The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance
Abstract
1. Introduction
2. CRPC and Its Subtypes
3. Non-Coding RNAs and CRPC
4. LncRNA and Its Interaction with miRNA
5. LncRNA–miRNA Interaction and Their Roles in AR-Independent/Crosstalk Mechanisms
5.1. Growth Factors
5.2. Oncogenic Signaling Pathways
5.3. Cell Cycle Dysregulation
5.4. Cytokines and Other Transmembrane Proteins
6. The Interplay between lncRNA and miRNA/Epigenetic Regulators in NEPC
6.1. General Introduction of NEPC
6.2. Genomic Mutation and NEPC
6.3. ENI and NEPC
6.4. The Interplay between lncRNA and miRNA in NEPC
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Gillessen, S.; Omlin, A.; Attard, G.; de Bono, J.S.; Efstathiou, E.; Fizazi, K.; Halabi, S.; Nelson, P.S.; Sartor, O.; Smith, M.R.; et al. Management of patients with advanced prostate cancer: Recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1589–1604. [Google Scholar] [CrossRef]
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004, 10, 33–39. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.J.; Han, M.; Freedland, S.J.; Humphreys, E.B.; DeWeese, T.L.; Partin, A.W.; Walsh, P.C. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008, 299, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Aly, M.; Leval, A.; Schain, F.; Liwing, J.; Lawson, J.; Vágó, E.; Nordström, T.; Andersson, T.M.; Sjöland, E.; Wang, C.; et al. Survival in patients diagnosed with castration-resistant prostate cancer: A population-based observational study in Sweden. Scand. J. Urol. 2020, 54, 115–121. [Google Scholar] [CrossRef]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: Extended analysis of the phase 3 PREVAIL study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- Scher, H.I.; Sawyers, C.L. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005, 23, 8253–8261. [Google Scholar] [CrossRef]
- Hoang, D.T.; Iczkowski, K.A.; Kilari, D.; See, W.; Nevalainen, M.T. Androgen receptor-dependent and -independent mechanisms driving prostate cancer progression: Opportunities for therapeutic targeting from multiple angles. Oncotarget 2017, 8, 3724–3745. [Google Scholar] [CrossRef]
- Wang, X.; Qi, M.; Zhang, J.; Sun, X.; Guo, H.; Pang, Y.; Zhang, Q.; Chen, X.; Zhang, R.; Liu, Z.; et al. Differential response to neoadjuvant hormonal therapy in prostate cancer: Predictive morphological parameters and molecular markers. Prostate 2019, 79, 709–719. [Google Scholar] [CrossRef]
- De Winter, J.A.; Trapman, J.; Brinkmann, A.O.; Boersma, W.J.; Mulder, E.; Schroeder, F.H.; Claassen, E.; van der Kwast, T.H. Androgen receptor heterogeneity in human prostatic carcinomas visualized by immunohistochemistry. J. Pathol. 1990, 160, 329–332. [Google Scholar] [CrossRef]
- Choucair, K.; Ejdelman, J.; Brimo, F.; Aprikian, A.; Chevalier, S.; Lapointe, J. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 2012, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Deng, Q.; Chao, H.P.; Liu, X.; Lu, Y.; Lin, K.; Liu, B.; Tang, G.W.; Zhang, D.; Tracz, A.; et al. Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses. Nat. Commun. 2018, 9, 3600. [Google Scholar] [CrossRef]
- Vellky, J.E.; Ricke, W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Mizokami, A. Undesirable status of prostate cancer cells after intensive inhibition of AR signaling: Post-AR era of CRPC treatment. Biomedicines 2021, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 2017, 32, 474–489.e6. [Google Scholar] [CrossRef]
