kurpe, S.R.; et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021, 22, 9776. [Google Scholar] [CrossRef]
- Mikhaylina, A.O.; Nikonova, E.Y.; Kostareva, O.S.; Tishchenko, S.V. Regulation of Ribosomal Protein Synthesis in Prokaryotes. Mol. Biol. 2021, 55, 16–36. [Google Scholar] [CrossRef]
- Machulin, A.V.; Deryusheva, E.I.; Selivanova, O.M.; Galzitskaya, O.V. The number of domains in the ribosomal protein S1 as a hallmark of the phylogenetic grouping of bacteria. PLoS ONE 2019, 14, e0221370. [Google Scholar] [CrossRef]
- Deryusheva, E.; Machulin, A.; Matyunin, M.; Galzitskaya, O. Sequence and evolutionary analysis of bacterial ribosomal S1 proteins. Proteins Struct. Funct. Bioinform. 2021, 89, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Deryusheva, E.I.; Machulin, A.V.; Matyunin, M.A.; Galzitskaya, O.V. Investigation of the Relationship between the S1 Domain and Its Molecular Functions Derived from Studies of the Tertiary Structure. Molecules 2019, 24, 3681. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.B.; Korostelev, A.A. Structural dynamics of protein S1 on the 70S ribosome visualized by ensemble cryo-EM. Methods 2018, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Galzitskaya, O.V. Exploring Amyloidogenicity of Peptides From Ribosomal S1 Protein to Develop Novel AMPs. Front. Mol. Biosci. 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wahiduzzaman; Prakash, A.; Tomar, A.K.; Srivastava, A.; Kundu, B.; Lynn, A.M.; Imtaiyaz Hassan, M. Exploring the aggregation-prone regions from structural domains of human TDP-43. Biochim. Biophys. Acta-Proteins Proteom. 2019, 1867, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Dzhus, U.F.; Selivanova, O.M.; Balobanov, V.A.; Surin, A.K.; Galzitskaya, O.V. Comparative Analysis of Aggregation of Thermus thermophilus Ribosomal Protein bS1 and Its Stable Fragment. Biochemistry 2020, 85, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Deryusheva, E.I.; Machulin, A.V.; Selivanova, O.M.; Glyakina, A.V.; Gorbunova, E.Y.; Mustaeva, L.G.; Azev, V.N.; Rekstina, V.V.; Kalebina, T.S.; et al. Amyloidogenic Propensities of Ribosomal S1 Proteins: Bioinformatics Screening and Experimental Checking. Int. J. Mol. Sci. 2020, 21, 5199. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, M.; Ahmed, M.; Lyngsø, J.; Vad, B.S.; Bøggild, A.; Fillipsen, A.; Pedersen, J.S.; Otzen, D.E.; Akbey, Ü. Predicted Loop Regions Promote Aggregation: A Study of Amyloidogenic Domains in the Functional Amyloid FapC. J. Mol. Biol. 2020, 432, 2232–2252. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Dzhus, U.F.; Glukhov, A.S.; Selivanova, O.M.; Surin, A.K.; Galzitskaya, O.V. Identification of Amyloidogenic Regions in Pseudomonas aeruginosa Ribosomal S1 Protein. Int. J. Mol. Sci. 2021, 22, 7291. [Google Scholar] [CrossRef] [PubMed]
- Kurpe, S.R.; Grishin, S.Y.; Glyakina, A.V.; Slizen, M.V.; Panfilov, A.V.; Kochetov, A.P.; Surin, A.K.; Kobyakova, M.I.; Fadeev, R.S.; Galzitskaya, O.V. Antibacterial effects of peptides synthesized based on the sequence of ribosome protein S1. Biomed. Khim. 2021, 67, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Dey, S.; Sil, P.; Paul, S.S.; Bhattacharyya, D.; Bhunia, A.; Sengupta, J.; Chattopadhyay, K. Conformational distortion in a fibril-forming oligomer arrests alpha-Synuclein fibrillation and minimizes its toxic effects. Commun. Biol. 2021, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Dovidchenko, N.V.; Glyakina, A.V.; Selivanova, O.M.; Grigorashvili, E.I.; Suvorina, M.Y.; Dzhus, U.F.; Mikhailina, A.O.; Shiliaev, N.G.; Marchenkov, V.V.; Surin, A.K.; et al. One of the possible mechanisms of amyloid fibrils formation based on the sizes of primary and secondary folding nuclei of Aβ40 and Aβ42. J. Struct. Biol. 2016, 194, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Zottig, X.; Sebastiao, M.; Arnold, A.A.; Marcotte, I.; Bourgault, S. Identification of transmissible proteotoxic oligomer-like fibrils that expand conformational diversity of amyloid assemblies. Commun. Biol. 2021, 4, 939. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, R. The Toxicity and Polymorphism of β-Amyloid Oligomers. Int. J. Mol. Sci. 2020, 21, 4477. [Google Scholar] [CrossRef] [PubMed]
- Galzitskaya, O.V. Oligomers Are Promising Targets for Drug Development in the Treatment of Proteinopathies. Front. Mol. Neurosci. 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Galzitskaya, O.V.; Nakamura, H.; Higo, J. Beta-Hairpins, alpa-helices, and the intermediates among the secondary structures in the energy landscape of a peptide from a distal beta-hairpin of SH3 domain. J. Comput. Chem. 2003, 24, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Higo, J. Free-energy landscape of a chameleon sequence in explicit water and its inherent α/β bifacial property. Protein Sci. 2009, 12, 2542–2548. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L. Artificial intelligence for microbial biotechnology: Beyond the hype. Microb. Biotechnol. 2021, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.M.; Pappu, R.V. AlphaFold and Implications for Intrinsically Disordered Proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef]
- Pinheiro, F.; Santos, J.; Ventura, S. AlphaFold and the amyloid landscape. J. Mol. Biol. 2021, 433, 167059. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Janda, J.M.; Bottone, E.J. Pseudomonas aeruginosa: Changes in antibiotic susceptibility, enzymatic activity, and antigenicity among colonial morphotypes. J. Clin. Microbiol. 1982, 15, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Bayer, A.S.; Weidenmaier, C.; Grau, T.; Wanner, S.; Stefani, S.; Cafiso, V.; Bertuccio, T.; Yeaman, M.R.; Nast, C.C.; et al. Phenotypic and Genotypic Characterization of Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus Strains: Relative Roles of mprF and dlt Operons. PLoS ONE 2014, 9, e107426. [Google Scholar] [CrossRef]
- Boyen, F.; Verstappen, K.M.H.W.; De Bock, M.; Duim, B.; Weese, J.S.; Schwarz, S.; Haesebrouck, F.; Wagenaar, J.A. In vitro antimicrobial activity of miconazole and polymyxin B against canine meticillin-resistant Staphylococcus aureus and meticillin-resistant Staphylococcus pseudintermedius isolates. Vet. Dermatol. 2012, 23, 381-e70. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; He, Y.; Liu, K.; Zhang, F.; Zhang, H.; Lu, Y.; Yang, C.; Wang, Z.; Fareed, M.S.; et al. An optimized analog of antimicrobial peptide Jelleine-1 shows enhanced antimicrobial activity against multidrug resistant P. aeruginosa and negligible toxicity in vitro and in vivo. Eur. J. Med. Chem. 2021, 219, 113433. [Google Scholar] [CrossRef]
- Tonk, M.; Valdés, J.J.; Cabezas-Cruz, A.; Vilcinskas, A. Potent Activity of Hybrid Arthropod Antimicrobial Peptides Linked by Glycine Spacers. Int. J. Mol. Sci. 2021, 22, 8919. [Google Scholar] [CrossRef]
- Soltaninejad, H.; Zare-Zardini, H.; Ordooei, M.; Ghelmani, Y.; Ghadiri-Anari, A.; Mojahedi, S.; Hamidieh, A.A. Antimicrobial Peptides from Amphibian Innate Immune System as Potent Antidiabetic Agents: A Literature Review and Bioinformatics Analysis. J. Diabetes Res. 2021, 2021, 2894722. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Wu, R.; Niekerk, L.-A.; Klein, A.; Keyster, M. Biomedical Relevance of Novel Anticancer Peptides in the Sensitive Treatment of Cancer. Biomolecules 2021, 11, 1120. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef]
