Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals
Abstract
:1. Scope of the Review
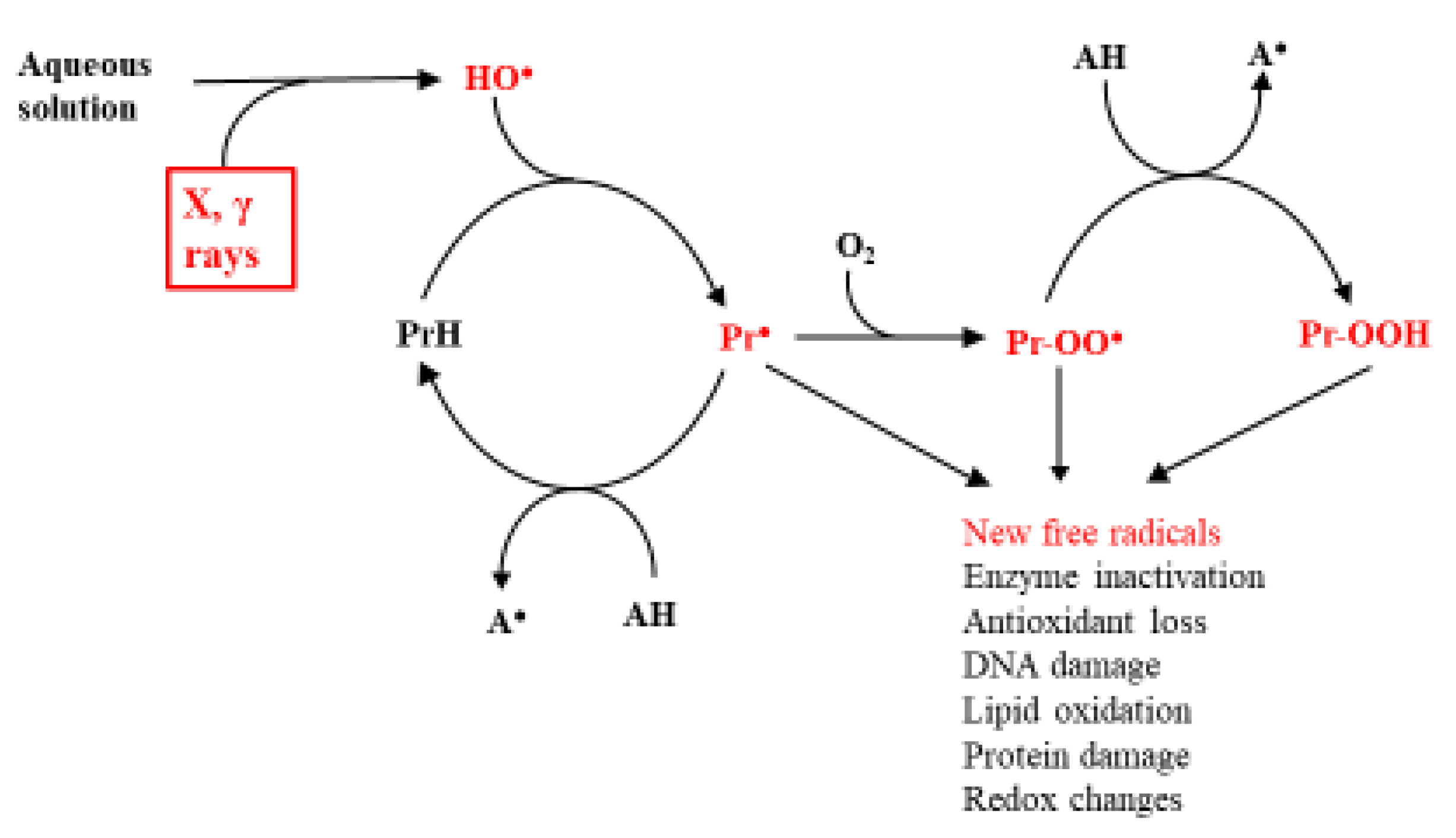
2. Ionizing Radiations Generate Radicals in Aqueous Solutions
2.1. Radiation-Generated Free Radicals and Their Targets in Biology
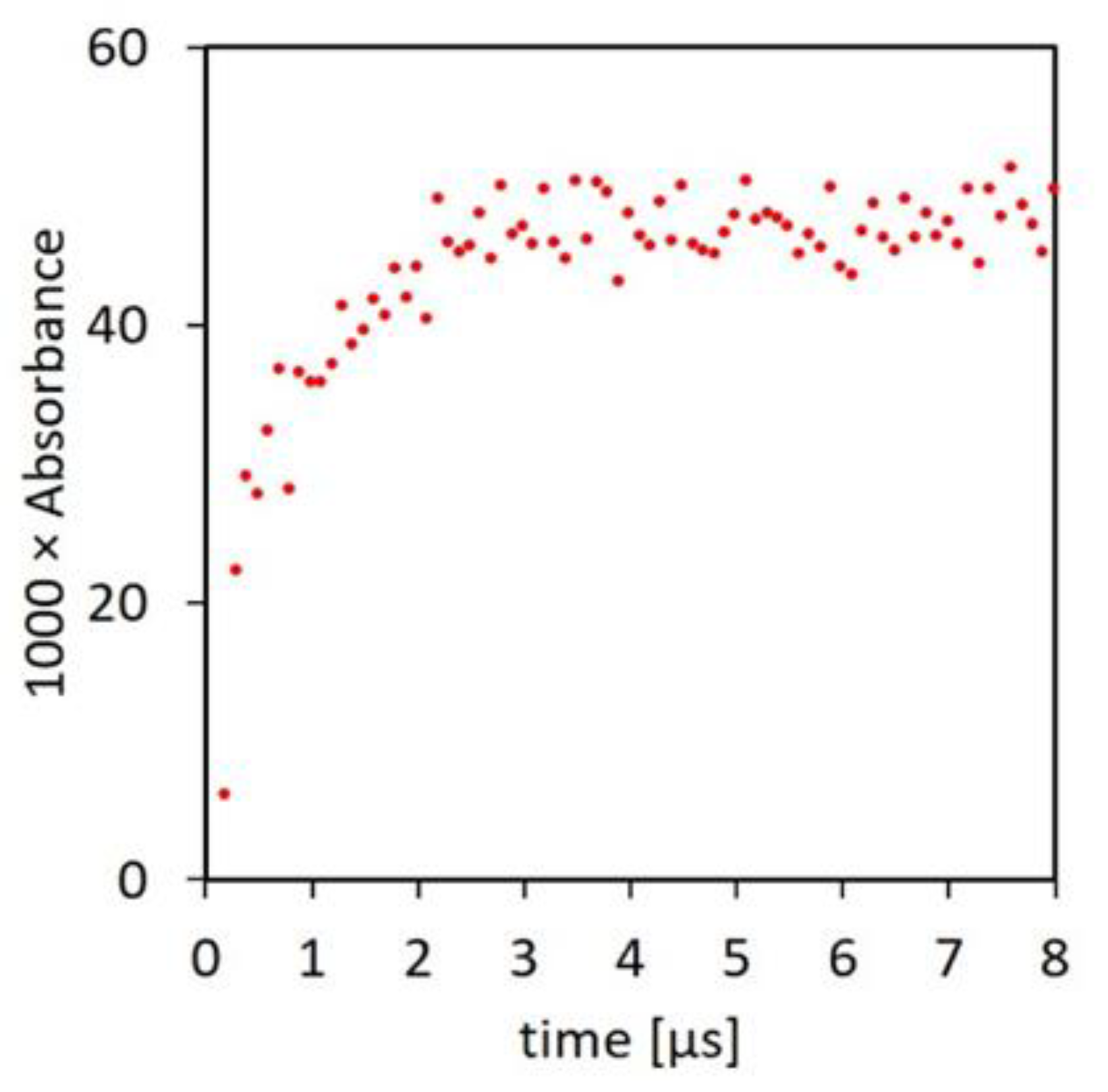
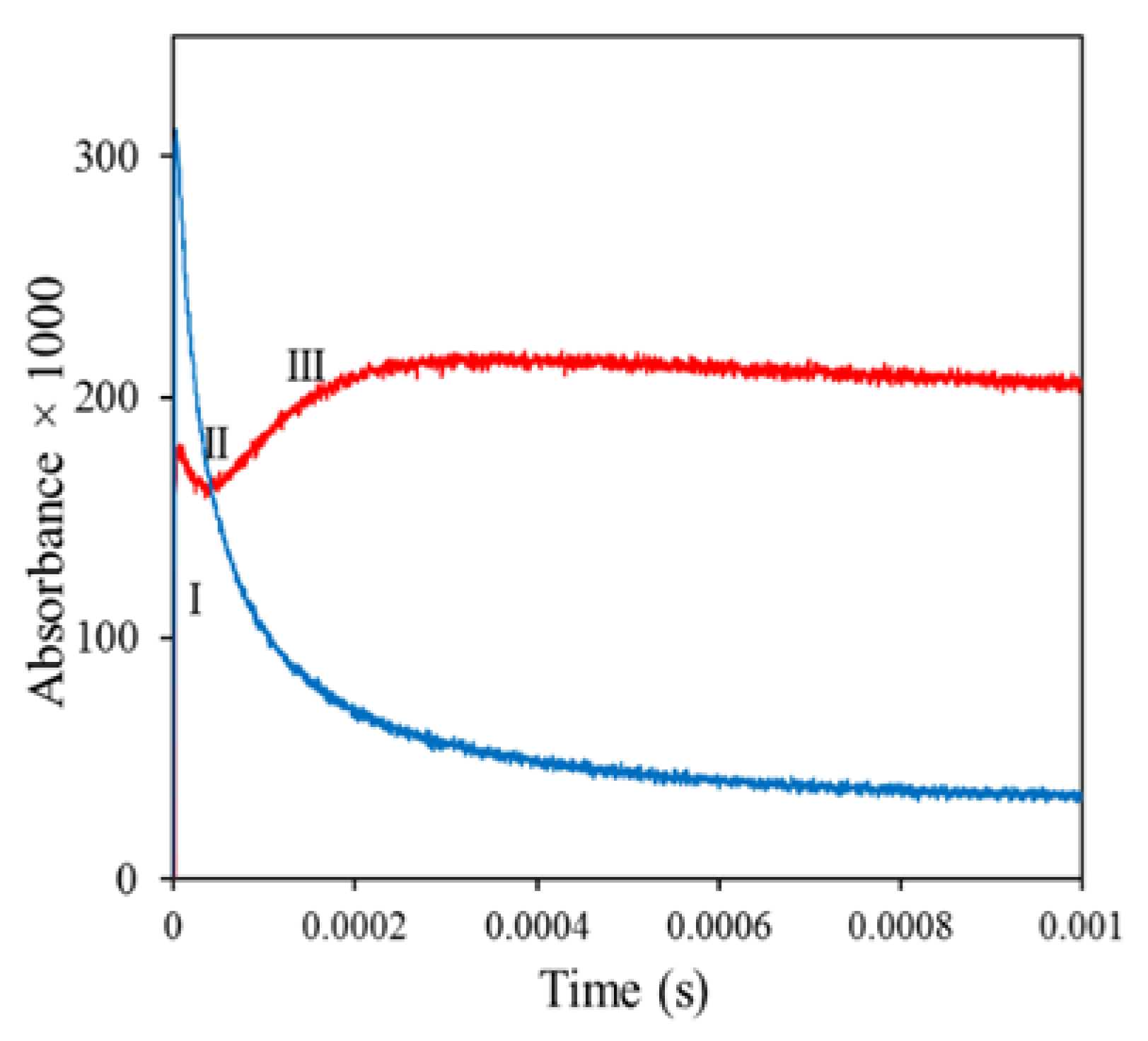
2.2. Damage Transfer in Proteins
3. Repair of Protein Radicals
3.1. Inhibition of Radiation Damage
3.2. Kinetics of Reduction of Pr● by Asc, Urate and GSH
3.3. Fast Inactivation of C-Centered Radicals by Aromatic Compounds
3.4. Polyphenols and Their Metabolites as Biological Antioxidants: New Insights
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor and Francis, Inc.: London, UK, 1987. [Google Scholar]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin, Germany, 2006; p. 523. [Google Scholar]
- O’Neill, P.; Wardman, P. Radiation chemistry comes before radiation biology. Int. J. Radiat. Biol. 2009, 85, 9–25. [Google Scholar] [CrossRef]
- Buxton, G.; Greenstock, C.; Helman, W.; Ross, A. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OHbul/O-bul) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Bielski, B.H.J.; Gebicki, J.M. Application of radiation chemistry to biology. In Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1977; Volume 3, pp. 1–51. [Google Scholar]
- Bensasson, R.; Land, E.; Truscott, T. Flash Photolysis and Pulse Radiolysis; Pergamon Press: Oxford, UK, 1983. [Google Scholar]
- Nauser, T.; Casi, G.; Koppenol, W.H.; Schoneich, C. Reversible intramolecular hydrogen transfer between cysteine thiyl radicals and glycine and alanine in model peptides: Absolute rate constants derived from pulse radiolysis and laser flash photolysis. J. Phys. Chem. B 2008, 112, 15034–15044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pryor, W.A. Cancer and free radicals. Basic Life Sci. 1986, 39, 45–59. [Google Scholar] [PubMed]
- Halliwell, B. Albumin--an important extracellular antioxidant? Biochem. Pharmacol. 1988, 37, 569–571. [Google Scholar] [CrossRef]
- Distel, L.; Distel, B.; Schussler, H. Formation of DNA double-strand breaks and DNA-protein crosslinks by irradiation of DNA in the presence of a protein. Radiat. Phys. Chem. 2002, 65, 141–149. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Allen, A.O. Relationship between critical micelle concentration and rate of radiolysis of aqueous sodium linoleate. J. Phys. Chem. 1969, 73, 2443–2444. [Google Scholar] [CrossRef]