- Deng, Q.; Tang, D.G. Androgen receptor and prostate cancer stem cells: Biological mechanisms and clinical implications. Endocr.-Relat. Cancer 2015, 22, T209–T220. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wyatt, A.W.; Xue, H.; Wang, Y.; Dong, X.; Haegert, A.; Wu, R.; Brahmbhatt, S.; Mo, F.; Jong, L.; et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014, 74, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbé, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Toivanen, R.; Mitrofanova, A.; Floch, N.; Hayati, S.; Sun, Y.; Le Magnen, C.; Chester, D.; Mostaghel, E.A.; Califano, A.; et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017, 7, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.W.; Wang, L.Y.; Hung, C.L.; Kung, H.J.; Hsieh, C.L. Non-coding RNAs in castration-resistant prostate cancer: Regulation of androgen receptor signaling and cancer metabolism. Int. J. Mol. Sci. 2015, 16, 28943–28978. [Google Scholar] [CrossRef]
- Kojima, S.; Goto, Y.; Naya, Y. The roles of microRNAs in the progression of castration-resistant prostate cancer. J. Hum. Genet. 2017, 62, 25–31. [Google Scholar] [CrossRef]
- Ding, L.; Wang, R.; Shen, D.; Cheng, S.; Wang, H.; Lu, Z.; Zheng, Q.; Wang, L.; Xia, L.; Li, G. Role of noncoding RNA in drug resistance of prostate cancer. Cell Death Dis. 2021, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Santosh, B.; Varshney, A.; Yadava, P.K. Non-coding RNAs: Biological functions and applications. Cell Biochem. Funct. 2015, 33, 14–22. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta—Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Hu, W.; Liu, C.; Bi, Z.Y.; Zhou, Q.; Zhang, H.; Li, L.L.; Zhang, J.; Zhu, W.; Song, Y.Y.; Zhang, F.; et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Baird, A.M.; Aird, J.; Greene, J.; Kapoor, D.; Gray, S.G.; McDermott, R.; Finn, S.P. RNAs as candidate diagnostic and prognostic markers of prostate cancer-from cell line models to liquid biopsies. Diagnostics 2018, 8, 60. [Google Scholar] [CrossRef]
- Ramnarine, V.R.; Kobelev, M.; Gibb, E.A.; Nouri, M.; Lin, D.; Wang, Y.; Buttyan, R.; Davicioni, E.; Zoubeidi, A.; Collins, C.C. The evolution of long noncoding RNA acceptance in prostate cancer initiation, progression, and its clinical utility in disease management. Eur. Urol. 2019, 76, 546–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Pitchiaya, S.; Cieślik, M.; Niknafs, Y.S.; Tien, J.C.; Hosono, Y.; Iyer, M.K.; Yazdani, S.; Subramaniam, S.; Shukla, S.K.; et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat. Genet. 2018, 50, 814–824. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Jin, C.; Yang, J.C.; Tanasa, B.; Li, W.; Merkurjev, D.; Ohgi, K.A.; Meng, D.; Zhang, J.; et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature 2013, 500, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, A.; Banerjee, A.; Basu, K.; Pal, D.K.; Ghosh, A. PRNCR1: A long non-coding RNA with a pivotal oncogenic role in cancer. Hum. Genet. 2021, 1–15. [Google Scholar] [CrossRef]
- Aird, J.; Baird, A.M.; Lim, M.; McDermott, R.; Finn, S.P.; Gray, S.G. Carcinogenesis in prostate cancer: The role of long non-coding RNAs. Noncoding RNA Res. 2018, 3, 29–38. [Google Scholar] [CrossRef]
- Ma, G.; Tang, M.; Wu, Y.; Xu, X.; Pan, F.; Xu, R. LncRNAs and miRNAs: Potential biomarkers and therapeutic targets for prostate cancer. Am. J. Transl. Res. 2016, 8, 5141–5150. [Google Scholar]
- Yang, Y.; Liu, K.Y.; Liu, Q.; Cao, Q. Androgen receptor-related non-coding RNAs in prostate cancer. Front. Cell Dev. Biol. 2021, 9, 660853. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Wong, J.H.; Tsui, S.K.W.; Zuo, T.; Chan, T.F.; Ng, T.B. LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: Updates in recent years. Appl. Microbiol. Biotechnol. 2019, 103, 4649–4677. [Google Scholar] [CrossRef]
