Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway
Abstract
1. Introduction
2. Results
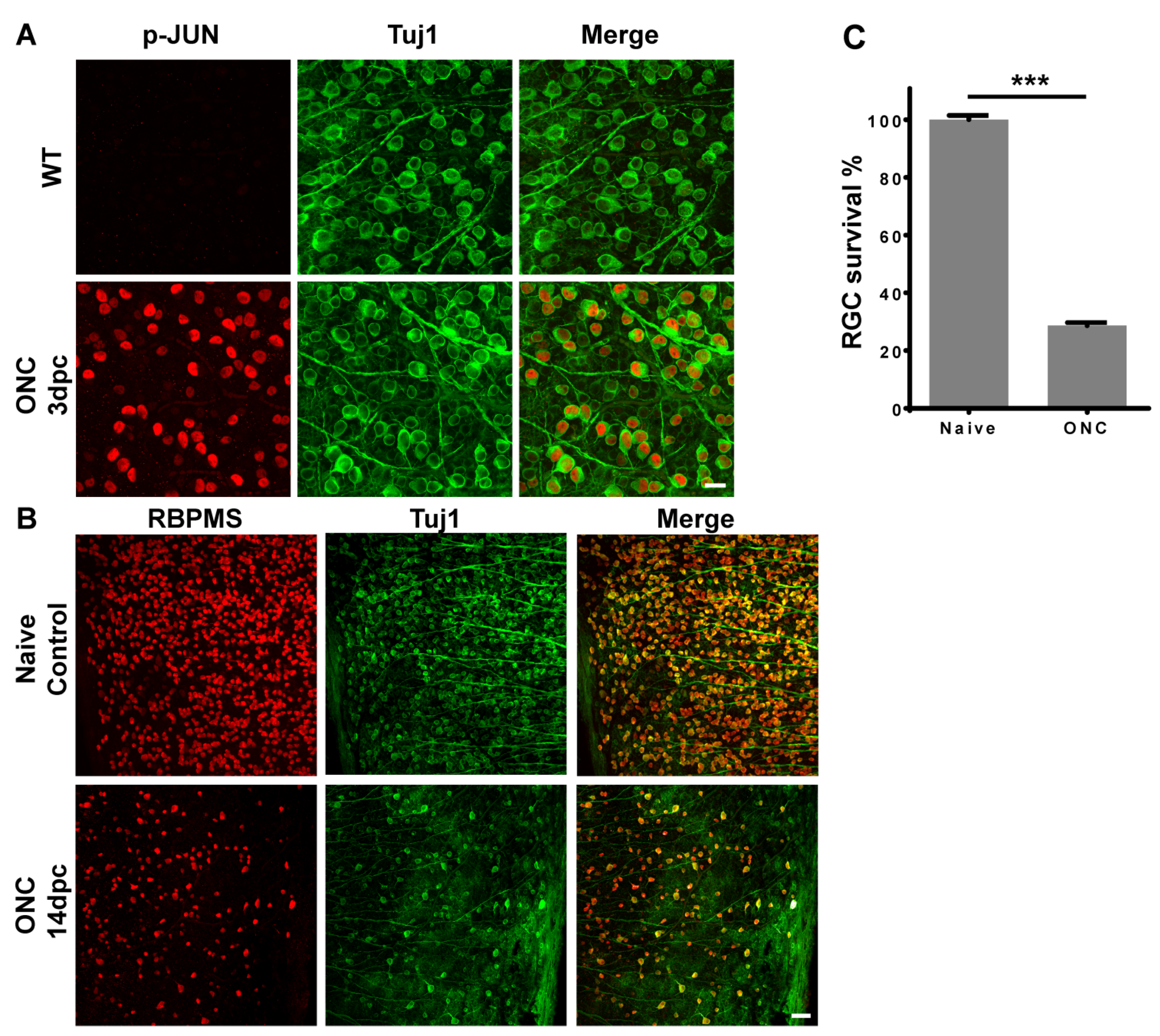
2.1. Optic Nerve Injury Activated JNK-JUN Pathway and Caused RGC Death
2.2. Effects of Citrus Components on Inhibiting Stress Induced Pro-Apoptotic JNK-JUN Pathway
2.3. Effects of Citrus Components on RGC Survival after Optic Nerve Injury
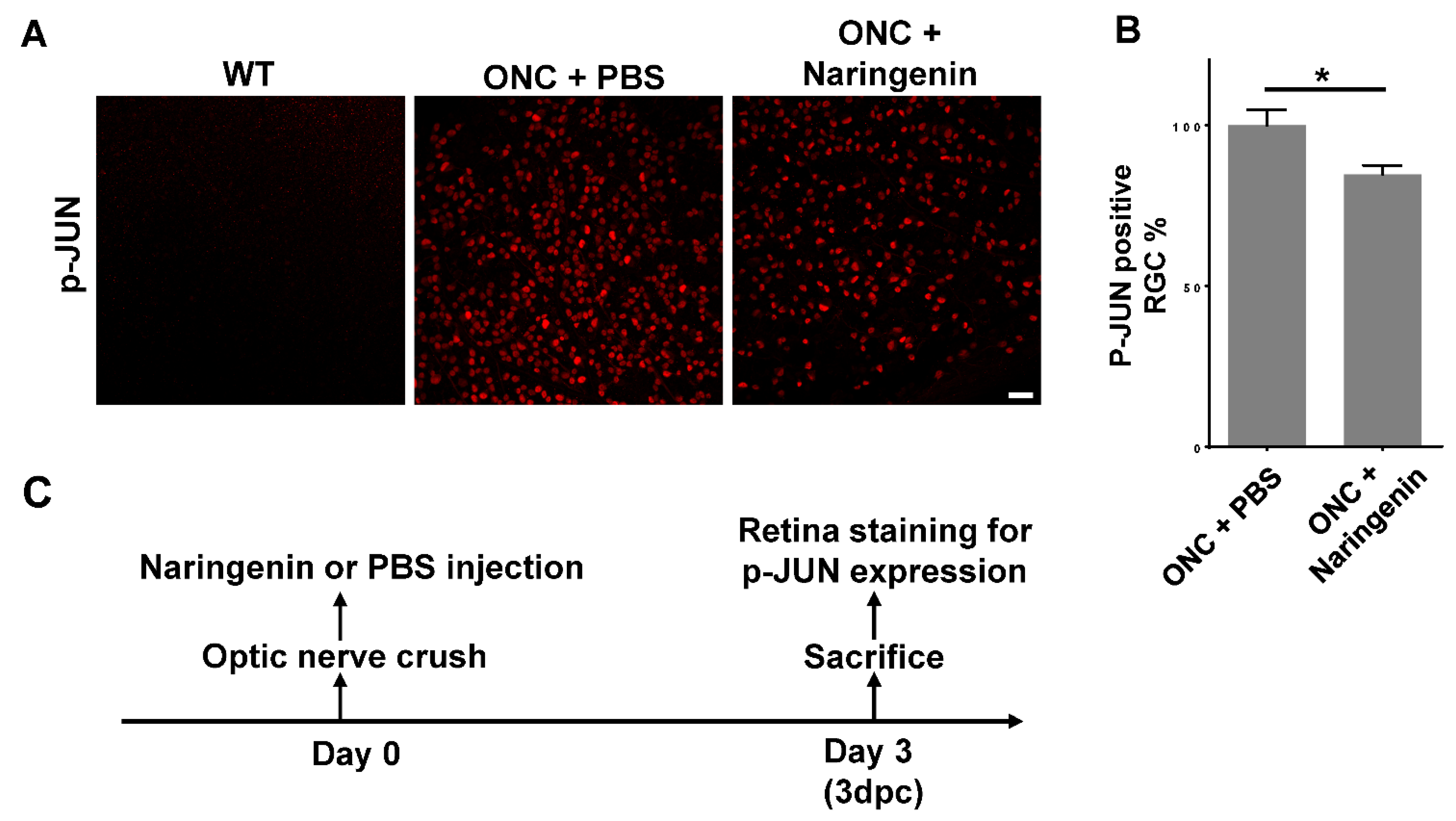
2.4. Naringenin Promoted RGC Survival by Inhibiting JNK-JUN Pathway In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Optic Nerve Crush
4.4. Intravitreal Injection
4.5. Cell Cultures and Treatments
4.6. Cell Toxicity Analysis of Compounds
4.7. Western Blot
4.8. Retina Immunohistochemistry Staining and RGC Counting
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Cai, H.; Zhan, Z.; Luo, Y.; Huang, Y.; Chen, D.; Chen, B. Herbal medicine (zhishi xiebai guizhi decoction) for unstable angina: Protocol for a systematic review and meta-analysis. Medicine 2018, 97, e13965. [Google Scholar] [CrossRef]
- Putri, H.; Nagadi, S.; Larasati, Y.A.; Wulandari, N.; Hermawan, A.; Nugroho, A.E. Cardioprotective and hepatoprotective effects of Citrus hystrix peels extract on rats model. Asian Pac. J. Trop. Biomed. 2013, 3, 371–375. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Wang, M.; He, D.; Liu, L.; Shu, Y.; Song, Z.; Li, H.; Liu, Y.; Lu, A. Anti-inflammatory effects of Zhishi and Zhiqiao revealed by network pharmacology integrated with molecular mechanism and metabolomics studies. Phytomedicine 2018, 50, 61–72. [Google Scholar] [CrossRef]
- Wang, J.; Ying, Q.; Niu, X.L.; Tang, H.; Meydani, S.N.; Wu, D.Y. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2017, 54, 130–139. [Google Scholar] [CrossRef]
- Tiza, N.U.; Thato, M.; Raymond, D.; Jeremy, K.; Burtram, C.F. Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus. Afr. J. Microbiol. Res. 2015, 9, 1513–1518. [Google Scholar] [CrossRef][Green Version]
