The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment
Abstract
1. Introduction
2. The Role of Mitochondria in Tumorigenesis
2.1. Mitochondrial DNA Alterations in TC
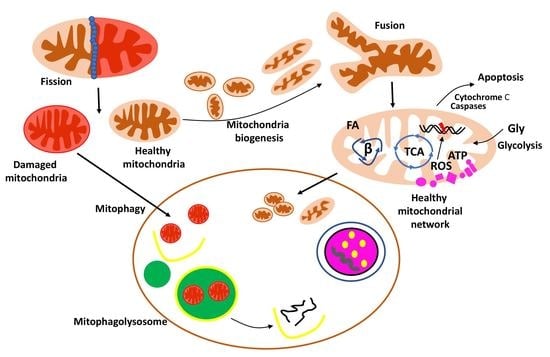
2.2. Mitochondrial Quality Control and Mitophagy in TC
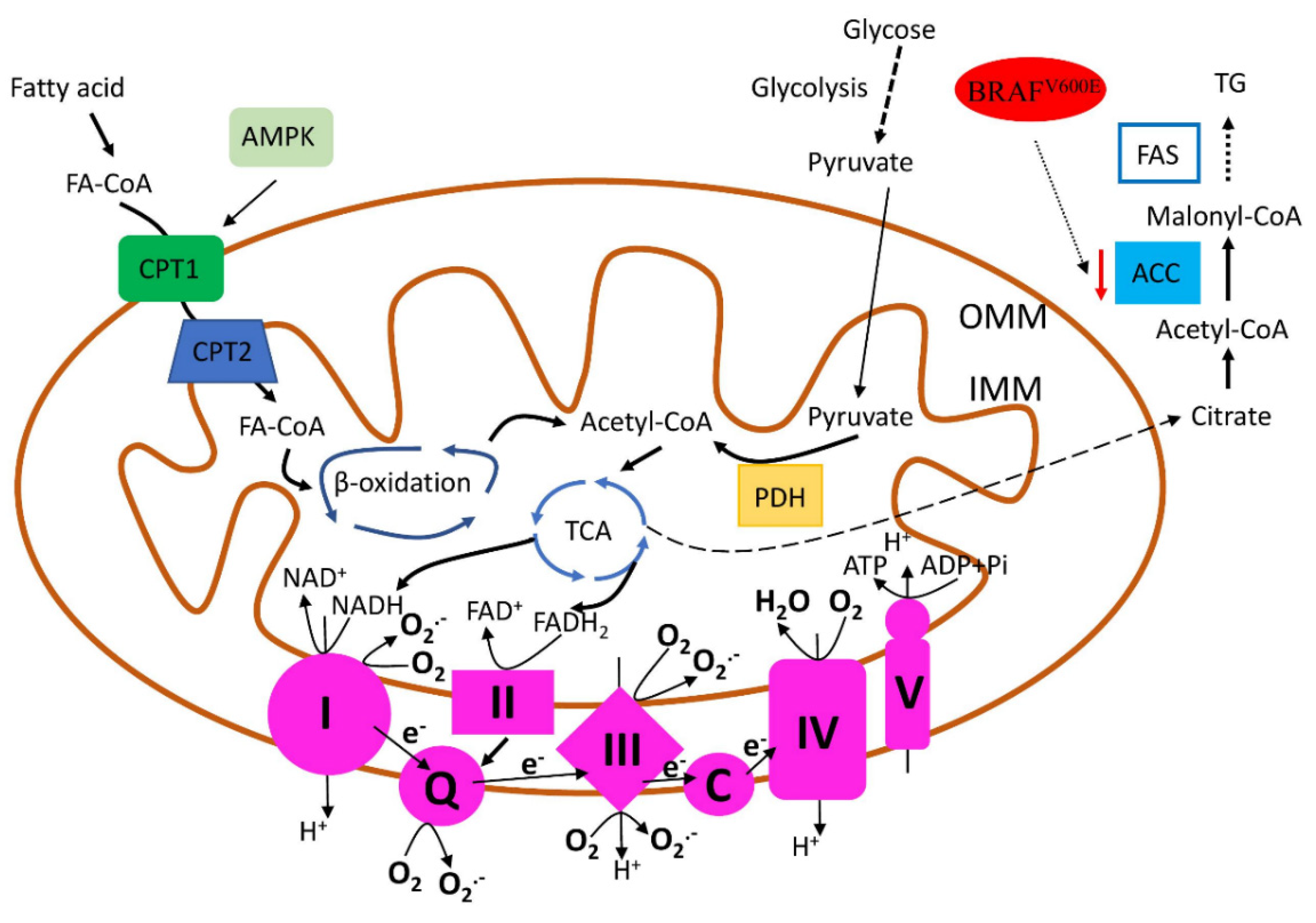
2.3. Mitochondrial Biogenesis and Metabolism in TC
2.4. Mitochondria-Dependent Apoptosis in TC
3. Thyroid Cancer Treatment
3.1. Mitochondrial Apoptosis Targeted Drugs
Natural Substances with Pro-Apoptotic Properties
3.2. Mitochondrial Biogenesis and Metabolism Targeted Drugs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ACC | acetyl-CoA carboxylase |
| ACC | adrenocortical carcinoma |
| AIF | apoptosis-inducing factor |
| ALK | anaplastic lymphoma kinase |
| AMPK | 5′AMP-Activated Protein Kinase |
| ATC | anaplastic thyroid carcinoma |
| BCL2L13 | BCL2 Like 13 |
| BNIP3 | BCL2 Interacting Protein 3 |
| BRAF | rapidly accelerated fibrosarcoma type-B |
| CCN2 | connective tissue growth factor |
| CDK2 | Cyclin-Dependent Kinase 2 |
| COX | cytochrome c oxidase |
| CPT1 | carnitine palmitoyltransferase |
| CV | classical variant |
| DRP1 | dynamin-related protein 1 |
| EIF1AX | Eukaryotic Translation Initiation Factor 1A Pseudogene 1 |
| FAO | fatty acid oxidation |
| FAS | fatty acid synthesis |
| FIS1 | mitochondrial fission 1 |
| FNMTC | familial non-medullary thyroid cancer |
| FTC | follicular thyroid carcinoma |
| FUNDC1 | FUN14 Domain Containing 1 |
| FV | follicular variant |
| GPD2 | mitochondrial glycerol-3- phosphate dehydrogenase |
| GRB7 | Growth Factor Receptor Bound Protein 7 |
| HIFs | hypoxia-inducible transcription factor |
| IFT88 | Intraflagellar Transport 88 |
| JNK-MIEF1 | c-Jun N-terminal kinases/Mitochondrial Elongation Factor 1 |
| KIF3A | Kinesin Family Member 3A |
| MAPK | mitogen-activated protein kinase |
| MFN2 | mitofusin 2 |
| Mst1 | Macrophage Stimulating 1 |
| Mst2 | Macrophage stimulating 2 |
| MTC | medullary thyroid carcinomas |
| mtDNA-CN | mtDNA copy number |
| MYO1F | Myosin IF |
| NIS | Sodium/Iodide Symporter, or SLC5A5, Solute Carrier Family 5 Member 5 |
| NIX | NIP-3-Like Protein X |
| NO | nitric oxide |
| NOXs | NADPH Oxidase |
| NTRK | neurotrophic receptor tyrosine kinase |
| OPA1 | optic atrophy 1 |
| Parkin | Parkin E3 Ubiquitin Protein Ligase |
| PAX8 | Paired Box Gene 8 |
| PDTC | poorly differentiated thyroid carcinoma |
| PGC-1α | PPARG Coactivator 1 Alpha |
| PI3K | Phosphoinositide 3-Kinase Alpha |
| PINK1 | PTEN Induced Kinase 1 |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PTC | papillary thyroid carcinoma |
| RAS | rat sarcoma |
| RET | rearranged during transfection |
| RPS6KB1 | Ribosomal protein S6 kinase beta-1 |
| STAT3 | Signal Transducer And Activator Of Transcription 3 |
| TC | thyroid cancer |
| TCV | tall cell variant |
| TERT | Telomerase Reverse Transcriptase |
| TKI | tyrosine kinase inhibitors |
| TP53 | Tumour Protein P53 |
| TRPV1 | transient receptor potential vanilloid type 1 |
| TSH | thyroid-stimulating hormone |
| VDAC1 | Voltage-Dependent Anion Channel 1 |