- Sun, B.; Liu, C.; Li, H.; Zhang, L.; Luo, G.; Liang, S.; Lü, M. Research progress on the interactions between long non-coding RNAs and microRNAs in human cancer. Oncol. Lett. 2020, 19, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, T.; Liu, L.; Zhang, W.; Zhao, C.; Li, S.; Li, J.; Rao, N.; Le, T.D. LMSM: A modular approach for identifying lncRNA related miRNA sponge modules in breast cancer. PLoS Comput. Biol. 2020, 16, e1007851. [Google Scholar] [CrossRef]
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: Deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Q.; Wang, C.Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. LncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194–1206. [Google Scholar] [CrossRef]
- Mayr, C.; Bartel, D.P. Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009, 138, 673–684. [Google Scholar] [CrossRef]
- Park, H.J.; Ji, P.; Kim, S.; Xia, Z.; Rodriguez, B.; Li, L.; Su, J.; Chen, K.; Masamha, C.P.; Baillat, D.; et al. 3’ UTR shortening represses tumor-suppressor genes in trans by disrupting ceRNA crosstalk. Nat. Genet. 2018, 50, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Xu, C.; Li, Y.; Cai, X.; Ren, S.; Liu, H.; Wang, Y.; Wang, F.; Chen, R.; Qu, M.; et al. A feed-forward regulatory loop between androgen receptor and PlncRNA-1 promotes prostate cancer progression. Cancer Lett. 2016, 374, 62–74. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Liu, C.; Wang, C.; Ling, Z.; Wang, Y.; Wang, Y.; Zhang, M.; Chen, S.; Xu, B.; Guan, H.; et al. LncRNA CCAT1 promotes prostate cancer cell proliferation by interacting with DDX5 and MIR-28-5P. Mol. Cancer Ther. 2019, 18, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, H.Y.; Yuan, T.; Zhou, W.D.; Xiang, Z.D.; Jiang, Q.Q.; Wu, D.L. Long non-coding RNA AFAP1-AS1 facilitates prostate cancer progression by regulating miR-15b/IGF1R axis. Curr. Pharm. Des. 2021, 27, 4261–4269. [Google Scholar] [CrossRef]
- Huang, S.; Liao, Q.; Li, W.; Deng, G.; Jia, M.; Fang, Q.; Ji, H.; Meng, M. The lncRNA PTTG3P promotes the progression of CRPC via upregulating PTTG1. Bull. Cancer 2021, 108, 359–368. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Xiao, N.; Duan, J.; Wang, Z.; Wang, S. Long noncoding RNA SChLAP1 accelerates the proliferation and metastasis of prostate cancer via targeting miR-198 and promoting the MAPK1 pathway. Oncol. Res. 2018, 26, 131–143. [Google Scholar] [CrossRef]
- Bai, M.; He, C.; Shi, S.; Wang, M.; Ma, J.; Yang, P.; Dong, Y.; Mou, X.; Han, S. Linc00963 promote cell proliferation and tumor growth in castration-resistant prostate cancer by modulating miR-655/TRIM24 axis. Front. Oncol. 2021, 11, 636965. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Li, R.; Wang, L.; Gu, Y.; Zhao, Y.; Huang, K.H.; Cheng, T.; Yuan, Y.; Gao, S. Long non-coding RNA MYU promotes prostate cancer proliferation by mediating the miR-184/c-Myc axis. Oncol. Rep. 2018, 40, 2814–2825. [Google Scholar] [CrossRef]
- Qi, H.; Wen, B.; Wu, Q.; Cheng, W.; Lou, J.; Wei, J.; Huang, J.; Yao, X.; Weng, G. Long noncoding RNA SNHG7 accelerates prostate cancer proliferation and cycle progression through cyclin D1 by sponging miR-503. Biomed. Pharmacother. 2018, 102, 326–332. [Google Scholar] [CrossRef]
- Long, B.; Li, N.; Xu, X.X.; Li, X.X.; Xu, X.J.; Liu, J.Y.; Wu, Z.H. Long noncoding RNA LOXL1-AS1 regulates prostate cancer cell proliferation and cell cycle progression through miR-541-3p and CCND1. Biochem. Biophys. Res. Commun. 2018, 505, 561–568. [Google Scholar] [CrossRef]
