Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis
Abstract
:1. Introduction
2. Results
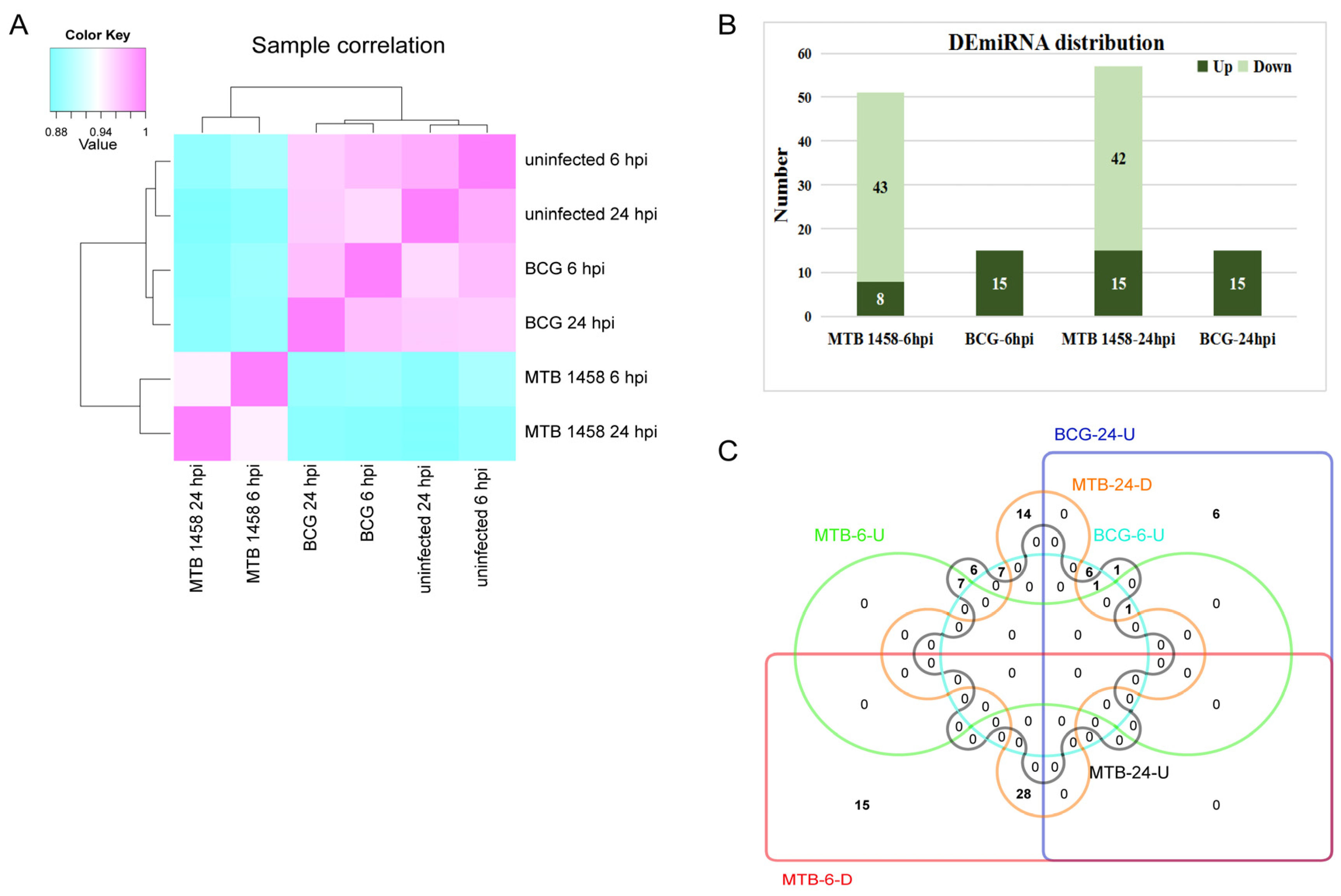
2.1. Expression Profiles of miRNAs in THP-1 Cells after Infection
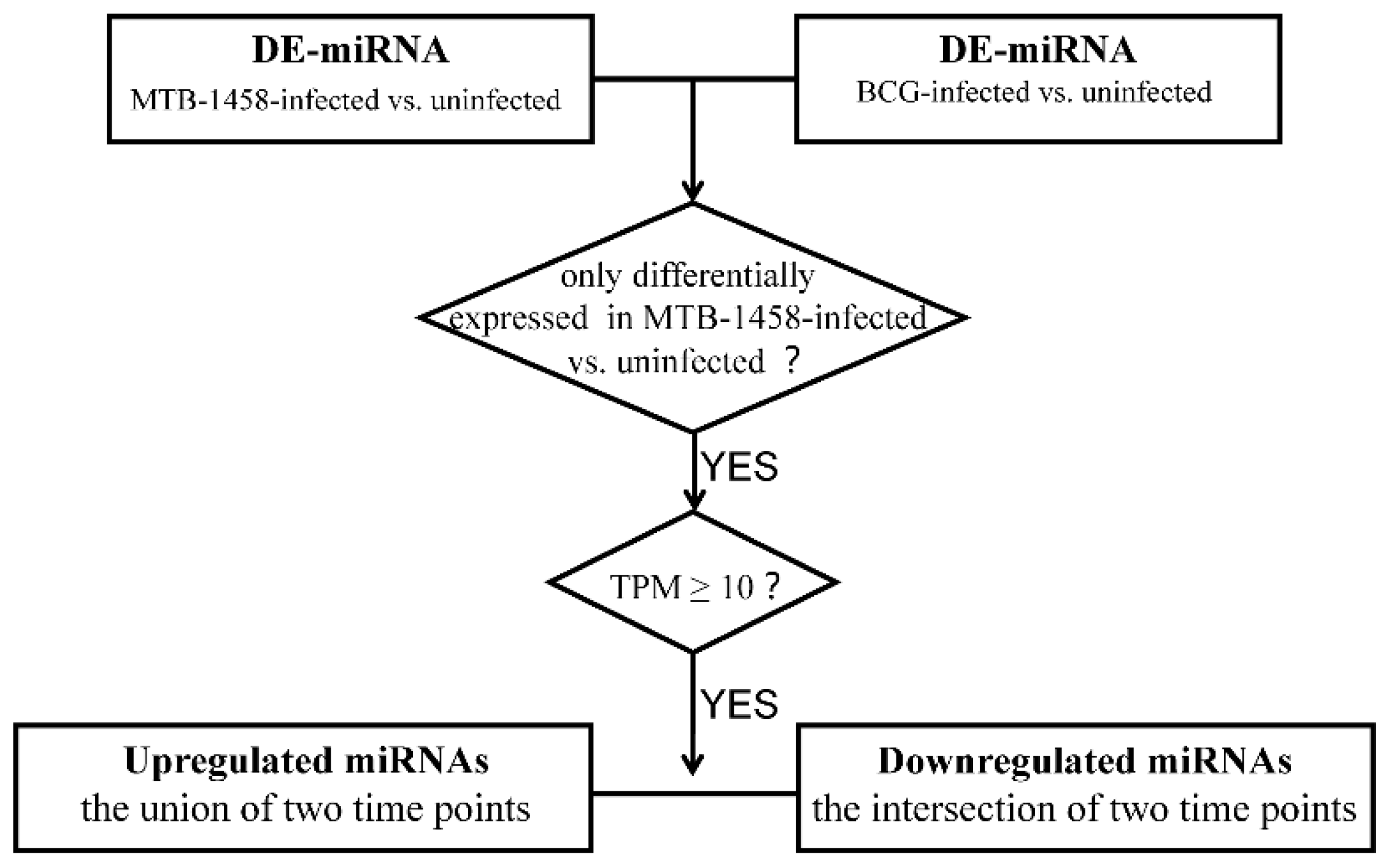
2.2. Screening miRNAs Only Differentially Expressed in MTB-1458 Groups
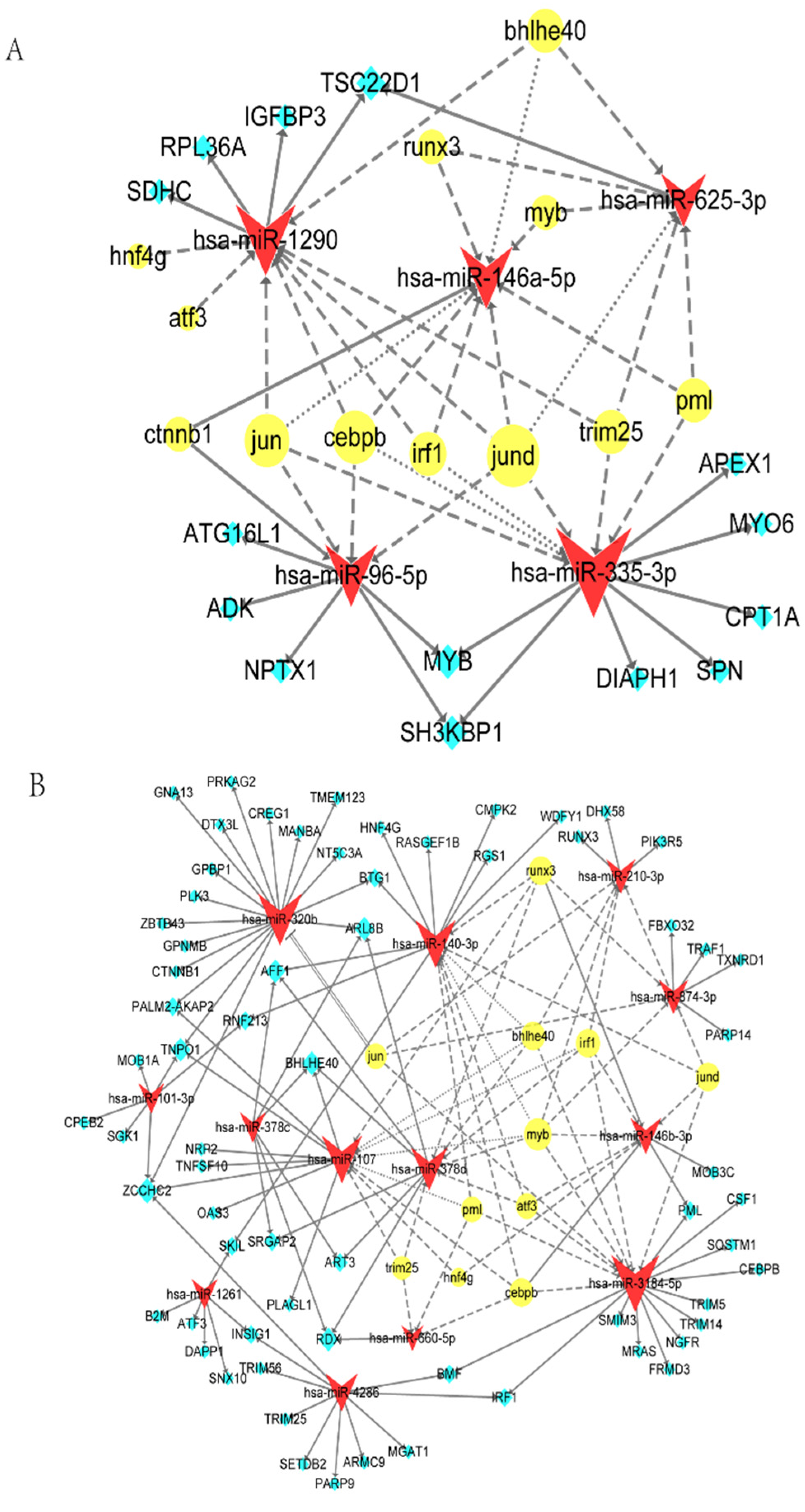
2.3. Target and TF Prediction of Unique DE-miRNAs in MTB-1458 Groups
2.4. Gene Ontology (GO) Classification and Kyoto Encyclopedia of Genes and Genomes (KEGG Pathways) Pathway Enrichment Analysis of the Candidate Targets
2.5. TF-miRNA-mRNA Network
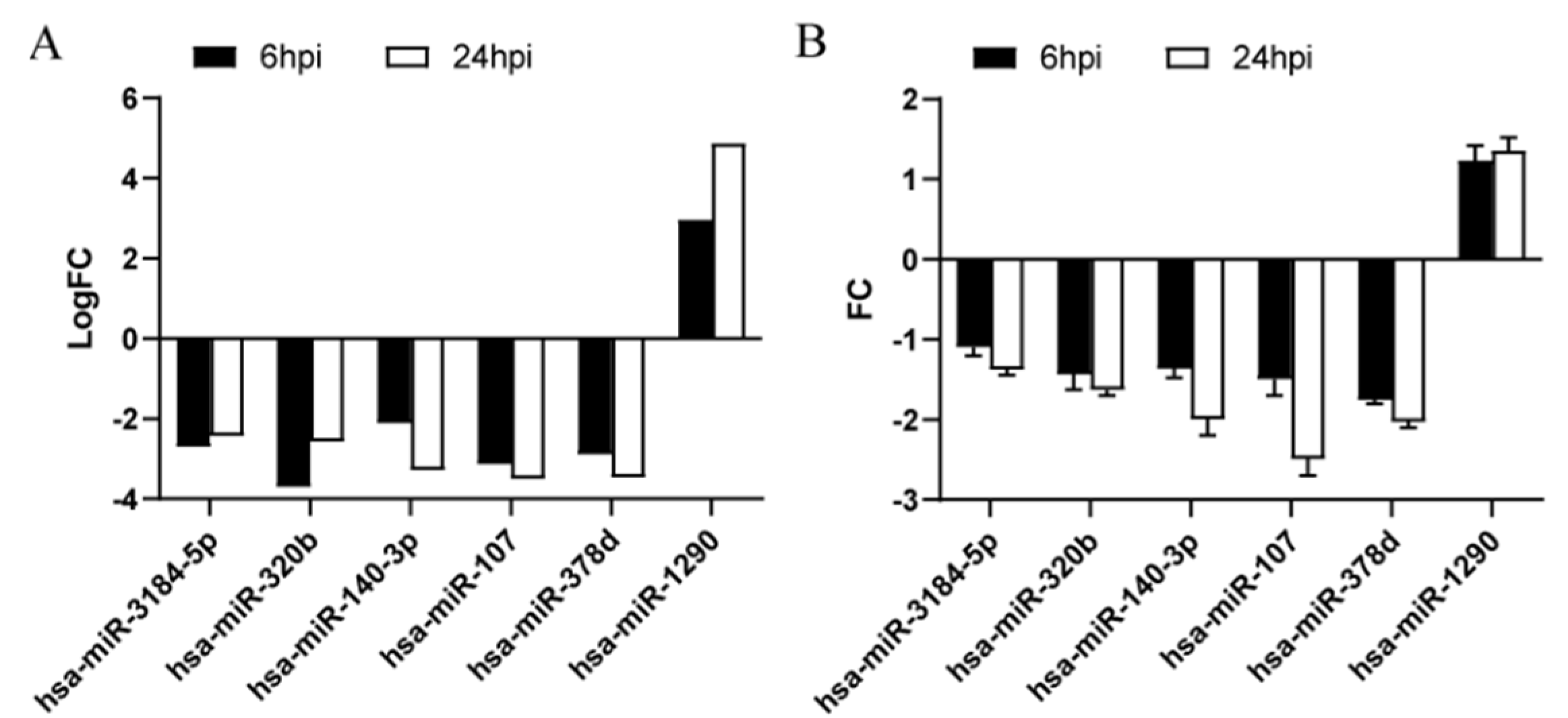
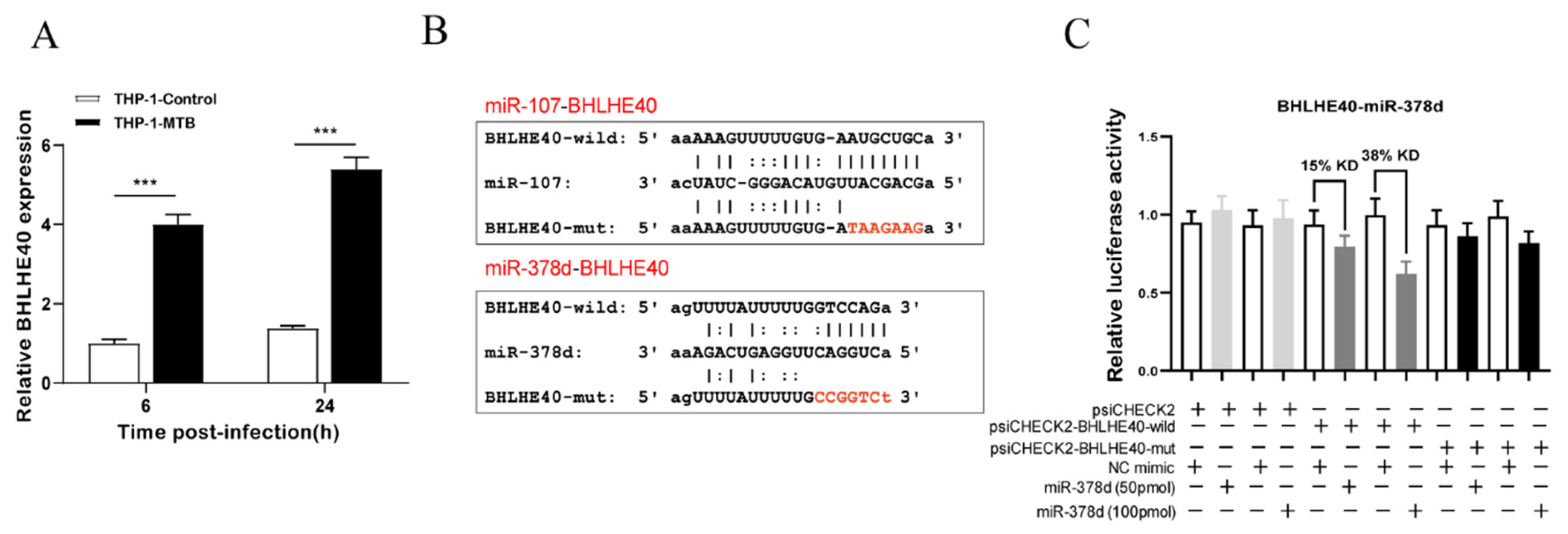
2.6. Key DE-miRNA Validation by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. BHLHE40-miR-378d-BHLHE40 Regulation Axis Verification
3. Discussion
3.1. miRNA Expression Profile Comparison between MTB-1458-Infected Macrophages and BCG-Infected Macrophages
3.2. Key DE-miRNAs According to the TF-miRNA-mRNA Network
4. Materials and Methods
4.1. Bacterial Strains and Culture
4.2. Cell Culture and Infection
4.3. Total RNA Extraction and Integrity Analysis
4.4. miRNA Profiling by High-Throughput Sequencing
4.5. Bioinformatic Analysis
4.6. miRNAs Screening Differentially Expressed Only in MTB-1458 Groups
4.7. miRNA and mRNA Expression Validation by qRT-PCR
4.8. Identification of the Relationship between BHLHE40 and miR-378d with Dual-Luciferase Reporter Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Zhang, X.; Li, S.; Luo, Y.; Chen, Y.; Cheng, S.; Zhang, G.; Hu, C.; Chen, H.; Guo, A. Mycobacterium bovis and BCG induce different patterns of cytokine and chemokine production in dendritic cells and differentiation patterns in CD4+ T cells. Microbiology 2013, 159, 366–379. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [Green Version]
- Golby, P.; Villarreal-Ramos, B.; Dean, G.; Jones, G.J.; Vordermeier, M. MicroRNA expression profiling of PPD-B stimulated PBMC from M. bovis-challenged unvaccinated and BCG vaccinated cattle. Vaccine 2014, 32, 5839–5844. [Google Scholar] [CrossRef]