| WDTC | well-differentiated thyroid carcinomas |
| Yap | Yes-Associated Protein 1 |
References
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid Cancer Incidence Trends by Histology in 25 Countries: A Population-Based Study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Nelkin, B. Recent Advances in the Biology and Therapy of Medullary Thyroid Carcinoma. F1000Research 2017, 6, 2184. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.J.; Grubbs, E.G.; Hofmann, M.-C. Thyroid C-Cell Biology and Oncogenic Transformation. In Medullary Thyroid Carcinoma; Raue, F., Ed.; Recent Results in Cancer Research; Springer: Cham, Switzerland, 2015; Volume 204, pp. 1–39. ISBN 978-3-319-22541-8. [Google Scholar]
- Khosravi, M.H.; Kouhi, A.; Saeedi, M.; Bagherihagh, A.; Amirzade-Iranaq, M.H. Thyroid Cancers: Considerations, Classifications, and Managements. In Diagnosis and Management of Head and Neck Cancer; Akarslan, Z., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3495-4. [Google Scholar]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Zaballos, M.A.; Santisteban, P. Key Signaling Pathways in Thyroid Cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, G.; Visani, M.; Repaci, A.; Rhoden, K.J.; de Biase, D.; Pession, A.; Giovanni, T. Molecular Pathology of Thyroid Tumours of Follicular Cells: A Review of Genetic Alterations and Their Clinicopathological Relevance. Histopathology 2018, 72, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.; Shin, D.W.; Park, Y.; Lim, H.; Yun, J.M.; Song, S.O.; Park, J.H.; Cho, B.; Guallar, E. Increased Cardiovascular Risk in Thyroid Cancer Patients Taking Levothyroxine: A Nationwide Cohort Study in Korea. Eur. J. Endocrinol. 2019, 180, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.K. Vemurafenib. Recent Results Cancer Res. 2018, 211, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Galdiero, M.R.; Varricchi, G.; Elia, G.; Ragusa, F.; Paparo, S.R.; Benvenga, S.; Antonelli, A. Molecular Targets of Tyrosine Kinase Inhibitors in Thyroid Cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel Treatments for Anaplastic Thyroid Carcinoma. Gland. Surg. 2020, 9, S28–S42. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Song, Y.S.; Park, Y.J.; Seo, J.-S. Recent Improvements in Genomic and Transcriptomic Understanding of Anaplastic and Poorly Differentiated Thyroid Cancers. Endocrinol. Metab. 2020, 35, 44. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Foo, B.J.-A.; Eu, J.Q.; Hirpara, J.L.; Pervaiz, S. Interplay between Mitochondrial Metabolism and Cellular Redox State Dictates Cancer Cell Survival. Oxid. Med. Cell Longev. 2021, 2021, 1341604. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Di Minno, A.; Songia, P.; Massaiu, I.; Alfieri, V.; Valerio, V.; Moschetta, D.; Andreini, D.; Alamanni, F.; Pepi, M.; et al. Sex-Specific Differences in Age-Related Aortic Valve Calcium Load: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2020, 61, 101077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Jia, X.; Hu, X.; Pang, P.; Zhao, S.; Wang, Y.; Wang, J.; Zhang, Y.; Lyu, Z. The Coexistence of Genetic Mutations in Thyroid Carcinoma Predicts Histopathological Factors Associated With a Poor Prognosis: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2020, 10, 540238. [Google Scholar] [CrossRef] [PubMed]