- Xiao, G.; Yao, J.; Kong, D.; Ye, C.; Chen, R.; Li, L.; Zeng, T.; Wang, L.; Zhang, W.; Shi, X.; et al. The long noncoding RNA TTTY15, which is located on the Y chromosome, promotes prostate cancer progression by sponging let-7. Eur. Urol. 2019, 76, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Lei, Y.; Wang, M.; Ma, J.; Yang, P.; Mou, X.; Dong, Y.; Han, S. Long non-coding RNA SNHG17 promotes cell proliferation and invasion in castration-resistant prostate cancer by targeting the miR-144/CD51 axis. Front. Genet. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Wang, X.; Tao, T.; Zhang, L.; Guan, H.; You, Z.; Lu, K.; Zhang, G.; Chen, S.; Wu, J.; et al. Involvement of aberrantly activated HOTAIR/EZH2/miR-193a feedback loop in progression of prostate cancer. J. Exp. Clin. Cancer Res. 2017, 36, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Zhang, L.; Xu, Z. MEF2activated long noncoding RNA PCGEM1 promotes cell proliferation in hormonerefractory prostate cancer through downregulation of miR148a. Mol. Med. Rep. 2018, 18, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Li, R.; Zhao, Y.; Gu, Y.; Liu, S.; Cheng, T.; Huang, K.; Yuan, Y.; Song, D.; et al. The long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2. Biochem. Biophys. Res. Commun. 2018, 502, 262–268. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, J.; Hu, Z.; Zhao, M.; Gu, Z.; Miao, S. Identification of potential prostate cancer-related pseudogenes based on competitive endogenous RNA network hypothesis. Med. Sci. Monit. 2018, 24, 4213–4239. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Radmayr, C.; Trapman, J.; Hittmair, A.; Bartsch, G.; Klocker, H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994, 54, 5474–5478. [Google Scholar] [CrossRef]
- Wu, J.D.; Haugk, K.; Woodke, L.; Nelson, P.; Coleman, I.; Plymate, S.R. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J. Cell Biochem. 2006, 99, 392–401. [Google Scholar] [CrossRef]
- Chien, W.; Pei, L. A novel binding factor facilitates nuclear translocation and transcriptional activation function of the pituitary tumor-transforming gene product. J. Biol. Chem. 2000, 275, 19422–19427. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Jin, G.; Zhou, X.; Li, M.; Ying, X.; Wang, L.; Wu, H.; Zhu, Q. Linc00963: A novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independence. Int. J. Oncol. 2014, 44, 2041–2049. [Google Scholar] [CrossRef]
- Groner, A.C.; Cato, L.; de Tribolet-Hardy, J.; Bernasocchi, T.; Janouskova, H.; Melchers, D.; Houtman, R.; Cato, A.C.B.; Tschopp, P.; Gu, L.; et al. TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell 2016, 29, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Rahl, P.B.; Young, R.A. MYC and transcription elongation. Cold Spring Harb. Perspect. Med. 2014, 4, a020990. [Google Scholar] [CrossRef]
- Campisi, J.; Gray, H.E.; Pardee, A.B.; Dean, M.; Sonenshein, G.E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell 1984, 36, 241–247. [Google Scholar] [CrossRef]
- Jackstadt, R.; Roh, S.; Neumann, J.; Jung, P.; Hoffmann, R.; Horst, D.; Berens, C.; Bornkamm, G.W.; Kirchner, T.; Menssen, A.; et al. AP4 is a mediator of epithelial-mesenchymal transition and metastasis in colorectal cancer. J. Exp. Med. 2013, 210, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. Alphav integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef]