- Fu, Y.; Yi, Z.; Li, J.; Li, R. Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J. Cell. Mol. Med. 2014, 18, 503–513. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chen, Y.M.; Lin, C.F.; Lo, C.M.; Liu, H.J.; Liao, T.L. MicroRNA-889 Inhibits Autophagy To Maintain Mycobacterial Survival in Patients with Latent Tuberculosis Infection by Targeting TWEAK. mBio 2020, 11, e03045-19. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Zhou, C.; Xiong, W.; Su, J.; He, J.; Zhang, S.; Du, X.; Liu, S.; Wang, J.; Ma, L. MiR-381-3p Regulates the Antigen-Presenting Capability of Dendritic Cells and Represses Antituberculosis Cellular Immune Responses by Targeting CD1c. J. Immunol. 2016, 197, 580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Yi, Z.; Fu, Y. Downregulation of miR-20b-5p facilitates Mycobacterium tuberculosis survival in RAW 264.7 macrophages via attenuating the cell apoptosis by Mcl-1 upregulation. J. Cell. Biochem. 2019, 120, 5889–5896. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Yang, R.; Zhao, W.; Hu, X.; Gan, J. Identification and comparison of novel circular RNAs with associated co-expression and competing endogenous RNA networks in pulmonary tuberculosis. Oncotarget 2017, 8, 113571–113582. [Google Scholar] [CrossRef] [Green Version]
- Furci, L.; Schena, E.; Miotto, P.; Cirillo, D.M. Alteration of human macrophages microRNA expression profile upon infection with Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2013, 2, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Saikolappan, S.; Dhandayuthapani, S. Differential expression of miRNAs by macrophages infected with virulent and avirulent Mycobacterium tuberculosis. Tuberculosis 2013, 93, S47–S50. [Google Scholar] [CrossRef]
- Huynh, J.P.; Lin, C.C.; Kimmey, J.M.; Jarjour, N.N.; Stallings, C.L. Bhlhe40 is an essential repressor of IL-10 during Mycobacterium tuberculosis infection. J. Exp. Med. 2018, 215, 1823–1838. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, C.; Wazir, J.; Su, Z.; Niu, M.; Song, S.; Wei, L.; Li, L.; Zhang, X.; Shi, X.; et al. Comparative transcriptomic analysis of THP-1-derived macrophages infected with Mycobacterium tuberculosis H37Rv, H37Ra and BCG. J. Cell. Mol. Med. 2021, 25, 10504–10520. [Google Scholar] [CrossRef]