- Younis, E. Oncogenesis of Thyroid Cancer. APJCP 2017, 18, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.K.; Kübler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270. [Google Scholar] [CrossRef]
- Pereira, L.; Soares, P.; Máximo, V.; Samuels, D.C. Somatic Mitochondrial DNA Mutations in Cancer Escape Purifying Selection and High Pathogenicity Mutations Lead to the Oncocytic Phenotype: Pathogenicity Analysis of Reported Somatic MtDNA Mutations in Tumors. BMC Cancer 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, W.; Ruan, G.; Liang, M.; Zheng, J.; Chen, Y.; Wu, H.; Fahey, T.; Guan, M.; Teng, L. A Comprehensive Characterization of Mitochondrial Genome in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2016, 17, 1594. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, Q.; Song, S.; Li, L.; Zhou, H.; Li, M.; Jiang, Z.; Zhou, C.; Chen, G.; Lyu, J.; et al. Oncocytic Tumors Are Marked by Enhanced Mitochondrial Content and MtDNA Mutations of Complex I in Chinese Patients. Mitochondrion 2019, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsybrovskyy, O.; De Luise, M.; Biase, D.; Caporali, L.; Fiorini, C.; Gasparre, G.; Carelli, V.; Hackl, D.; Imamovic, L.; Haim, S.; et al. Papillary Thyroid Carcinoma Tall Cell Variant Shares Accumulation of Mitochondria, Mitochondrial DNA Mutations, and Loss of Oxidative Phosphorylation Complex I Integrity with Oncocytic Tumors. J. Pathol. Clin. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Wang, O.; Xing, X.; Cui, M.; Wang, M.; Song, C.; Liao, Q. Spectrum of Mitochondrial Genomic Variation in Parathyroid Neoplasms. Endocrine 2021, 74, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cui, N.; Zhang, S.; Wang, X.; Ming, L. Leukocyte Mitochondrial DNA Copy Number and Risk of Thyroid Cancer: A Two-Stage Case-Control Study. Front. Endocrinol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Perdas, E.; Stawski, R.; Kaczka, K.; Nowak, D.; Zubrzycka, M. Altered Levels of Circulating Nuclear and Mitochondrial DNA in Patients with Papillary Thyroid Cancer. Sci. Rep. 2019, 9, 14438. [Google Scholar] [CrossRef]
- Cocoş, R.; Schipor, S.; Badiu, C.; Raicu, F. Mitochondrial DNA Haplogroup K as a Contributor to Protection against Thyroid Cancer in a Population from Southeast Europe. Mitochondrion 2018, 39, 43–50. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Ryu, S.W.; Lin, J.; Buentello, G.; Tibshirani, R.; Suliburk, J.; Eberlin, L.S. Cardiolipins Are Biomarkers of Mitochondria-Rich Thyroid Oncocytic Tumors. Cancer Res. 2016, 76, 6588–6597. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, B.; Pereira, J.B.; Correia, M.; Fernandes, V.; Eloy, C.; Sobrinho-Simões, M.; Soares, P.; Samuels, D.C.; Máximo, V.; Pereira, L. Genomic and Transcriptomic Characterization of the Mitochondrial-Rich Oncocytic Phenotype on a Thyroid Carcinoma Background. Mitochondrion 2019, 46, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-da-Silva, A.; Valacca, C.; Rios, E.; Pópulo, H.; Soares, P.; Sobrinho-Simões, M.; Scorrano, L.; Máximo, V.; Campello, S. Mitochondrial Dynamics Protein Drp1 Is Overexpressed in Oncocytic Thyroid Tumors and Regulates Cancer Cell Migration. PLoS ONE 2015, 10, e0122308. [Google Scholar] [CrossRef]