- Wolters, H.; Kelholt, D.; Konings, A.W. Effect of membrane fatty acid substitution and temperature on repair of sublethal damage in mammalian cells. Radiat. Res. 1985, 102, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wolters, H.; Konings, A.W. Membrane radiosensitivity of fatty acid supplemented fibroblasts as assayed by the loss of intracellular potassium. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 48, 963–973. [Google Scholar] [CrossRef]
- Wolters, H.; Konings, A.W. Radiation effects on membranes. III. The effect of X irradiation on survival of mammalian cells substituted by polyunsaturated fatty acids. Radiat. Res. 1982, 92, 474–482. [Google Scholar] [CrossRef]
- Goulet, D.L.; Fisher, G.J.; Pageau, R.; van Lier, J.E. Effect of membrane fatty acid composition on radiosensitivity of V79 Chinese hamster cells. Biochim. Biophys. Acta 1986, 875, 414–417. [Google Scholar] [CrossRef]
- Du, J.; Gebicki, J.M. Proteins are major initial cell targets of hydroxyl free radicals. Int. J. Biochem. Cell Biol. 2004, 36, 2334–2343. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 4th ed.; Clarendon Press: Oxford, UK, 2007. [Google Scholar]
- Robinson, M.G.; Weiss, J.J.; Wheeler, C.M. Irradiation deoxyribonucleohistone solutions. I. Amino acid destruction. Biochim. Biophys. Acta 1966, 124, 176–180. [Google Scholar] [CrossRef]
- Sak, A.; Stuschke, M.; Wurm, R.; Budach, V. Protection of DNA from radiation-induced double-strand breaks: Influence of replication and nuclear proteins. Int. J. Radiat. Biol. 2000, 76, 749–756. [Google Scholar]
- Samuni, A.; Chevion, M.; Halpern, Y.S.; Ilan, Y.A.; Czapski, G. Radiation-induced damage in T4 bacteriophage: The effect of superoxide radicals and molecular oxygen. Radiat. Res. 1978, 75, 489–496. [Google Scholar] [CrossRef]
- Ljungman, M.; Hanawalt, P.C. Efficient protection against oxidative DNA damage in chromatin. Mol. Carcinog. 1992, 5, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, M.; Nyberg, S.; Nygren, J.; Eriksson, M.; Ahnstrom, G. DNA-bound proteins contribute much more than soluble intracellular compounds to the intrinsic protection against radiation-induced DNA strand breaks in human cells. Radiat. Res. 1991, 127, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Begusova, M.; Eon, S.; Sy, D.; Culard, F.; Charlier, M.; Spotheim-Maurizot, M. Radiosensitivity