- Zaffuto, E.; Pompe, R.; Zanaty, M.; Bondarenko, H.D.; Leyh-Bannurah, S.R.; Moschini, M.; Dell’Oglio, P.; Gandaglia, G.; Fossati, N.; Stabile, A.; et al. Contemporary incidence and cancer control outcomes of primary neuroendocrine prostate cancer: A SEER database analysis. Clin. Genitourin. Cancer 2017, 15, e793–e800. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Zhang, T.; Small, E.J.; Armstrong, A.J. Neuroendocrine prostate cancer: Subtypes, biology, and clinical outcomes. J. Natl. Compr. Cancer Netw. JNCCN 2014, 12, 719–726. [Google Scholar] [CrossRef]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef]
- Tan, H.L.; Sood, A.; Rahimi, H.A.; Wang, W.; Gupta, N.; Hicks, J.; Mosier, S.; Gocke, C.D.; Epstein, J.I.; Netto, G.J.; et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 2014, 20, 890–903. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Y.; Wu, C.; Magyar, C.E.; Li, X.; Cheng, L.; Yao, J.L.; Shen, S.; Osunkoya, A.O.; Liang, C.; et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocr.-Relat. Cancer 2012, 19, 321–331. [Google Scholar] [CrossRef]
- Cheng, L.; MacLennan, G.T.; Lopez-Beltran, A.; Montironi, R. Anatomic, morphologic and genetic heterogeneity of prostate cancer: Implications for clinical practice. Expert Rev. Anticancer Ther. 2012, 12, 1371–1374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosquera, J.M.; Beltran, H.; Park, K.; MacDonald, T.Y.; Robinson, B.D.; Tagawa, S.T.; Perner, S.; Bismar, T.A.; Erbersdobler, A.; Dhir, R.; et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 2013, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Phillips, J.W.; Smith, B.A.; Park, J.W.; Stoyanova, T.; McCaffrey, E.F.; Baertsch, R.; Sokolov, A.; Meyerowitz, J.G.; Mathis, C.; et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell 2016, 29, 536–547. [Google Scholar] [CrossRef]
- Zhang, X.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lucas, J.M.; Lam, H.M.; Dumpit, R.; Corey, E.; Chéry, L.; et al. SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin. Cancer Res. 2015, 21, 4698–4708. [Google Scholar] [CrossRef]
- Lee, A.R.; Gan, Y.; Tang, Y.; Dong, X. A novel mechanism of SRRM4 in promoting neuroendocrine prostate cancer development via a pluripotency gene network. EBioMedicine 2018, 35, 167–177. [Google Scholar] [CrossRef]
- Li, Y.; Donmez, N.; Sahinalp, C.; Xie, N.; Wang, Y.; Xue, H.; Mo, F.; Beltran, H.; Gleave, M.; Wang, Y.; et al. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur. Urol. 2017, 71, 68–78. [Google Scholar] [CrossRef]
- Bohrer, L.R.; Chen, S.; Hallstrom, T.C.; Huang, H. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: A potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology 2010, 151, 5136–5145. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat. Commun. 2018, 9, 4080. [Google Scholar] [CrossRef]
- Chang, Y.T.; Lin, T.P.; Campbell, M.; Pan, C.C.; Lee, S.H.; Lee, H.C.; Yang, M.H.; Kung, H.J.; Chang, P.C. REST is a crucial regulator for acquiring EMT-like and stemness phenotypes in hormone-refractory prostate cancer. Sci. Rep. 2017, 7, 42795. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Hao, J.; Li, T.G.; Qi, X.; Zhao, D.F.; Zhao, G.Q. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006, 290, 81–91. [Google Scholar] [CrossRef]
- Soundararajan, R.; Paranjape, A.N.; Maity, S.; Aparicio, A.; Mani, S.A. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim. Biophys. Acta—Rev. Cancer 2018, 1870, 229–238. [Google Scholar] [CrossRef]