- Nakamura, Y.; Arakawa, H. Discovery of Mieap-Regulated Mitochondrial Quality Control as a New Function of Tumor Suppressor P53. Cancer Sci. 2017, 108, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Mussazhanova, Z.; Shimamura, M.; Kurashige, T.; Ito, M.; Nakashima, M.; Nagayama, Y. Causative Role for Defective Expression of Mitochondria-Eating Protein in Accumulation of Mitochondria in Thyroid Oncocytic Cell Tumors. Cancer Sci. 2020, 111, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Dumont, S.; Le Pennec, S.; Donnart, A.; Teusan, R.; Steenman, M.; Chevalier, C.; Houlgatte, R.; Savagner, F. Transcriptional Orchestration of Mitochondrial Homeostasis in a Cellular Model of PGC-1-Related Coactivator-Dependent Thyroid Tumor. Oncotarget 2018, 9, 15883–15894. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.; Neureiter, D.; Sperl, W.; Mayr, J.; Kofler, B. Alterations of Oxidative Phosphorylation Complexes in Papillary Thyroid Carcinoma. Cells 2018, 7, 40. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Martín, M. Long-Tailed Unconventional Class I Myosins in Health and Disease. Int. J. Mol. Sci. 2020, 21, 2555. [Google Scholar] [CrossRef] [PubMed]
- Diquigiovanni, C.; Bergamini, C.; Evangelisti, C.; Isidori, F.; Vettori, A.; Tiso, N.; Argenton, F.; Costanzini, A.; Iommarini, L.; Anbunathan, H.; et al. Mutant MYO1F Alters the Mitochondrial Network and Induces Tumor Proliferation in Thyroid Cancer. Int. J. Cancer 2018, 143, 1706–1719. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Chandel, N.S.; Hay, N. AMPK Regulates NADPH Homeostasis to Promote Tumour Cell Survival during Energy Stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D. Carnitine Palmitoyltransferase 1C: From Cognition to Cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, Y.; Su, D.; Gong, B.; He, X.; Zhou, X.; Pang, Z.; Cheng, L.; Chen, Y.; Yao, Z. Cpt1c Regulated by AMPK Promotes Papillary Thyroid Carcinomas Cells Survival under Metabolic Stress Conditions. J. Cancer 2017, 8, 3675–3681. [Google Scholar] [CrossRef]
- Valvo, V.; Iesato, A.; Kavanagh, T.R.; Priolo, C.; Zsengeller, Z.; Pontecorvi, A.; Stillman, I.E.; Burke, S.D.; Liu, X.; Nucera, C. Fine-Tuning Lipid Metabolism by Targeting Mitochondria-Associated Acetyl-CoA-Carboxylase 2 in BRAFV600E Papillary Thyroid Carcinoma. Thyroid 2021, 31, 1335–1358. [Google Scholar] [CrossRef]
- Hunkeler, M.; Hagmann, A.; Stuttfeld, E.; Chami, M.; Guri, Y.; Stahlberg, H.; Maier, T. Structural Basis for Regulation of Human Acetyl-CoA Carboxylase. Nature 2018, 558, 470–474. [Google Scholar] [CrossRef]
