Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice
Abstract
:1. Introduction
2. Results
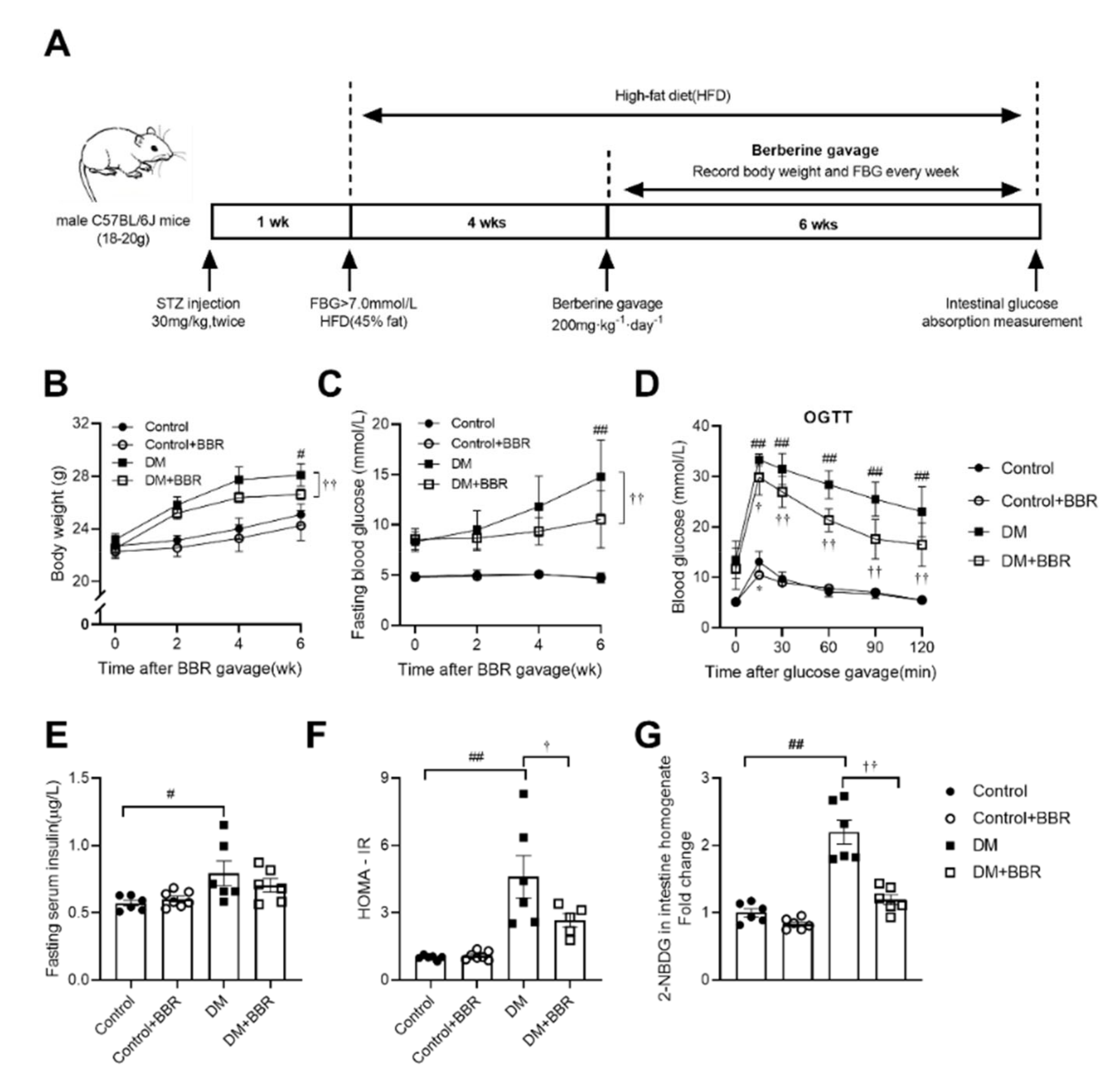
2.1. Berberine Alleviated