- Chiaverotti, T.; Couto, S.S.; Donjacour, A.; Mao, J.H.; Nagase, H.; Cardiff, R.D.; Cunha, G.R.; Balmain, A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am. J. Pathol. 2008, 172, 236–246. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.K.; Witte, O.N.; Huang, J. FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Mod. Pathol. 2017, 30, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jin, H.; Zhao, J.C.; Yang, Y.A.; Li, Y.; Yang, X.; Dong, X.; Yu, J. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 2017, 36, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Rotinen, M.; You, S.; Yang, J.; Coetzee, S.G.; Reis-Sobreiro, M.; Huang, W.C.; Huang, F.; Pan, X.; Yáñez, A.; Hazelett, D.J.; et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat. Med. 2018, 24, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ci, X.; Ahmed, M.; Hua, J.T.; Soares, F.; Lin, D.; Puca, L.; Vosoughi, A.; Xue, H.; Li, E.; et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun. 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A. BRN2 is a neuroendocrine driver. Nat. Rev. Urol. 2017, 14, 10. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Quigley, D.A.; Huang, J.; Zhang, L.; Beer, T.M.; Rettig, M.B.; Reiter, R.E.; Gleave, M.E.; Thomas, G.V.; Foye, A.; et al. Whole-genome and transcriptional analysis of treatment-emergent small-cell neuroendocrine prostate cancer demonstrates intraclass heterogeneity. Mol. Cancer Res. MCR 2019, 17, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Clegg, N.; Ferguson, C.; True, L.D.; Arnold, H.; Moorman, A.; Quinn, J.E.; Vessella, R.L.; Nelson, P.S. Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate 2003, 55, 55–64. [Google Scholar] [CrossRef]
- Ostano, P.; Mello-Grand, M.; Sesia, D.; Gregnanin, I.; Peraldo-Neia, C.; Guana, F.; Jachetti, E.; Farsetti, A.; Chiorino, G. Gene expression signature predictive of neuroendocrine transformation in prostate adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1078. [Google Scholar] [CrossRef]
- Chang, Y.T.; Lin, T.P.; Tang, J.T.; Campbell, M.; Luo, Y.L.; Lu, S.Y.; Yang, C.P.; Cheng, T.Y.; Chang, C.H.; Liu, T.T.; et al. HOTAIR is a REST-regulated lncRNA that promotes neuroendocrine differentiation in castration resistant prostate cancer. Cancer Lett. 2018, 433, 43–52. [Google Scholar] [CrossRef]
- Mather, R.L.; Parolia, A.; Carson, S.E.; Venalainen, E.; Roig-Carles, D.; Jaber, M.; Chu, S.C.; Alborelli, I.; Wu, R.; Lin, D.; et al. The evolutionarily conserved long non-coding RNA LINC00261 drives neuroendocrine prostate cancer proliferation and metastasis via distinct nuclear and cytoplasmic mechanisms. Mol. Oncol. 2021, 15, 1921–1941. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, Q.; Liu, X.; Sun, Q.; Zhao, X.; Deng, R.; Wang, Y.; Huang, J.; Xu, M.; Yan, J.; et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014, 281, 3766–3775. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Chen, X.; Fu, C.L.; Zhang, W.; Zhang, D.L.; Pang, C.; Liu, M.; Wang, J.Y. FENDRR reduces tumor invasiveness in prostate cancer PC-3 cells by targeting CSNK1E. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7327–7337. [Google Scholar] [CrossRef]
- Zhang, G.; Han, G.; Zhang, X.; Yu, Q.; Li, Z.; Li, Z.; Li, J. Long non-coding RNA FENDRR reduces prostate cancer malignancy by competitively binding miR-18a-5p with RUNX1. Biomarkers 2018, 23, 435–445. [Google Scholar] [CrossRef]
- Chang, J.; Xu, W.; Du, X.; Hou, J. MALAT1 silencing suppresses prostate cancer progression by upregulating miR-1 and downregulating KRAS. OncoTargets Ther. 2018, 11, 3461–3473. [Google Scholar] [CrossRef]