- Porter, A.; Wong, D.J. Perspectives on the Treatment of Advanced Thyroid Cancer: Approved Therapies, Resistance Mechanisms, and Future Directions. Front. Oncol. 2020, 10, 592202. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-El-Hassani, R.; Schlumberger, M.; Dupuy, C. NADPH Oxidases: New Actors in Thyroid Cancer? Nat. Rev. Endocrinol. 2016, 12, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Zana, M.; Péterfi, Z.; Kovács, H.A.; Tóth, Z.E.; Enyedi, B.; Morel, F.; Paclet, M.-H.; Donkó, Á.; Morand, S.; Leto, T.L.; et al. Interaction between P22phox and Nox4 in the Endoplasmic Reticulum Suggests a Unique Mechanism of NADPH Oxidase Complex Formation. Free Radic. Biol. Med. 2018, 116, 41–49. [Google Scholar] [CrossRef]
- Tang, P.; Dang, H.; Huang, J.; Xu, T.; Yuan, P.; Hu, J.; Sheng, J. NADPH Oxidase NOX4 Is a Glycolytic Regulator through MROS-HIF1α Axis in Thyroid Carcinomas. Sci. Rep. 2018, 8, 15897. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo Reguera, D.; Čunátová, K.; Vrbacký, M.; Pecinová, A.; Houštěk, J.; Mráček, T.; Pecina, P. Cytochrome c Oxidase Subunit 4 Isoform Exchange Results in Modulation of Oxygen Affinity. Cells 2020, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; Reynolds, S.; Mendonca-Torres, M.; Thakur, S.; Klubo-Gwiezdzinska, J.; Ylli, D.; Wartofsky, L.; et al. Cytochrome C Oxidase Subunit 4 (COX4): A Potential Therapeutic Target for the Treatment of Medullary Thyroid Cancer. Cancers 2020, 12, 2548. [Google Scholar] [CrossRef]
- Wanderoy, S.; Hees, J.T.; Klesse, R.; Edlich, F.; Harbauer, A.B. Kill One or Kill the Many: Interplay between Mitophagy and Apoptosis. Biol. Chem. 2020, 402, 73–88. [Google Scholar] [CrossRef]
- Scott, A.J.; Walker, S.A.; Krank, J.J.; Wilkinson, A.S.; Johnson, K.M.; Lewis, E.M.; Wilkinson, J.C. AIF Promotes a JNK1-Mediated Cadherin Switch Independently of Respiratory Chain Stabilization. J. Biol. Chem. 2018, 293, 14707–14722. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.-L. Targeting the Hippo Pathway in Cancer, Fibrosis, Wound Healing and Regenerative Medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Cui, Y.; Liu, S.; Sun, H. Mst1 Overexpression Combined with Yap Knockdown Augments Thyroid Carcinoma Apoptosis via Promoting MIEF1-Related Mitochondrial Fission and Activating the JNK Pathway. Cancer Cell Int. 2019, 19, 143. [Google Scholar] [CrossRef]
- Meng, C.; Tian, G.; Xu, C.; Li, X.; Zhang, Y.; Wang, Y.; Qin, J.; Fok, E.K.L.; Hinton, B.T.; Mak, K.K.; et al. Hippo Kinases MST1 and MST2 Control the Differentiation of the Epididymal Initial Segment via the MEK-ERK Pathway. Cell Death Differ. 2020, 27, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Sun, X.; Wang, P.; Zhang, S.; Wang, X.; Wu, H.; Hong, L.; Xie, C.; Li, X.; Zhao, H.; et al. Kinases Mst1 and Mst2 Positively Regulate Phagocytic Induction of Reactive Oxygen Species and Bactericidal Activity. Nat. Immunol. 2015, 16, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, X.; Han, L.; Di, X. Mst2 Overexpression Inhibits Thyroid Carcinoma Growth and Metastasis by Disrupting Mitochondrial Fitness and Endoplasmic Reticulum Homeostasis. J. Oncol. 2021, 2021, 1262291. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.K.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef]
