The Combination of Bacillus Thuringiensis and Its Engineered Strain Expressing dsRNA Increases the Toxicity against Plutella Xylostella
Abstract
:1. Introduction
2. Results
2.1. Engineered Bt Expressing dsRNA of PxAK
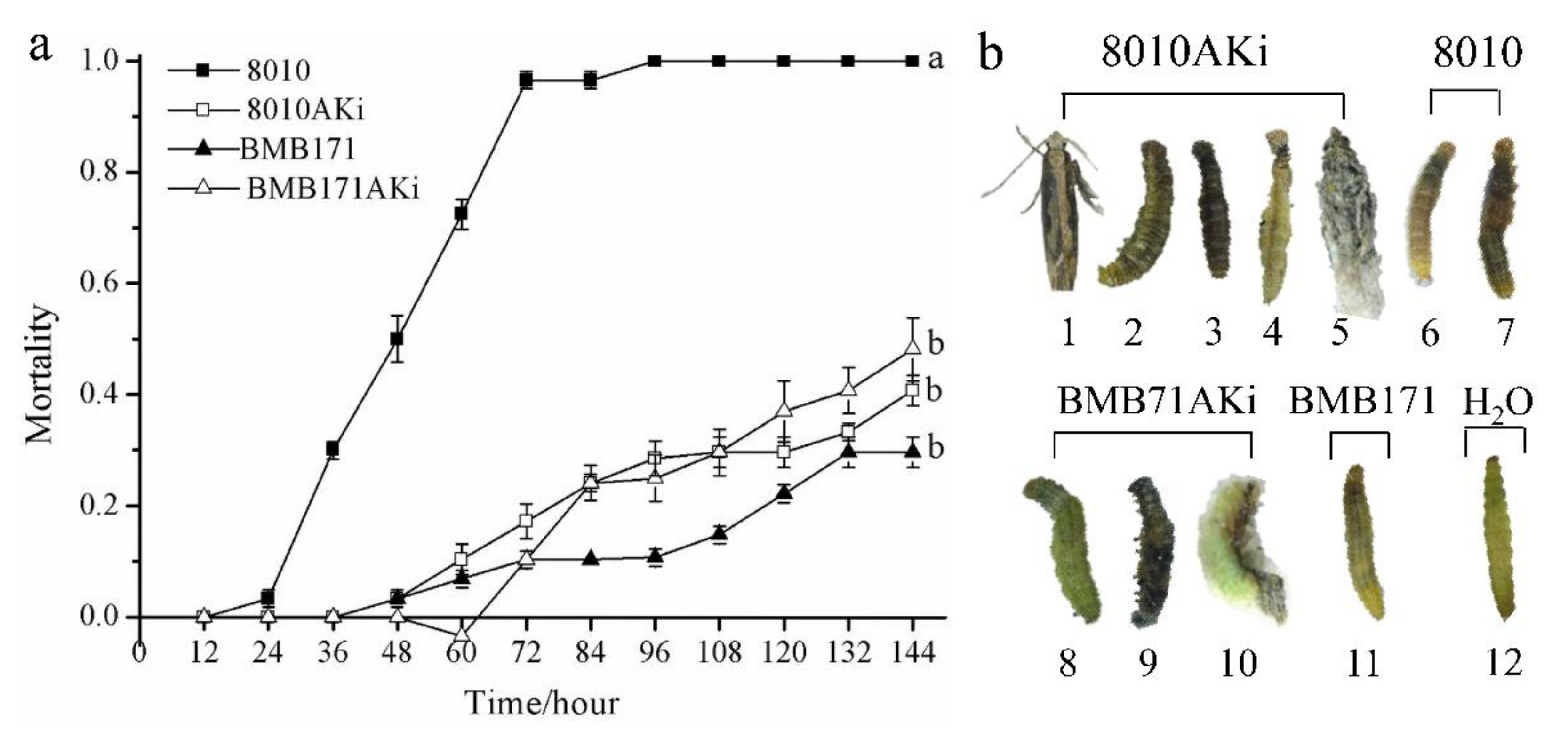
2.2. Toxicity of Engineered Bt against P. xylostella
2.3. Toxicity of the Mixtures of Bt 8010 and Engineered Bt against P. Xylostella
2.4. Growth and Development Characteristics of P. xylostella Feeding on the Mixtures of Bt and Engineered Bt
2.5. Effects of the Mixtures of Bt and Engineered Bt on P. xylostella Population Dynamics Parameters
3. Discussion
4. Materials and Methods
4.1. Insects and Bacteria
4.2. Construction of RNAi vector of PxAK
4.3. Construction of Engineered Bt
4.4. Feeding Bioassay
4.5. Quantification of PxAK Gene Expression
4.6. Life Table Analysis of Laboratory Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, D.; Gatehouse, J. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Burand, J.; Hunter, W. RNAi: Future in insect management. J. Invertebr. Pathol. 2013, 112 (Suppl. S1), S68–S74. [Google Scholar] [CrossRef]
- Miguel, K.; Scott, J. The next generation of insecticides: dsRNA is stable as a foliar applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef]
- Gordon, K.; Waterhouse, P. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.; Hasse, C.; Ruf, S.; Heckel, D.; Bock, R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef]
- Baum, J.; Bogaert, T.; Clinton, W.; Heck, G.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Cai, W.; Wang, J.; Hong, G.; Tao, X.; Wang, L.; Huang, Y.; Chen, X. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Zhao, Y.; Li, Y.; Liu, Y.; Sun, J. Silencing the HaAK gene by transgenic plant-mediated RNAi impairs larval growth of Helicoverpa armigera. Int. J. Biol. Sci. 2015, 11, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Liu, Z.X.; Chen, J.Z.; Sun, G.X.; Jiang, Y.X.; Li, M.W.; Xiong, L.; Chen, S.P.; Zhou, Y.Q.; Asad, M.; et al. Silencing arginine kinase/integrin β1 subunit by transgenic plant expressing dsRNA inhibits the development and survival of Plutella xylostella. Pest Manag. Sci. 2020, 76, 1761–1771. [Google Scholar] [CrossRef]