- Luo, J.; Wang, K.; Yeh, S.; Sun, Y.; Liang, L.; Xiao, Y.; Xu, W.; Niu, Y.; Cheng, L.; Maity, S.N.; et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Venalainen, E.; Ci, X.; Cheng, H.; Pikor, L.; Parolia, A.; Xue, H.; Nur Saidy, N.R.; Lin, D.; Lam, W.; et al. The role of epigenetics and long noncoding RNA MIAT in neuroendocrine prostate cancer. Epigenomics 2016, 8, 721–731. [Google Scholar] [CrossRef]
- Ramnarine, V.R.; Alshalalfa, M.; Mo, F.; Nabavi, N.; Erho, N.; Takhar, M.; Shukin, R.; Brahmbhatt, S.; Gawronski, A.; Kobelev, M.; et al. The long noncoding RNA landscape of neuroendocrine prostate cancer and its clinical implications. GigaScience 2018, 7, giy050. [Google Scholar] [CrossRef]
- Mirza, S.; Katafiasz, B.J.; Kumar, R.; Wang, J.; Mohibi, S.; Jain, S.; Gurumurthy, C.B.; Pandita, T.K.; Dave, B.J.; Band, H.; et al. Alteration/deficiency in activation-3 (Ada3) plays a critical role in maintaining genomic stability. Cell Cycle 2012, 11, 4266–4274. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.; Rogerson, L.; Ryan-Munden, C.; Alkharaif, D.; Stockley, J.; Heer, R.; Sahadevan, K.; O’Neill, D.; Jones, D.; Darby, S.; et al. The lysine demethylase, KDM4B, is a key molecule in androgen receptor signalling and turnover. Nucleic Acids Res. 2013, 41, 4433–4446. [Google Scholar] [CrossRef]
- Yang, J.; AlTahan, A.M.; Hu, D.; Wang, Y.; Cheng, P.H.; Morton, C.L.; Qu, C.; Nathwani, A.C.; Shohet, J.M.; Fotsis, T.; et al. The role of histone demethylase KDM4B in Myc signaling in neuroblastoma. J. Natl. Cancer Inst. 2015, 107, djv080. [Google Scholar] [CrossRef]
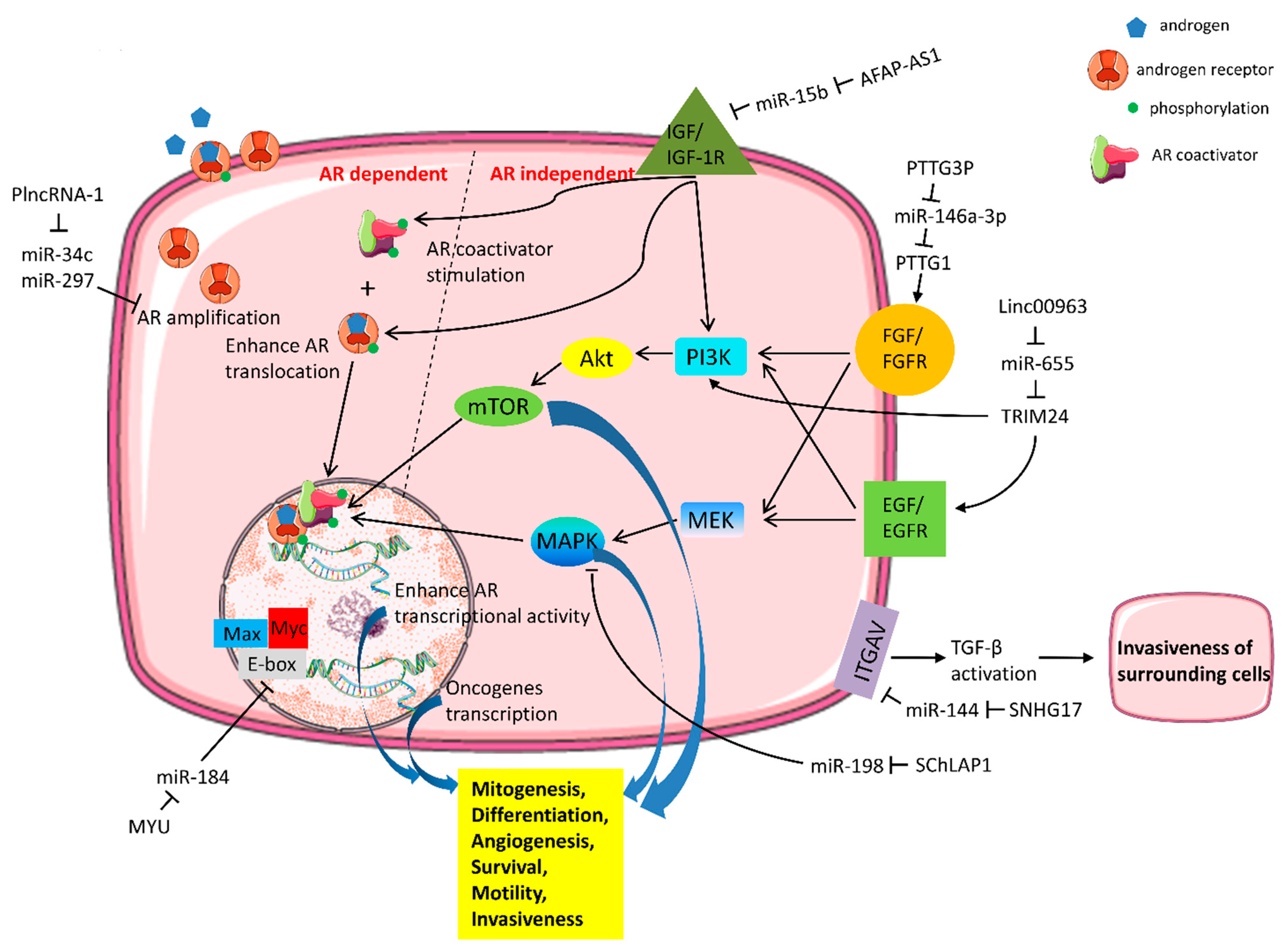

| Mechanism of Castration Resistance | Subject Investigated (Cell Lines/Tissue) | lncRNA | miRNA | Interactions | Reference | |
|---|---|---|---|---|---|---|
| AR amplification | 22RV1/human PCa | PlncRNA-1 | miR-34c/miR-297 | PlncRNA-1 promotes AR expression via competitive inhibition of miRNA-34c/miR-297 targeting AR. | [57] | |
| Increased AR transcriptional activity | PC3, DU145/human PCa | CCAT1 | miR-28-5P | In cytoplasm: competing for miR-28-5P to promote cell proliferation and colony formation. | [58] | |
| In nucleus: CCAT1 acts as a scaffold for DDX5(P68) and AR transcriptional complex to facilitate expression of AR-regulated castration resistance gene (UBE2C). | ||||||
| Signal cascades independent of AR and crosstalk with AR | Growth factors | C4-2, PC3, DU145 | AFAP1-AS1 | miR-15b | AFAP1-AS1 upregulates IGF1R by competitively binding with miR-15b to de-repress IGF1R. | [59] |
| human PCa | PTTG3P | miR-146a-3p | PTTG3P upregulates PTTG1 to stimulate FGF expression by competing for miR-146a-3p. | [60] | ||
| Other oncogenic signal pathways | PC3 | SChLAP1 | miR-198 | SChLAP1 regulates the miR-198 expression and influences cancer progression by the MAPK1 pathway. | [61] | |
| human PCa | Linc00963 | miR-655 | Linc00963 competitively binds with miR-655 and upregulates TRIM24 expression to activate the PI3K/AKT pathway. | [62] | ||
| PC3, DU145/human PCa | MYU(VPS9D1-AS1) | miR-184 | MYU upregulates the MYC expression by competitively binding with miR-184. | [63] | ||
| Cell cycle dysregulation | DU145/human PCa | SNHG7 | miR-503 | MiR-503 targets 3′-UTR of SNHG and inhibits cell cycle proteins (CDK4, CDK6, Cyclin D), inducing G0/G1 cell cycle arrest. | [64] | |
| PC3, DU145 | LOXL1-AS1 | miR-541-3p | LOXL1-AS1 interferes with miR-541-3p targeting cell cycle regulator Cyclin D and promotes cell proliferation. | [65] | ||
| DU145/human PCa | TTTY15 | let-7 | FOXA1, acting as a transcription factor of TTTY15, promotes PCa progression by sponging let-7 and upregulating CDK6 and FN1. | [66] | ||