of DNA in a specific protein-DNA complex: The lac repressor-lac operator complex. Int. J. Radiat. Biol. 2001, 77, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Begusova, M.; Giliberto, S.; Gras, J.; Sy, D.; Charlier, M.; Spotheim-Maurizot, M. DNA radiolysis in DNA-protein complexes: A stochastic simulation of attack by hydroxyl radicals. Int. J. Radiat. Biol. 2003, 79, 385–391. [Google Scholar] [CrossRef]
- Begusova, M.; Sy, D.; Charlier, M.; Spotheim-Maurizot, M. Radiolysis of nucleosome core DNA: A modelling approach. Int. J. Radiat. Biol. 2000, 76, 1063–1073. [Google Scholar] [CrossRef]
- Gebicki, J.M. The role of proteins in biological damage induced by oxidative stress. In Protein Oxidation and Disease; Pietzsch, J., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 7–38. [Google Scholar]
- Pryor, W.A. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef] [Green Version]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Davies, M.J.; Dean, R.T. Radical-Mediated Protein Oxidation; Oxford University Press: Oxford, UK, 1997; pp. 1–443. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta 2001, 1504, 196–219. [Google Scholar] [CrossRef] [Green Version]
- Wardman, P. The importance of radiation chemistry to radiation and free radical biology (The 2008 Silvanus Thompson Memorial Lecture). Br. J. Radiol. 2009, 82, 89–104. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Takamoto, K.; Chance, M.R. Radiolytic modification of basic amino acid residues in peptides: Probes for examining protein-protein interactions. Anal. Chem. 2003, 75, 6995–7007. [Google Scholar] [CrossRef] [PubMed]
- Prutz, W.A.; Butler, J.; Land, E.J.; Swallow, A.J. Direct demonstration of electron transfer between tryptophan and tyrosine in proteins. Biochem. Biophys. Res. Commun. 1980, 96, 408–414. [Google Scholar] [CrossRef]
- Butler, J.; Land, E.J.; Prutz, W.A.; Swallow, A.J. Charge transfer between tryptophan and tyrosine in proteins. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1982, 705, 150–162. [Google Scholar] [CrossRef]
- Bobrowski, K.; Holcman, J.; Poznanski, J.; Ciurak, M.; Wierzchowski, K.L. Pulse radiolysis of intramolecular electron transfer in model peptides and proteins. 5. Trp-Tyr. radical transformation in H-Trp-(Pro)n-Tyr-OH series of peptides. J. Phys. Chem. 1992, 96, 10036–10043. [Google Scholar] [CrossRef]