Hyperglycemia, Improved Insulin Sensitivity and Reduced Intestinal Glucose Absorption in Diabetic Mice
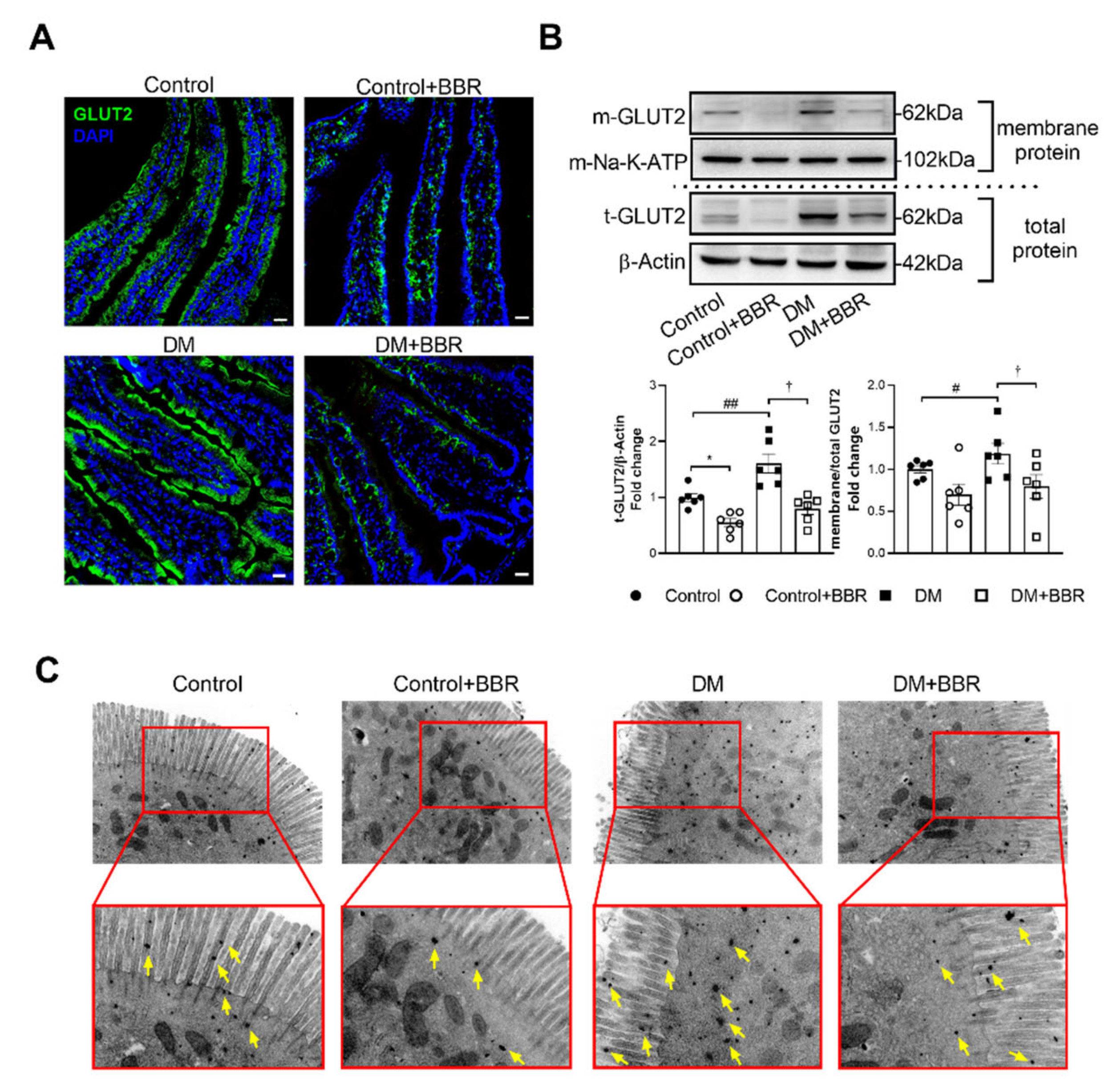
2.2. Berberine Decreased GLUT2 