| Cytokine | C4-2, PC3, DU145 | SNHG17 | miR-144 | SNHG17 acts as a ceRNA to upregulate CD51 (integrin alpha-V) expression through competitively sponging miR-144. | [67] | |
| Unidentified mechanisms | PC3, DU145 | HOTAIR | miR-34a | MiR-34a directly targets HOTAIR and inhibits cell growth. | [68] | |
| PC3 | HOTAIR | miR-193a | HOTAIR couples with EZH2 to repress miR-193a by trimethylation of H3K27me3; miR-193a directly targets HOTAIR to reduce HOTAIR level in miR-193a overexpressed cells. | [69] | ||
| PC3 | PCGEM-1 | miR-148a | Putative PCGEM1 binding site is identified in the 5′-UTR of miR-148a; PCGEM-1 expression represses miR-148a and cell apoptosis. | [70] | ||
| PC3, DU145/human PCa | PCSEAT | miR-143-3p-/miR-24-2-5p | PCSEAT competitively sponges miR-143-3p/miR-24-2-5p and decreases PCSEAT-mediated cell proliferation. | [71] | ||
| PC3, DU145/human PCa | Linc00308 | miR-137 | LncRNA-miRNA-mRNA networks regulate tumor suppressor gene PTGS2 and DUSP2 and affect survival. | [72] | ||
| Linc00355 | miR-122/miR-506 | |||||
| OSTN-AS1 | miR-137/miR-506 | |||||
| The Interplay between lncRNA and miRNA | ||||
|---|---|---|---|---|
| lncRNA | miRNA | Target | Pathway and Influence | Reference |
| HOTAIR | miR-31-5p | REST, EZH2 | A direct interaction network among AR, HOTAIR, ESR1, and miR-31-5p was proposed. MiR-31-5p inhibits the expression of AR, and then AR transcriptionally inhibits HOTAIR. | [111,112] |
| LINC00261 | miR-8485 | CBX2, FOXA2 | In the cytoplasm, LINC00261 binds to miR-8485, which reduces the inhibition of CBX2. In the nucleus, LINC00261 activates FOXA2 expression via the SMAD2/3 transcriptional complex. | [113] |
| H19 | miR-675 | TGF-β1 | H19 and miR-675 negatively regulate the expression of TGFβ1, inhibiting prostate cancer migration. | [114] |
| FENDRR (FOXF1-AS1) | miR-301b-3p | CSNK1E, PRC2 | Reduce tumor invasion by targeting CSNK1E. | [115] |
| miR-18a-5p | RUNX1 | FENDRR inhibits tumor cell proliferation by binding to miR-18a-5p with RUNX1. | [116] | |
| MALAT1 | miR-1 | MALAT1 acts as a sponge of miR-1, resulting in downregulating KRAS in AR independent prostate cancer. | [117] | |
| The interplay between lncRNA and epigenetic regulators | ||||
| LncRNA-p21 | EZH2 | LncRNA-p21 promotes EZH2 to enhance STAT3 methylation and drives neuroendocrine transdifferentiation. | [118] | |
| MIAT | Polycomb genes | MIAT interacts with polycomb genes and is positively associated with Rb mutation and CBX2 expression. | [111,119] | |
| LINC00514 | TADA3 | LINC00514 interacts with TADA3 and reduces p53 activity. | [120] | |
| SSTR5-AS1 | KDM4B | SSTR5-AS1 binds with KDM4B. KDM4B interacts with N-Myc, which drives the progression of NEPC. | [120] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.-Y.; Wu, K.-Y.; Lin, T.-Y.; Chen, C.-C. The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance. Int. J. Mol. Sci. 2022, 23, 392. https://doi.org/10.3390/ijms23010392
Hu C-Y, Wu K-Y, Lin T-Y, Chen C-C. The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance. International Journal of Molecular Sciences. 2022; 23(1):392. https://doi.org/10.3390/ijms23010392
Chicago/Turabian StyleHu, Che-Yuan, Kuan-Yu Wu, Tsung-Yen Lin, and Chien-Chin Chen. 2022. "The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance" International Journal of Molecular Sciences 23, no. 1: 392. https://doi.org/10.3390/ijms23010392
APA StyleHu, C.-Y., Wu, K.-Y., Lin, T.-Y., & Chen, C.-C. (2022). The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance. International Journal of Molecular Sciences, 23(1), 392. https://doi.org/10.3390/ijms23010392