- Bobrowski, K.; Holcman, J.; Poznanski, J.; Wierzchowski, K.L. Pulse radiolysis studies of intramolecular electron transfer in model peptides and proteins. 7. Trp-->TyrO radical transformation in hen egg-white lysozyme. Effects of pH, temperature, Trp62 oxidation and inhibitor binding. Biophys. Chem. 1997, 63, 153–166. [Google Scholar] [CrossRef]
- Bobrowski, K.; Poznanski, J.; Holcman, J.; Wierzchowski, K.L. Pulse radiolysis studies of intramolecular electron transfer in model peptides and proteins. 8. Trp [NH•+]-->Tyr [O•] radical transformation in H-Trp-(Pro) n-Tyr-OH, n = 3–5, series of peptides. J. Phys. Chem. B 1999, 103, 10316–10324. [Google Scholar] [CrossRef]
- Bobrowski, K.; Wierzchowski, K.L.; Holcman, J.; Ciurak, M. Intramolecular electron transfer in peptides containing methionine, tryptophan and tyrosine: A pulse radiolysis study. Int. J. Radiat. Biol. 1990, 57, 919–932. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E.; Lin, S.W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [CrossRef]
- Domazou, A.; Gebicki, J.M.; Nauser, T.; Koppenol, W.H. Repair of protein radicals by antioxidants. Isr. J. Chem. 2014, 54, 254–264. [Google Scholar] [CrossRef]
- Simpson, J.A.; Narita, S.; Gieseg, S.; Gebicki, S.; Gebicki, J.M.; Dean, R.T. Long-lived reactive species on free-radical-damaged proteins. Biochem. J. 1992, 282, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Gebicki, J.M. Protein hydroperoxides as new reactive oxygen species. Redox Rep. 1997, 3, 99–110. [Google Scholar] [CrossRef]
- Luxford, C.; Dean, R.T.; Davies, M.J. Radicals derived from histone hydroperoxides damage nucleobases in RNA and DNA. Chem. Res. Toxicol. 2000, 13, 665–672. [Google Scholar] [CrossRef]
- Willson, R. Organic peroxy free radicals as ultimate agents in oxygen toxicity. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 41–72. [Google Scholar]
- Gebicki, S.; Gebicki, J.M. Crosslinking of DNA and proteins induced by protein hydroperoxides. Biochem. J. 1999, 338, 629–636. [Google Scholar] [CrossRef]
- Gieseg, S.; Duggan, S.; Gebicki, J.M. Peroxidation of proteins before lipids in U937 cells exposed to peroxyl radicals. Biochem. J. 2000, 350, 215–218. [Google Scholar] [CrossRef]