- Tian, H.; Peng, H.; Yao, Q.; Chen, H.; Xie, Q.; Tang, B.; Zhang, W. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e13. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Park, Y.; Kim, Y. A transformed bacterium expressing double-stranded RNA specific to integrin beta1 enhances Bt toxin efficacy against a polyphagous insect pest, Spodoptera exigua. PLoS ONE 2015, 10, e0132631. [Google Scholar] [CrossRef]
- Timmons, L.; Court, D.; Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263, 103–112. [Google Scholar] [CrossRef]
- Yang, D.; Buchholz, F.; Huang, Z.D.; Goga, A.; Chen, C.Y.; Brodsky, F.M.; Bishop, J.M. Short RNA duplexes produced by hydrolysis with Escherichia coli RNase III mediate effective RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9942–9947. [Google Scholar] [CrossRef] [Green Version]
- Newmark, P.; Reddien, P.; Cebria, F.; Alvarado, S. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. S1), 11861–11865. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-H.; Zhu, H.-M.; Luo, H.-S.; Wang, K.-K.; Yang, X.-B.; Jiang, L.; Xia, Q.-Y. RNA interference of FTZ-F1 gene mediated by bacterially expressed dsRNA in the silkworm, Bombyx mori. Acta Entomol. Sin. 2011, 54, 596–601. [Google Scholar]
- Huang, L.; Jin, J.; Deighan, P.; Kiner, E.; McReynolds, L.; Lieberman, J. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat. Biotechnol. 2013, 31, 350–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlong, M.; Wright, D.; Dosdall, L. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Li, Z.Y.; Feng, X.; Liu, S.S.; You, M.S.; Furlong, M.J. Biology, Ecology, and Management of the Diamondback Moth in China. Annu. Rev. Entomol. 2016, 61, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Arthropod Pesticide Resistance Database (APRD). 2020. Available online: https://www.pesticideresistance.org/ (accessed on 3 November 2020).