- Yuan, Z.; Prasla, Z.; Lee, F.E.; Bedi, B.; Sutliff, R.L.; Sadikot, R.T. MicroRNA-155 Modulates Macrophages’ Response to Non-Tuberculous Mycobacteria through COX-2/PGE2 Signaling. Pathogens 2021, 10, 920. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, J.; Yan, J.; Mao, E.; Chen, H.; Wang, C. Circ_0001490/miR-579-3p/FSTL1 axis modulates the survival of mycobacteria and the viability, apoptosis and inflammatory response in Mycobacterium tuberculosis-infected macrophages. Tuberculosis 2021, 131, 102123. [Google Scholar] [CrossRef]
- Fu, B.; Lin, X.; Tan, S.; Zhang, R.; Xue, W.; Zhang, H.; Zhang, S.; Zhao, Q.; Wang, Y.; Feldman, K.; et al. MiR-342 controls Mycobacterium tuberculosis susceptibility by modulating inflammation and cell death. EMBO Rep. 2021, 22, e52252. [Google Scholar] [CrossRef]
- Qu, Y.; Gao, Q.; Wu, S.; Xu, T.; Jiang, D.; Xu, G. MicroRNA-142-3p inhibits autophagy and promotes intracellular survival of Mycobacterium tuberculosis by targeting ATG16L1 and ATG4c. Int. Immunopharmacol. 2021, 101, 108202. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Fang, Y.; Gong, S.; Li, M.; Wu, M.; Lai, X.; Zeng, G.; Wang, Y.; Yang, K.; et al. microRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci. Rep. 2016, 6, 23351. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yue, Y.; Xu, W.; Xiong, S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS ONE 2013, 8, e81438. [Google Scholar] [CrossRef]
- Li, X.; Huang, S.; Yu, T.; Liang, G.; Liu, H.; Pu, D.; Peng, N. MiR-140 modulates the inflammatory responses of Mycobacterium tuberculosis-infected macrophages by targeting TRAF6. J. Cell. Mol. Med. 2019, 23, 5642–5653. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Xiao, Y.; Kong, D.; Liu, H.; Chen, X.; Chen, Y.; Zhu, T.; Peng, Y.; Zhai, W.; Hu, C.; et al. Down-Regulation of miR-378d Increased Rab10 Expression to Help Clearance of Mycobacterium tuberculosis in Macrophages. Front. Cell. Infect. Microbiol. 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.L.; Pi, J.; Zhang, J.A.; Yang, E.Z.; Xu, H.; Luo, H.; Shen, L.; Peng, Y.; Liu, G.B.; Song, C.M.; et al. Circular RNA TRAPPC6B inhibits intracellular Mycobacterium tuberculosis growth while inducing autophagy in macrophages by targeting microRNA-874-3p. Clin. Transl. Immunol. 2021, 10, e1254. [Google Scholar] [CrossRef]