- Schaich, K.M. Free radical initiation in proteins and amino acids by ionizing and ultraviolet radiations and lipid oxidation--part III: Free radical transfer from oxidizing lipids. Crit. Rev. Food Sci. Nutr. 1980, 13, 189–244. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry and Pathology; Fink, G., Ed.; Elsevier Science and Technology: New York, NY, USA, 2019; pp. 153–163. [Google Scholar]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef]
- Jin, F.; Leitich, J.; von Sonntag, C. The superoxide radical reacts with tyrosine-derived phenoxyl radicals by addition rather than by electron transfer. J. Chem. Soc. Perkin Trans. II 1993, 1583–1588. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Butler, J.; van der Vliet, A.; Cross, C.E.; Halliwell, B. Nitric oxide rapidly scavenges tyrosine and tryptophan radicals. Biochem. J. 1995, 310, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, N.H.; Yandell, J.K. Outer-Sphere Electron-Transfer Reactions of Ascorbate Anions. Aust. J. Chem. 1982, 35, 1133–1144. [Google Scholar]
- Buffington, G.D.; Doe, W. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res. 1995, 22, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 1999, 92, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kume-Kick, J.; Rice, M. Estrogen-dependent modulation of rat brain ascorbate levels and ischemia-induced ascorbate loss. Brain Res. 1998, 803, 105–113. [Google Scholar] [CrossRef]
- Locatelli, F.; Canaud, B.; Eckardt, K.U.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transpl. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Moor, E.; Shohami, E.; Kanevski, E.; Grigoriadis, N.; Symeonidou, C.; Kohen, R. Impairment of the ability of the injured aged brain in elevating urate and ascorbate. Exp. Gerontol. 2006, 41, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Misso, N.L.; Brooks-Wildhaber, J.; Ray, S.; Vally, H.; Thompson, P.J. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur. Respir. J. 2005, 26, 257–264. [Google Scholar] [CrossRef]
- Davies, M.J.; Fu, S.; Wang, H.; Dean, R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999, 27, 1151–1163. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T.; Domazou, A.; Steinmann, D.; Bounds, P.L.; Koppenol, W.H. Reduction of protein radicals by GSH and ascorbate: Potential biological significance. Amino Acids 2010, 39, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Domazou, A.S.; Koppenol, W.H.; Gebicki, J.M. Efficient repair of protein radicals by ascorbate. Free Radic. Biol. Med. 2009, 46, 1049–1057. [Google Scholar] [CrossRef]