- Newsholme, E.A.; Beis, I.; Leech, A.R.; Zammit, V.A. The role of creatine kinase and arginine kinase in muscle. Biochem. J. 1978, 172, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Uda, K.; Fujimoto, N.; Akiyama, Y.; Mizuta, K.; Tanaka, K.; Ellington, W.R.; Suzuki, T. Evolution of the arginine kinase gene family. Comp. Biochem. Physiol. Part D Genomics Proteomics 2006, 1, 209–218. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Liu, Z.; Li, F.; Yu, Z. Screening of acrystalliferous mutants from Bacillus thuringiensis and their transformation properties. Acta Microbiol. Sin. 2000, 40, 85–90. [Google Scholar] [CrossRef]
- Lereclus, D.; Arantès, O.; Chaufaux, J.; Lecadet, M. Transformation and expression of a cloned δ-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 1989, 51, 211–217. [Google Scholar] [CrossRef]
- Arantes, O.; Lereclus, D. Construction of cloning vectors for Bacillus thuringiensis. Gene 1991, 108, 115–119. [Google Scholar] [CrossRef]
- Mahillon, J.; Chungjatupornchai, W.; Decock, J.; Dierickx, S.; Michiels, F.; Peferoen, M.; Joos, H. Transformation of Bacillus thuringiensis by electroporation. FEMS Microbiol. Lett. 2010, 58, 171–177. [Google Scholar]
- De Souza, M.T.; Lecadet, M.M.; Lereclus, D. Full expression of the cryIIIA toxin gene of Bacillus thuringiensis requires a distant upstream DNA sequence affecting transcription. J. Bacteriol. 1993, 175, 2952–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.M.; Collier, G.E. Early gene interaction during prepupal expression of Drosophila arginine kinase. Dev. Genet. 1992, 13, 302–305. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, H.; Meng, X.K.; Qian, K.; Liu, Y.P.; Song, Q.S.; Stanley, D.; Wu, J.C.; Park, Y.; Wang, J.J. Broad-complex transcription factor mediates opposing hormonal regulation of two phylogenetically distant arginine kinase genes in Tribolium castaneum. Commun. Biol. 2020, 3, e12. [Google Scholar] [CrossRef]
- Guan, X. Study on Bacillus Thuringiensis 8010; Science Press: Beijing, China, 1997. [Google Scholar]
- Caccia, S.; Astarita, F.; Barra, E.; Di Lelio, I.; Varricchio, P.; Pennacchio, F. Enhancement of Bacillus thuringiensis toxicity by feeding Spodoptera littoralis larvae with bacteria expressing immune suppressive dsRNA. J. Pest Sci. 2020, 93, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Al Baki, A.; Jung, J.K.; Kim, Y. Alteration of insulin signaling to control insect pest by using transformed bacteria expressing dsRNA. Pest Manag. Sci. 2020, 76, 1020–1030. [Google Scholar] [CrossRef]
- You, M.S.; Yue, Z.; He, W.Y.; Yang, X.H.; Yang, G.; Xie, M.; Zhan, D.L.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, Y.; Zeng, B.; Liu, Z.; Xu, X.; Meng, Q.; Huang, Y.; Yang, G.; Vasseur, L.; Gurr, G.; et al. Functional characterization of Pol III U6 promoters for gene knockdown and knockout in Plutella xylostella. Insect Biochem. Mol. Biol. 2017, 89, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dower, W.J.; Miller, J.F.; Ragsdale, C.W. High efficiency transformation of E. coliby high voltage electroporation. Nucleic Acids Res. 1988, 16, 6127–6145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, L.; Prefontaine, G.; Brousseau, R. Transformation of Bacillus thuringiensis vegetative cells by electroporation. FEMS Microbiol. Lett. 1989, 51, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R.; Cutting, S.M. Molecular Biological Methods for Bacillus; Wiley: Chichester, UK, 1991. [Google Scholar]
- Püntener, W. Manual for Field Trials in Plant Protection, 2nd ed.; Ciba Geigy Limited: Basel, Switzerland, 1981; p. 205. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. Computer Program for The Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung City, Taiwan, 2016; Available online: http://140.120.197.173/Ecology/index.htm (accessed on 10 August 2018).