- Manzo, G.; Ferguson, P.M.; Gustilo, V.B.; Hind, C.K.; Clifford, M.; Bui, T.T.; Drake, A.F.; Atkinson, R.A.; Sutton, J.M.; Batoni, G.; et al. Minor sequence modifications in temporin B cause drastic changes in antibacterial potency and selectivity by fundamentally altering membrane activity. Sci. Rep. 2019, 9, 1385. [Google Scholar] [CrossRef]
- Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides and Cell-Penetrating Peptides for Treating Intracellular Bacterial Infections. Front. Cell. Infect. Microbiol. 2021, 10, 2931. [Google Scholar] [CrossRef]
- Barani, G.; Kneib-Cordonier, N.; Mullen, D.G. Solid-phase peptide synthesis: A silver anniversary report. Int. J. Pept. Protein Res. 2009, 30, 705–739. [Google Scholar] [CrossRef] [PubMed]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Reid, G.E.; Simpson, R.J. Automated solid-phase peptide synthesis: Use of 2-(1H-benzotriazol-1-yl)-1,1,3,3,-tetramethyluronium tetrafluoroborate for coupling of ert- butyloxycarbonyl amino acids. Anal. Biochem. 1992, 200, 301–309. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Baba, T.; Sugiyama, H.; Seto, S. Rearrangement of α to β-Aspartyl Peptide with Anhydrous Hydrogen Fluoride. Chem. Pharm. Bull. 1973, 21, 207–209. [Google Scholar] [CrossRef][Green Version]
- Yajima, H.; Fujii, N. Studies on peptides. 103. Chemical synthesis of a crystalline protein with the full enzymic activity of ribonuclease A. J. Am. Chem. Soc. 1981, 103, 5867–5871. [Google Scholar] [CrossRef]
- Jubilut, G.N.; Cilli, E.M.; Tominaga, M.; Miranda, A.; Okada, Y.; Nakaie, C.R. Evaluation of the Trifluoromethanosulfonic Acid/Trifluoroacetic Acid/Thioanisole Cleavage Procedure for Application in Solid-Phase Peptide Synthesis. Chem. Pharm. Bull. 2001, 49, 1089–1092. [Google Scholar] [CrossRef]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the Protocol for the LIVE/DEAD® BacLightTM Bacterial Viability Kit for Rapid Determination of Bacterial Load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed]


| G1 Phase | S Phase | G2 Phase | M Phase | |
|---|---|---|---|---|
| Peptides + BT-474 | 73 ± 5% | 16 ± 3% | 7 ± 2% | 4 ± 1% |
| Control (BT-474) | 72 ± 5% | 20 ± 3% | 5 ± 1% | 3 ± 1% |
| Peptides + fibroblasts | 72 ± 4% | 17 ± 3% | 8 ± 2% | 3 ± 1% |
| Control (fibroblasts) | 78 ± 5% | 13 ± 2% | 7 ± 2% | 2 ± 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Panfilov, A.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Glyakina, A.V.; et al. Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 524. https://doi.org/10.3390/ijms23010524
Kravchenko SV, Domnin PA, Grishin SY, Panfilov AV, Azev VN, Mustaeva LG, Gorbunova EY, Kobyakova MI, Surin AK, Glyakina AV, et al. Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. International Journal of Molecular Sciences. 2022; 23(1):524. https://doi.org/10.3390/ijms23010524
Chicago/Turabian StyleKravchenko, Sergey V., Pavel A. Domnin, Sergei Y. Grishin, Alexander V. Panfilov, Viacheslav N. Azev, Leila G. Mustaeva, Elena Y. Gorbunova, Margarita I. Kobyakova, Alexey K. Surin, Anna V. Glyakina, and et al. 2022. "Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus" International Journal of Molecular Sciences 23, no. 1: 524. https://doi.org/10.3390/ijms23010524
APA StyleKravchenko, S. V., Domnin, P. A., Grishin, S. Y., Panfilov, A. V., Azev, V. N., Mustaeva, L. G., Gorbunova, E. Y., Kobyakova, M. I., Surin, A. K., Glyakina, A. V., Fadeev, R. S., Ermolaeva, S. A., & Galzitskaya, O. V. (2022). Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. International Journal of Molecular Sciences, 23(1), 524. https://doi.org/10.3390/ijms23010524