- Ni, B.; Rajaram, M.V.; Lafuse, W.P.; Landes, M.B.; Schlesinger, L.S. Mycobacterium tuberculosis decreases human macrophage IFN-gamma responsiveness through miR-132 and miR-26a. J. Immunol. 2014, 193, 4537–4547. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhang, Y.; Yu, H.; Tian, R.; Li, F. Identification of unique key genes and miRNAs in latent tuberculosis infection by network analysis. Mol. Immunol. 2019, 112, 103–114. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, J.; Liu, X.; Xue, Y.; Liu, L.; Ma, J.; He, Q.; Li, Z.; Cai, H.; Liu, Y. Knockdown of USF1 Inhibits the Vasculogenic Mimicry of Glioma Cells via Stimulating SNHG16/miR-212-3p and linc00667/miR-429 Axis. Mol. Ther.-Nucleic Acids 2019, 14, 465–482. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Kim, E.H.; Lee, Y.J.; Sai, S.; Lim, S.H.; Park, J.W.; Chung, H.K.; Kim, J.; Vares, G.; Takahashi, A.; et al. Synergistic Autophagy Effect of miR-212-3p in Zoledronic Acid-Treated In Vitro and Orthotopic In Vivo Models and in Patient-Derived Osteosarcoma Cells. Cancers 2019, 11, 1812. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Zw, A.; Rui, Z.A.; Wei, X.A.; Yy, A.; Ning, S.A.; Jq, A. Dexmedetomidine exerts cardioprotective effect through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the NF-κB pathway-ScienceDirect. Biomed. Pharmacother. 2021, 133, 110993. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L. Depleting microRNA-146a-3p attenuates lipopolysaccharide-induced acute lung injury via up-regulating SIRT1 and mediating NF-κB pathway. J. Drug Target. 2021, 29, 420–429. [Google Scholar] [CrossRef]
- Keane, J.; Remold, H.G.; Kornfeld, H. Virulent Mycobacterium tuberculosis Strains Evade Apoptosis of Infected Alveolar Macrophages. J. Immunol. 2000, 164, 2016–2020. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 21, 2879–2888. [Google Scholar] [CrossRef] [Green Version]