- Chin, M.T.; Conway, S.J. Role of Tafazzin in Mitochondrial Function, Development and Disease. J. Dev. Biol. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, J.; Gao, K.; Zhang, P.; Yao, L.; Tang, Y.; Tang, L.; Ma, J.; Xiao, J.; Zhang, E.; et al. Dysregulation of INF2-Mediated Mitochondrial Fission in SPOP-Mutated Prostate Cancer. PLoS Genet. 2017, 13, e1006748. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; An, D.; Yuan, H.; Li, Z.; Song, Y.; Liu, Z. Suppression of Tafazzin Promotes Thyroid Cancer Apoptosis via Activating the JNK Signaling Pathway and Enhancing INF2-mediated Mitochondrial Fission. J. Cell Physiol. 2019, 234, 16238–16251. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, P.; Yang, X.; Peng, S.; Hu, X.; Bao, G. Growth Factor Receptor Bound Protein-7 Regulates Proliferation, Cell Cycle, and Mitochondrial Apoptosis of Thyroid Cancer Cells via MAPK/ERK Signaling. Mol. Cell Biochem. 2020, 472, 209–218. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tai, Y.-L.; Shen, T.-L. Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells 2019, 8, 435. [Google Scholar] [CrossRef]
- Lee, J.; Yi, S.; Chang, J.Y.; Kim, J.T.; Sul, H.J.; Park, K.C.; Zhu, X.; Cheng, S.-Y.; Kero, J.; Kim, J.; et al. Loss of Primary Cilia Results in the Development of Cancer in the Murine Thyroid Gland. Mol. Cells 2019, 42, 113–122. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.C.; Sul, H.J.; Hong, H.J.; Kim, K.-H.; Kero, J.; Shong, M. Loss of Primary Cilia Promotes Mitochondria-Dependent Apoptosis in Thyroid Cancer. Sci. Rep. 2021, 11, 4181. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.; Kim, Y.H.; Suh, S.; Goh, T.S.; Jeong, D.C.; Kim, S.J.; Kim, I.J.; Han, M.-E.; Oh, S.-O. Development of a Risk Scoring System for Patients with Papillary Thyroid Cancer. J. Cell Mol. Med. 2019, 23, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Kaae, A.C.; Kreissl, M.C.; Krüger, M.; Infanger, M.; Grimm, D.; Wehland, M. Kinase-Inhibitors in Iodine-Refractory Differentiated Thyroid Cancer-Focus on Occurrence, Mechanisms, and Management of Treatment-Related Hypertension. Int. J. Mol. Sci. 2021, 22, 12217. [Google Scholar] [CrossRef]
- Starenki, D.; Hong, S.-K.; Wu, P.-K.; Park, J.-I. Vandetanib and Cabozantinib Potentiate Mitochondria-Targeted Agents to Suppress Medullary Thyroid Carcinoma Cells. Cancer Biol. Ther. 2017, 18, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Bikas, A.; Patel, A.; Kushchayeva, Y.; Costello, J.; McDaniel, D.; Burman, K.; Vasko, V. Nelfinavir Inhibits Proliferation and Induces DNA Damage in Thyroid Cancer Cells. Endocr. Relat. Cancer 2017, 24, 147–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.; Cheng, S.-Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 4030–4043. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Torino, F.; Papi, G.; Locantore, P.; Pontecorvi, A.; Corsello, S.M. Role of Mitotane in Adrenocortical Carcinoma—Review and State of the Art. Eur. Endocrinol. 