Localization in BBM of Intestinal Epithelial Cells in Diabetic Mice
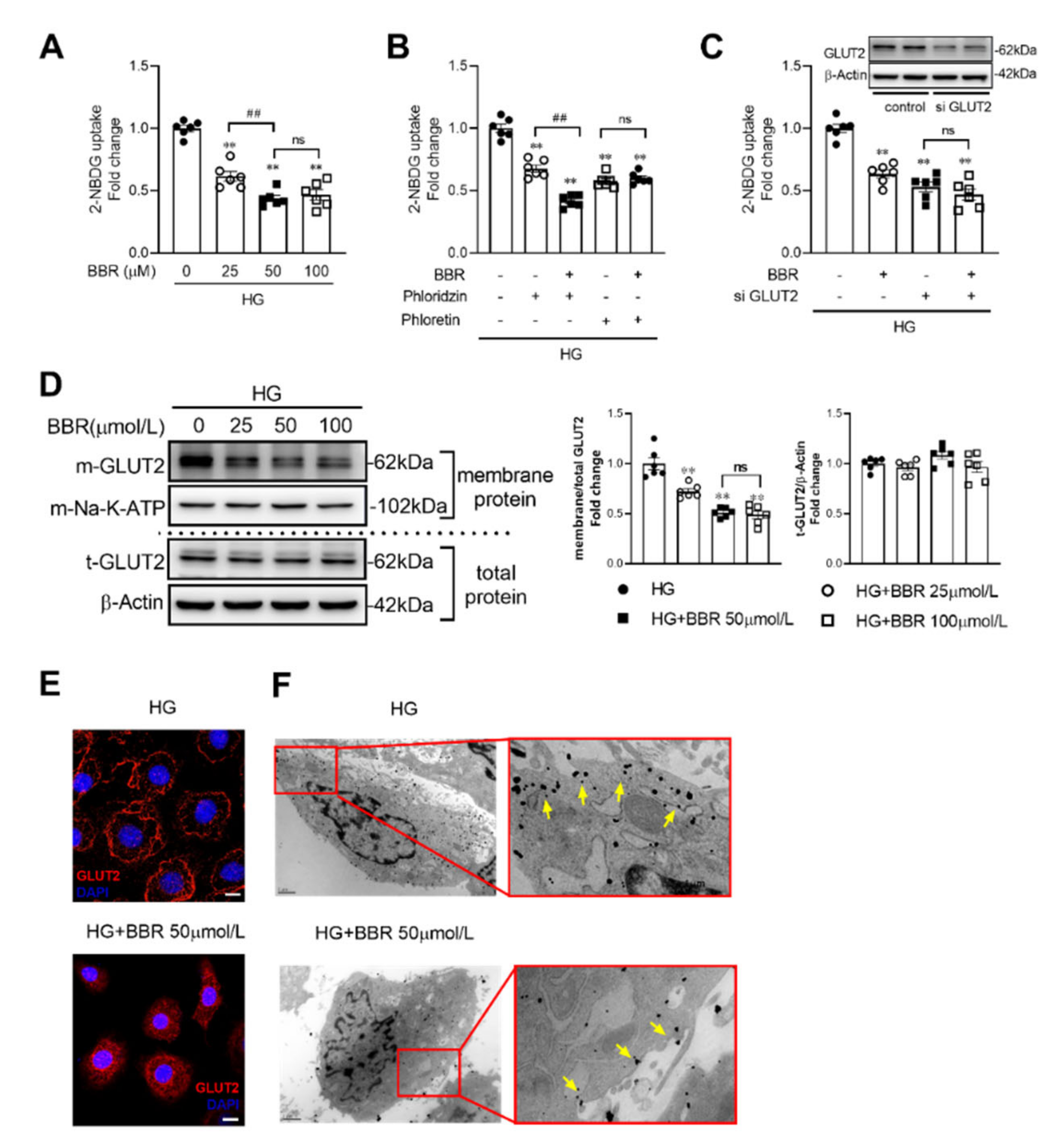
2.3. Berberine Decreased Glucose Absorption by Inhibiting GLUT2 