- Schoenen, H.; Bodendorfer, B.; Hitchens, K.; Manzanero, S.; Werninghaus, K.; Nimmerjahn, F.; Agger, E.M.; Stenger, S.; Andersen, P.; Ruland, J. Cutting Edge: Mincle Is Essential for Recognition and Adjuvanticity of the Mycobacterial Cord Factor and its Synthetic Analog Trehalose-Dibehenate. J. Immunol. 2010, 184, 2756–2760. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.B.; Azad, A.K.; Torrelles, J.B.; Kaufman, T.M.; Beharka, A.; Tibesar, E.; DesJardin, L.E.; Schlesinger, L.S. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 2005, 202, 987–999. [Google Scholar] [CrossRef]
- Rajaram, M.V.S.; Brooks, M.N.; Morris, J.D.; Torrelles, J.B.; Azad, A.K.; Schlesinger, L.S. Mycobacterium tuberculosis Activates Human Macrophage Peroxisome Proliferator-Activated Receptor gamma Linking Mannose Receptor Recognition to Regulation of Immune Responses. J. Immunol. 2010, 185, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Shah, P.K.; Amin, S.B.; Samur, M.K.; Huang, N.; Wang, X.; Misra, V.; Ji, H.; Gabuzda, D.; Li, C. Integrative analysis of gene and miRNA expression profiles with transcription factor-miRNA feed-forward loops identifies regulators in human cancers. Nucleic Acids Res. 2012, 40, e135. [Google Scholar] [CrossRef]
- Du, X.; Li, Q.; Yang, L.; Zeng, Q.; Wang, S.; Li, Q. Transcriptomic Data Analyses Reveal That Sow Fertility-Related lincRNA NORFA Is Essential for the Normal States and Functions of Granulosa Cells. Front. Cell Dev. Biol. 2021, 9, 610553. [Google Scholar] [CrossRef]
- Zandi, E.; Ayatollahi Mehrgardi, A.; Esmailizadeh, A. Mammary tissue transcriptomic analysis for construction of integrated regulatory networks involved in lactogenesis of Ovis aries. Genomics 2020, 112, 4277–4287. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, G.; Deng, X.; Yu, Q.; Hu, Y.; Sun, H.; Wang, Z.; Chen, H.; Jia, C.; Wang, D. Analysis of miRNA expression profiling in human macrophages responding to Mycobacterium infection: Induction of the immune regulator miR-146a. J. Infect. 2014, 68, 553–561. [Google Scholar] [CrossRef]