2018, 14, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; Kaltsas, G.; Hoperia, V.; Wartofsky, L.; Burman, K.; Vasko, V. Mitotane Induces Mitochondrial Membrane Depolarization and Apoptosis in Thyroid Cancer Cells. Int. J. Oncol. 2019, 55, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.J.; Löbenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide Repositioning for Treating Cancer: Challenges and Nano-Based Drug Delivery Opportunities. Eur. J. Pharm. Biopharm. 2019, 141, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, T.; Li, Y.; Wang, C.; Wang, X.; Zhang, M.; Xie, Y.; Li, S.; An, Z.; Ye, T. Niclosamide Induces Apoptosis through Mitochondrial Intrinsic Pathway and Inhibits Migration and Invasion in Human Thyroid Cancer in Vitro. Biomed. Pharmacother. 2017, 92, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-K.; Hong, S.-K.; Chen, W.; Becker, A.E.; Gundry, R.L.; Lin, C.-W.; Shao, H.; Gestwicki, J.E.; Park, J.-I. Mortalin (HSPA9) Facilitates BRAF-Mutant Tumor Cell Survival by Suppressing ANT3-Mediated Mitochondrial Membrane Permeability. Sci. Signal. 2020, 13, eaay1478. [Google Scholar] [CrossRef] [PubMed]
- Starenki, D.; Sosonkina, N.; Hong, S.-K.; Lloyd, R.V.; Park, J.-I. Mortalin (GRP75/HSPA9) Promotes Survival and Proliferation of Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2069. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Kotamraju, S.; Karunakaran, C.; Kalivendi, S.V.; Thomas, S.; Joseph, J.; Kalyanaraman, B. Mitochondria Superoxide Dismutase Mimetic Inhibits Peroxide-Induced Oxidative Damage and Apoptosis: Role of Mitochondrial Superoxide. Free Radic. Biol. Med. 2005, 39, 567–583. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of Capsaicin as a Potential New Therapeutic Drug in Human Cancers. J. Clin. Pharm. 2020, 45, 16–28. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Capsaicin Inhibits the Metastasis of Human Papillary Thyroid Carcinoma BCPAP Cells through the Modulation of the TRPV1 Channel. Food Funct. 2018, 9, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiang, G.-H.; Tang, T.; Tang, Y.; Zhao, L.-Y.; Liu, D.; Zhang, Y.-R.; Tang, J.-T.; Zhou, S.; Wu, D.-H. Capsaicin and Dihydrocapsaicin Induce Apoptosis in Human Glioma Cells via ROS and Ca2+-mediated Mitochondrial Pathway. Mol. Med. Rep. 2016, 14, 4198–4208. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin Induces Mitochondrial Dysfunction and Apoptosis in Anaplastic Thyroid Carcinoma Cells via TRPV1-Mediated Mitochondrial Calcium Overload. Cell. Signal. 2020, 75, 109733. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine-A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Sharvan, R.; Gao, J.; Qu, S. Berberine Could Inhibit Thyroid Carcinoma Cells by Inducing Mitochondrial Apoptosis, G0/G1 Cell Cycle Arrest and Suppressing Migration via PI3K-AKT and MAPK Signaling Pathways. Biomed. Pharmacother. 2017, 95, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharm. 2021, 134, 111017. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Jung, I.; Kim, M.E.; Bae, S.K.; Lee, J.S. Anti-Cancer Activity of Myricetin against Human Papillary Thyroid Cancer Cells Involves Mitochondrial Dysfunction–Mediated Apoptosis. Biomed. Pharmacother. 2017, 91, 378–384. [Google Scholar] [CrossRef]