- Nauser, T.; Gebicki, J.M. Physiological concentrations of ascorbate cannot prevent the potentially damaging reactions of protein radicals in humans. Chem. Res. Toxicol. 2017, 30, 1702–1710. [Google Scholar] [CrossRef]
- Forni, L.; Monig, J.; Mora-Arellano, V.; Willson, R. Thiyl free radicals: Direct observations of electron transfer reactions with phenothiazines and ascorbate. J. Chem. Soc. Perkin Trans. II 1983, 961–965. [Google Scholar] [CrossRef]
- Hoey, B.M.; Butler, J. The repair of oxidized amino acids by antioxidants. Biochim. Biophys. Acta 1984, 791, 212–218. [Google Scholar]
- Jovanovic, S.V.; Simic, M.G. Repair of tryptophan radicals by antioxidants. J. Free Radic. Biol. Med. 1985, 1, 125–129. [Google Scholar] [CrossRef]
- Filipe, P.; Morliere, P.; Patterson, L.K.; Hug, G.L.; Maziere, J.C.; Maziere, C.; Freitas, J.P.; Fernandes, A.; Santus, R. Mechanisms of flavonoid repair reactions with amino acid radicals in models of biological systems: A pulse radiolysis study in micelles and human serum albumin. Biochim. Biophys. Acta 2002, 1572, 150–162. [Google Scholar] [CrossRef]
- Nauser, T.; Gebicki, J.M. Reaction rates of glutathione and ascorbate with alkyl radicals are too slow for protection against protein peroxidation in vivo. Arch. Biochem. Biophys. 2017, 633, 118–123. [Google Scholar] [CrossRef]
- Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations). Pure Appl. Chem. 1994, 66, 1077–1184. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, S.V.; Steenken, S.; Simic, M.G.; Hara, Y. Antioxidant properties of flavonoids: Reduction potentials and electron transfer reactions of flavonoid radicals. In Flavonoids in Health and Disease; Rice-Evans, C., Packer, L., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1998; pp. 137–161. [Google Scholar]
- Nauser, T.; Gebicki, J.M. Fast reaction of carbon free radicals with flavonoids and other aromatic compounds. Arch. Biochem. Biophys. 2019, 674, 108107. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–8346. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Harms, L.M.; Scalbert, A.; Zamora-Ros, R.; Rinaldi, S.; Jenab, M.; Murphy, N.; Achaintre, D.