- Hirai, S.I.; Ryseck, R.P.; Mechta, F.; Bravo, R.; Yaniv, M. Characterization of junD: A new member of the jun proto-oncogene family. EMBO J. 1989, 8, 1433. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, Y.; Zhou, Y.; Zhou, Z.; Yan, W. LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis. 2019, 10, 472. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, T.; Yang, Y.; Kang, W.; Cheng, S. YY1-induced upregulation of FOXP4-AS1 and FOXP4 promote the proliferation of esophageal squamous cell carcinoma cells. Cell Biol. Int. 2020, 44, 1447–1457. [Google Scholar] [CrossRef]
- Roy, S.; Guler, R.; Parihar, S.P.; Schmeier, S.; Kaczkowski, B.; Nishimura, H.; Shin, J.W.; Negishi, Y.; Ozturk, M.; Hurdayal, R. Batf2/Irf1 Induces Inflammatory Responses in Classically Activated Macrophages, Lipopolysaccharides, and Mycobacterial Infection. J. Immunol. 2015, 194, 6035–6044. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kumar, M.; Chakraborty, S.; Banerjee, S.K.; Kumar, R.; Gupta, P.; Jana, K.; Gupta, U.D.; Ghosh, Z.; Kundu, M.; et al. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPbeta regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 2017, 13, e1006410. [Google Scholar] [CrossRef]
- Sampath, P.; Periyasamy, K.M.; Ranganathan, U.D.; Bethunaickan, R. Monocyte and Macrophage miRNA: Potent Biomarker and Target for Host-Directed Therapy for Tuberculosis. Front. Immunol. 2021, 12, 667206. [Google Scholar] [CrossRef]
- Wallis, R.S.; Hafner, R. Advancing host-directed therapy for tuberculosis. Nat. Rev. Immunol. 2015, 15, 255–263. [Google Scholar] [CrossRef]
- Iannaccone, M.; Dorhoi, A.; Kaufmann, S.H. Host-directed therapy of tuberculosis: What is in it for microRNA? Expert Opin. Ther. Targets 2014, 18, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Aqdas, M.; Maurya, S.K.; Pahari, S.; Singh, S.; Khan, N.; Sethi, K.; Kaur, G.; Agrewala, J.N. Immunotherapeutic Role of NOD-2 and TLR-4 Signaling as an Adjunct to Antituberculosis Chemotherapy. ACS Infect. Dis. 2021, 7, 2999–3008. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Tu, L.; Xiong, X.; Hu, X.; Huang, J.; Xu, Z.; Zhang, X.; Hu, C.; Hu, X.; et al. (1)H-NMR spectroscopy revealed Mycobacterium tuberculosis caused abnormal serum metabolic profile of cattle. PLoS ONE 2013, 8, e74507. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Wang, R.; Deng, D.; Chen, Y.; Liu, H.; Wang, T.; Wang, J.; Zhu, X.; Zhu, X.; Zhu, Y.; et al. Comparative Genomics of a Bovine Mycobacterium tuberculosis Isolate and Other Strains Reveals Its Potential Mechanism of Bovine Adaptation. Front. Microbiol. 2017, 8, 2500. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, H.; Su, L.; Xiong, X.; Wang, J.; Xiao, Y.; Zhu, Y.; Peng, Y.; Dawood, A.; Hu, C.; et al. MicroRNA-18b-5p Downregulation Favors Mycobacterium tuberculosis Clearance in Macrophages via HIF-1alpha by Promoting an Inflammatory Response. ACS Infect. Dis. 2021, 7, 800–810. [Google Scholar] [CrossRef]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Cui, Q.; Wang, J.; Zhou, Y. TransmiR v2.0: An updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019, 47, D253–D258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2008, 37, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316-22. [Google Scholar] [CrossRef] [Green Version]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Input | Total | Unique | Multiple |
|---|---|---|---|---|
| MTB-1458-6 hpi | 2,278,861 | 799,656 (35.09%) | 783,022 (97.92%) | 16,634 (2.08%) |
| BCG-6 hpi | 4,949,944 | 3,517,427 (71.06%) | 3,427,613 (97.45%) | 89,814 (2.55%) |
| uninfection-6 hpi | 5,370,123 | 4,449,198 (82.85%) | 4,342,620 (97.60%) | 106,578 (2.40%) |
| MTB-1458-24 hpi | 2,514,040 | 843,526 (33.55%) | 827,655 (98.12%) | 15,871 (1.88%) |
| BCG-24 hpi | 4,561,834 | 3,332,746 (73.06%) | 3,164,199 (94.94%) | 168,547 (5.06%) |
| uninfection-24 hpi | 4,271,225 | 3,251,564 (76.13%) | 3,157,811 (97.12%) | 93,753 (2.88%) |
| miRNA | Target | TF |
|---|---|---|
| Down | ||
| miR-101-3p | ZCCHC2|SGK1|MOB1A|CPEB2|TNPO1|RNF213 | - |
| miR-107 | TNFSF10|ZCCHC2|PALM2-AKAP2|OAS3|NRP2|BHLHE40|TNPO1|PLAGL1 | BHLHE40|CEBPB|HNF4G|IRF1|MYB|PML|RUNX3|TRIM25 |
| miR-140-3p | CMPK2|RGS1|RASGEF1B|HNF4G|SKIL|AFF1|WDFY1|BTG1|RNF213 | ATF3|BHLHE40|CEBPB|JUND|MYB|PML|RUNX3 |
| miR-146b-3p | PML|MOB3C | ATF3|CEBPB|HNF4G|IRF1|JUND|MYB|RUNX3 |