- Jo, S.; Ha, T.K.; Han, S.-H.; Kim, M.E.; Jung, I.; Lee, H.-W.; Bae, S.K.; Lee, J.S. Myricetin Induces Apoptosis of Human Anaplastic Thyroid Cancer Cells via Mitochondria Dysfunction. Anticancer Res. 2017, 37, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells. Int. J. Mol. Sci. 2020, 21, 4177. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Song, M.; Zhou, M.; Hu, Y. Antibiotic Tigecycline Enhances Cisplatin Activity against Human Hepatocellular Carcinoma through Inducing Mitochondrial Dysfunction and Oxidative Damage. Biochem. Biophys. Res. Commun. 2017, 483, 17–23. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, W.; Huang, Q.; Wang, J.; Wang, Y.; Li, H.; Fu, X. Tigecycline as a Dual Inhibitor of Retinoblastoma and Angiogenesis via Inducing Mitochondrial Dysfunctions and Oxidative Damage. Sci. Rep. 2018, 8, 11747. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, F.; Chen, D.; Wang, L. Inhibition of Mitochondrial Respiration by Tigecycline Selectively Targets Thyroid Carcinoma and Increases Chemosensitivity. Clin. Exp. Pharm. Physiol. 2019, 46, 890–897. [Google Scholar] [CrossRef]
- Fu, C.; Xiao, X.; Xu, H.; Lu, W.; Wang, Y. Efficacy of Atovaquone on EpCAM+CD44+ HCT-116 Human Colon Cancer Stem Cells under Hypoxia. Exp. Med. 2020, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting Tumor Hypoxia and Mitochondrial Metabolism with Anti-Parasitic Drugs to Improve Radiation Response in High-Grade Gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 208. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yan, X.; Lu, L.; Su, C.; He, Y. Atovaquone Enhances Doxorubicin’s Efficacy via Inhibiting Mitochondrial Respiration and STAT3 in Aggressive Thyroid Cancer. J. Bioenerg. Biomembr. 2018, 50, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fei, H. Antimalarial Drug Artesunate Is Effective against Chemoresistant Anaplastic Thyroid Carcinoma via Targeting Mitochondrial Metabolism. J. Bioenerg. Biomembr. 2020, 52, 123–130. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Nikiforov, N.G.; Zhuravlev, A.D.; Orekhov, N.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 460. https://doi.org/10.3390/ijms23010460
Dabravolski SA, Nikiforov NG, Zhuravlev AD, Orekhov NA, Mikhaleva LM, Orekhov AN. The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. International Journal of Molecular Sciences. 2022; 23(1):460. https://doi.org/10.3390/ijms23010460
Chicago/Turabian StyleDabravolski, Siarhei A., Nikita G. Nikiforov, Alexander D. Zhuravlev, Nikolay A. Orekhov, Liudmila M. Mikhaleva, and Alexander N. Orekhov. 2022. "The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment" International Journal of Molecular Sciences 23, no. 1: 460. https://doi.org/10.3390/ijms23010460
APA StyleDabravolski, S. A., Nikiforov, N. G., Zhuravlev, A. D., Orekhov, N. A., Mikhaleva, L. M., & Orekhov, A. N. (2022). The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. International Journal of Molecular Sciences, 23(1), 460. https://doi.org/10.3390/ijms23010460