Translocation in IEC-6 Cells
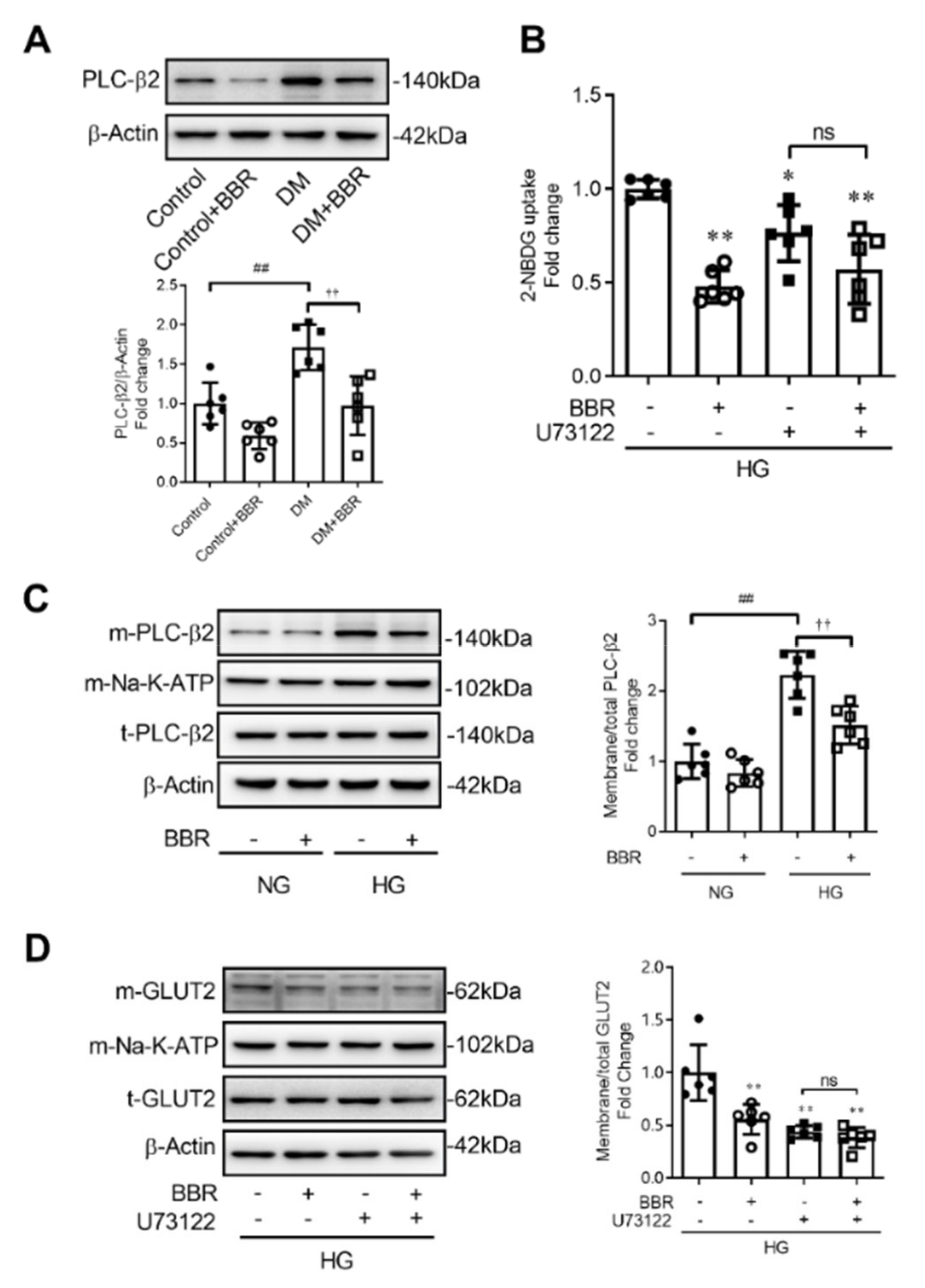
2.4. Berberine Decreased GLUT2 Translocation and Glucose Uptake through Depressing PLC-β2 - GLUT2 Signal Pathway