- Oluyede, D.M.; Lawal, A.O.; Adebimpe, M.O.; Olumegbon, L.T.; Elekofehinti, O.O. Biochemical and molecular effects of naringenin on the cardiovascular oxidative and pro-inflammatory effects of oral exposure to diesel exhaust particles in rats. Air Qual. Atmos. Health 2021, 14, 935–953. [Google Scholar] [CrossRef]
- Du, G.Y.; He, S.W.; Lu, Z.; Sun, C.X.; Mi, L.D.; Sun, Z.G. Hesperidin exhibits in vitro and in vivo antitumor effects in human osteosarcoma MG-63 cells and xenograft mice models via inhibition of cell migration and invasion, cell cycle arrest and induction of mitochondrial-mediated apoptosis. Oncol. Lett. 2018, 16, 6299–6306. [Google Scholar] [CrossRef]
- Chen, P.; Fan, R.S.; Qian, N. Synthesis and Structure Characterization of Water-soluble Hesperidin. Food Sci. 2007, 28, 143–147. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.-H.; Yun, S.-H.; Kim, S.-J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.Q.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923–3938. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Liu, L.; Yang, X.; Wu, F.; Gan, X.; Zhang, R.; He, Y.; Lv, Q.; Fu, H.; et al. Acupuncture Treatment Reverses Retinal Gene Expression Induced by Optic Nerve Injury via RNA Sequencing Analysis. Front. Integr. Neurosci. 2019, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Harder, J.M.; Kim, J.; Libby, R.T. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp. Eye Res. 2013, 112, 106–117. [Google Scholar] [CrossRef]
- Welsbie, D.S.; Mitchell, K.L.; Jaskula-Ranga, V.; Sluch, V.M.; Yang, Z.; Kim, J.; Buehler, E.; Patel, A.; Martin, S.E.; Zhang, P.-W.; et al. Enhanced Functional Genomic Screening Identifies Novel Mediators of Dual Leucine Zipper Kinase-Dependent Injury Signaling in Neurons. Neuron 2017, 94, 1142–1154.e6. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.T.; Courtney, M.J. Regulation of SAPKs in CNS neurons. Biochem. Soc. Trans. 1997, 25, S568. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Metzler, B.; Xu, Q. Discordant activation of stress-activated protein kinases or c-Jun NH2-terminal protein kinases in tissues of heat-stressed mice. J. Biol. Chem. 1997, 272, 9113–9119. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Davis, R.J. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem. Sci. 1998, 23, 481–485. [Google Scholar] [CrossRef]
- Syc-Mazurek, S.B.; Fernandes, K.A.; Libby, R.T. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis. 2017, 8, e2945. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Harder, J.M.; Fornarola, L.B.; Freeman, R.S.; Clark, A.F.; Pang, I.H.; John, S.W.; Libby, R.T. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol. Dis. 2012, 46, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Miao, L.; Liang, F.; Liu, X.; Xu, L.; Teng, X.; Wang, Q.; Ridder, W.H.; Shindler, K.S.; Sun, Y.; et al. Neuroprotection by eIF2alpha-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017, 8, e2936. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Miao, L.; Huang, H.; Liang, F.; Teng, X.; Xu, L.; Wang, Q.; Xiao, W.; Ridder, W.H.; et al. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. J. Neurosci. 2016, 36, 5891–5903. [Google Scholar] [CrossRef] [PubMed]
- Syc-Mazurek, S.B.; Fernandes, K.A.; Wilson, M.P.; Shrager, P.; Libby, R.T. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Mol. Neurodegener. 2017, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Bagowski, C.P.; Besser, J.; Frey, C.R.; Ferrell, J.E., Jr. The JNK cascade as a biochemical switch in mammalian cells: Ultrasensitive and all-or-none responses. Curr. Biol. 2003, 13, 315–320. [Google Scholar] [CrossRef][Green Version]