| Treatment | Intrinsic Rate of Increase, r (d−1) | Finite Rate of Increase, λ (d−1) | Net Reproductive Rate, R0 (Offspring) | Mean Generation Time, T (d) |
|---|---|---|---|---|
| H2O | 0.2427 ± 0.0090 ab | 1.2746 ± 0.0115 ab | 70.4682 ± 10.1416 ab | 17.5360 ± 0.1570 b |
| 8010 | 0.2529 ± 0.0107 a | 1.2878 ± 0.0137 a | 69.7647 ± 10.9253 ab | 16.7840 ± 0.2340 c |
| 8010AKi | 0.2510 ± 0.0089 a | 1.2852 ± 0.0114 a | 81.9904 ± 11.3744 a | 17.5590 ± 0.2170 b |
| 8010:8010AKi of 1:9 | 0.2065 ± 0.0115 c | 1.2293 ± 0.0141 c | 45.9127 ± 8.9724 bc | 18.5360 ± 0.2660 a |
| 8010:8010AKi of 3:7 | 0.2153 ± 0.0117 bc | 1.2403 ± 0.0145 bc | 52.2844 ± 10.1531 abc | 18.3760 ± 0.2280 a |
| 8010:8010AKi of 5:5 | 0.2383 ± 0.0115 abc | 1.2691 ± 0.0146 abc | 66.1250 ± 12.3838 abc | 17.5920 ± 0.2250 b |
| 8010:8010AKi of 7:3 | 0.2145 ± 0.0099 c | 1.2393 ± 0.0122 c | 56.4240 ± 9.3667 abc | 18.7990 ± 0.2530 a |
| 8010:8010AKi of 9:1 | 0.2097 ± 0.0147 c | 1.2333 ± 0.0180 c | 39.8571 ± 9.3817 c | 17.5760 ± 0.2460 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-X.; Chen, J.-Z.; Li, M.-W.; Zha, B.-H.; Huang, P.-R.; Chu, X.-M.; Chen, J.; Yang, G. The Combination of Bacillus Thuringiensis and Its Engineered Strain Expressing dsRNA Increases the Toxicity against Plutella Xylostella. Int. J. Mol. Sci. 2022, 23, 444. https://doi.org/10.3390/ijms23010444
Jiang Y-X, Chen J-Z, Li M-W, Zha B-H, Huang P-R, Chu X-M, Chen J, Yang G. The Combination of Bacillus Thuringiensis and Its Engineered Strain Expressing dsRNA Increases the Toxicity against Plutella Xylostella. International Journal of Molecular Sciences. 2022; 23(1):444. https://doi.org/10.3390/ijms23010444
Chicago/Turabian StyleJiang, Ying-Xia, Jin-Zhi Chen, Miao-Wen Li, Ben-Hu Zha, Peng-Rong Huang, Xue-Mei Chu, Jing Chen, and Guang Yang. 2022. "The Combination of Bacillus Thuringiensis and Its Engineered Strain Expressing dsRNA Increases the Toxicity against Plutella Xylostella" International Journal of Molecular Sciences 23, no. 1: 444. https://doi.org/10.3390/ijms23010444
APA StyleJiang, Y.-X., Chen, J.-Z., Li, M.-W., Zha, B.-H., Huang, P.-R., Chu, X.-M., Chen, J., & Yang, G. (2022). The Combination of Bacillus Thuringiensis and Its Engineered Strain Expressing dsRNA Increases the Toxicity against Plutella Xylostella. International Journal of Molecular Sciences, 23(1), 444. https://doi.org/10.3390/ijms23010444