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Plasma polyphenols associated with lower high-sensitivity C-reactive protein concentrations: A cross-sectional study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Br. J. Nutr. 2020, 123, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L.; Maiorino, M.I.; Marelli, G.; Parillo, M.; Pistis, D.; Tubili, C.; Vetrani, C.; et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr. Metab. Cardiovasc. Dis. 2020, 30, 355–367. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-Macromolecules Interactions in Human Diseases with Focus on Alzheimer, Atherosclerosis and Cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Williamson, G. At the interface of antioxidant signalling and cellular function: Key polyphenol effects. Mol. Nutr. Food Res. 2016, 60, 1770–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neta, P.; Simic, M.; Hoffman, Z. Pulse Radiolysis and Electron Spin Resonance Studies of Nitroaromatic Radical Anions. Optical Absorption Spectra, Kinetics, and One-Electron Redox Potentials. J. Phys. Chem. 1976, 80, 2018–2023. [Google Scholar] [CrossRef]
- Jagannadham, V.; Steenken, S. One-Electron Reduction of Nitrobenzenes by a-Hydroxy alkyl Radicals via Addition/Elimination. An Example of an Organic Inner-Sphere Electron-Transfer Reaction. J. Am. Chem. Soc. 1984, 106, 6542–6551. [Google Scholar] [CrossRef]
- Santschi, N.; Nauser, T. An experimental radical electrophilicity index. Chem. Phys. Chem. Comm. 2017, 18, 2973–2976. [Google Scholar] [CrossRef]
- Nauser, T.; Carreras, A. Carbon-centered radical add reversibly to histidine—Implications. Chem. Comm. 2014, 50, 14349–14351. [Google Scholar] [CrossRef] [Green Version]
- Nauser, T.; Gebicki, J.M. Addition of carbon-centered radicals to aromatic antioxidants: Mechanistic aspects. Phys. Chem. Chem. Phys. 2020, 22, 24572–24582. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Garrido, I.; Monagas, M.; Gomez-Cordoves, C.; Medina-Remon, A.; Andres-Lacueva, C.; Bartolome, B. Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. J. Agric. Food Chem. 2009, 57, 10134–10142. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, H.; Shibata, M.; Myojin, Y.; Ito, H.; Sugimoto, Y.; Tai, A.; Hatano, T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011, 21, 5901–5904. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Diaz-Rubio, M.E.; Saura-Calixto, F. Contribution of Macromolecular Antioxidants to Dietary Antioxidant Capacity: A Study in the Spanish Mediterranean Diet. Plant Foods Hum. Nutr. 2015, 70, 365–370. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Espin, J.C.; Thomas-Barberan, F.A. Non-extractabelle polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Domazou, A.S.; Zelenay, V.; Koppenol, W.H.; Gebicki, J.M. Efficient depletion of ascorbate by amino acid and protein radicals under oxidative stress. Free Radic. Biol. Med. 2012, 53, 1565–1573. [Google Scholar] [CrossRef]


| Radical | Reaction with | ||
|---|---|---|---|
| GSH | H2Ur− | HAsc− | |
| N-Ac-Trp●-NH2 | (1.1 ± 0.3) × 105 | 1.9 × 107 | 1.4 × 108 |
| Lysozyme-Trp● | (1.05 ± 0.05) × 105 | 1.2 × 107 | 8.3 × 107 |
| Radical | Generated by | k/106 | Reference (M−1 s−1) |
|---|---|---|---|
| Trp● | N3● | 83 ± 7 | [67] |
| Trp● | Br2●− | 100 | [68] |
| Ac-Trp● amide | N3● | 140 ± 10 | [64] |
| TyrO● | N3● | 11.3 ± 10 | [67] |
| Ac-TyrO● amide | N3● | 260 ± 20 | [64] |
| Insulin-TyrO● | N3● | 29 ± 1 | [64] |
| Chymotrypsin- TyrO● | N3● | 40 | [64] |
| Chymotrypsin-Trp● | N3● | 160 ± 10 | [64] |
| Pepsin-TyrO● | N3● | 35 ± 9 | [64] |
| Pepsin-Trp● | N3● | 180 ± 10 | [64] |
| β-Lactoglobulin-TyrO● | N3● | 0.4 ± 0.06 | [64] |
| β-Lactoglobulin-Trp● | N3● | 22 ± 2 | [64] |
| Lysozyme-TyrO● | N3● | 11 | [67] |
| Lysozyme-Trp● | N3● | 83 | [67] |
| Lysozyme-Trp● | N3● | 80 | [69] |
| Ac-Gly● amide | HO● | 2 | [70] |
| Cyclo(Gly●)2 | HO● | 2–5 | [70] |
| Ac-Ala● | HO● | 1 | [70] |
| (Phe..OH)● | HO● | 1.3 | [70] |
| γ-GluAla●Gly | HO● | 2.4 ± 0.1 | [70] |
| Lysozyme● | HO● | 160 and (5–20) | [65] |
| Chymotrypsin● | HO● | 250 and (5–20) | [65] |
| Ovalbumin● | HO● | 110 and (5–20) | [65] |
| HSA● | HO● | 120 and (5–20) | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebicki, J.M.; Nauser, T. Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals. Int. J. Mol. Sci. 2022, 23, 396. https://doi.org/10.3390/ijms23010396
Gebicki JM, Nauser T. Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals. International Journal of Molecular Sciences. 2022; 23(1):396. https://doi.org/10.3390/ijms23010396
Chicago/Turabian StyleGebicki, Janusz M., and Thomas Nauser. 2022. "Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals" International Journal of Molecular Sciences 23, no. 1: 396. https://doi.org/10.3390/ijms23010396
APA StyleGebicki, J. M., & Nauser, T. (2022). Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals. International Journal of Molecular Sciences, 23(1), 396. https://doi.org/10.3390/ijms23010396