| miR-210-3p | DHX58|RUNX3|PIK3R5 | BHLHE40|IRF1|JUN|JUND|MYB |
| miR-320b | CREG1|DTX3L|ZCCHC2|PRKAG2|NT5C3A|PALM2-AKAP2|TMEM123|GNA13|PLK3|ARL8B|CTNNB1|MANBA|BTG1|TNPO1|GPBP1|ZBTB43|GPNMB | JUN |
| miR-378c | AFF1|RDX|SRGAP2|ART3|BHLHE40|ARL8B | - |
| miR-378d | AFF1|RDX|SRGAP2|ART3|BHLHE40|ARL8B | ATF3|BHLHE40|IRF1|MYB|RUNX3|TRIM25 |
| miR-660-5p | RDX | CEBPB|PML|TRIM25 |
| miR-874-3p | FBXO32|PARP14|TXNRD1|TRAF1 | JUN|JUND|MYB|RUNX3 |
| miR-1261 | ATF3|SKIL|DAPP1|INSIG1|B2M|SNX10 | - |
| miR-3184-5p | MRAS|IRF1|SQSTM1|TRIM5|CSF1|TRIM14|PML|SMIM3|FRMD3|BMF|NGFR|CEBPB | ATF3|BHLHE40|CEBPB|IRF1|JUN|JUND|MYB|PML |
| miR-4286 | ZCCHC2|IRF1|PARP9|MGAT1|ARMC9|TRIM25|INSIG1|TRIM56|BMF|SETDB2 | - |
| Up | ||
| miR-1290 | SDHC|TSC22D1|IGFBP3|RPL36A | ATF3|BHLHE40|CEBPB|HNF4G|IRF1|JUN|JUND|TRIM25 |
| miR-96-5p | MYB|SH3KBP1|ATG16L1|ADK|NPTX1 | CEBPB|CTNNB1|JUN|JUND |
| miR-335-3p | MYB|DIAPH1|APEX1|SPN|CPT1A|SH3KBP1|MYO6 | CEBPB|IRF1|JUN|JUND|PML|TRIM25 |
| miR-625-3p | TSC22D1 | BHLHE40|JUND|MYB|PML|RUNX3|TRIM25 |
| miR-146a-5p | - | BHLHE40|CEBPB|CTNNB1|IRF1|JUN|JUND|MYB|PML|RUNX3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Liu, H.; Su, L.; Dawood, A.; Hu, C.; Chen, X.; Chen, H.; Chen, Y.; Guo, A. Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis. Int. J. Mol. Sci. 2022, 23, 382. https://doi.org/10.3390/ijms23010382
Zhu T, Liu H, Su L, Dawood A, Hu C, Chen X, Chen H, Chen Y, Guo A. Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis. International Journal of Molecular Sciences. 2022; 23(1):382. https://doi.org/10.3390/ijms23010382
Chicago/Turabian StyleZhu, Tingting, Han Liu, Li Su, Ali Dawood, Changmin Hu, Xi Chen, Huanchun Chen, Yingyu Chen, and Aizhen Guo. 2022. "Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis" International Journal of Molecular Sciences 23, no. 1: 382. https://doi.org/10.3390/ijms23010382
APA StyleZhu, T., Liu, H., Su, L., Dawood, A., Hu, C., Chen, X., Chen, H., Chen, Y., & Guo, A. (2022). Identification of Unique Key miRNAs, TFs, and mRNAs in Virulent MTB Infection Macrophages by Network Analysis. International Journal of Molecular Sciences, 23(1), 382. https://doi.org/10.3390/ijms23010382