- Jin, Y.; Wang, H. Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury through Nrf2/HO-1 Pathway. Neurotox. Res. 2019, 36, 796–805. [Google Scholar] [CrossRef]
- Shin, J.E.; Cho, Y.; Beirowski, B.; Milbrandt, J.; Cavalli, V.; DiAntonio, A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012, 74, 1015–1022. [Google Scholar] [CrossRef]
- Holland, S.M.; Collura, K.M.; Ketschek, A.; Noma, K.; Ferguson, T.A.; Jin, Y.; Gallo, G.; Thomas, G.M. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 763–768. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Cell Autonomous Neuroprotection by the Mitochondrial Uncoupling Protein 2 in a Mouse Model of Glaucoma. Front. Neurosci. 2019, 13, 201. [Google Scholar] [CrossRef]
- Bray, E.R.; Yungher, B.J.; Levay, K.; Ribeiro, M.; Dvoryanchikov, G.; Ayupe, A.C.; Thakor, K.; Marks, V.; Randolph, M.; Danzi, M.C.; et al. Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron 2019, 103, 642–657.e7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, H.; Yang, C.; He, Y.; Arai, T.; Huang, Q.; Liu, X.; Miao, L. Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway. Int. J. Mol. Sci. 2022, 23, 385. https://doi.org/10.3390/ijms23010385
Chen J, Li H, Yang C, He Y, Arai T, Huang Q, Liu X, Miao L. Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway. International Journal of Molecular Sciences. 2022; 23(1):385. https://doi.org/10.3390/ijms23010385
Chicago/Turabian StyleChen, Jie, Hui Li, Changming Yang, Yinjia He, Tatsuo Arai, Qiang Huang, Xiaodong Liu, and Linqing Miao. 2022. "Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway" International Journal of Molecular Sciences 23, no. 1: 385. https://doi.org/10.3390/ijms23010385
APA StyleChen, J., Li, H., Yang, C., He, Y., Arai, T., Huang, Q., Liu, X., & Miao, L. (2022). Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway. International Journal of Molecular Sciences, 23(1), 385. https://doi.org/10.3390/ijms23010385