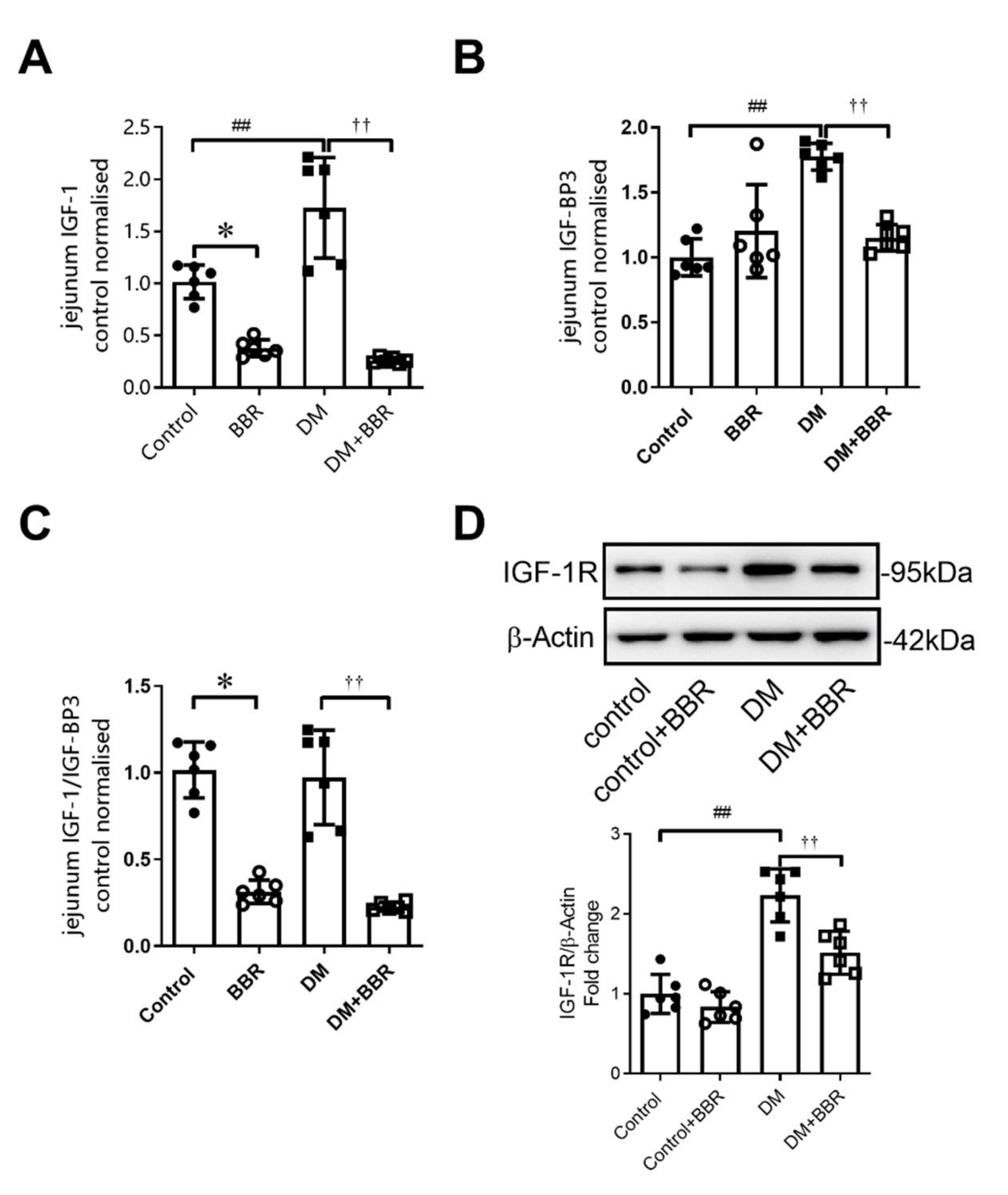
2.5. Berberine Treatment for 6 Weeks Decreased Intestinal IGF-1, IGFBP-3 Levels, and IGF-1R Expression in Diabetic Mice
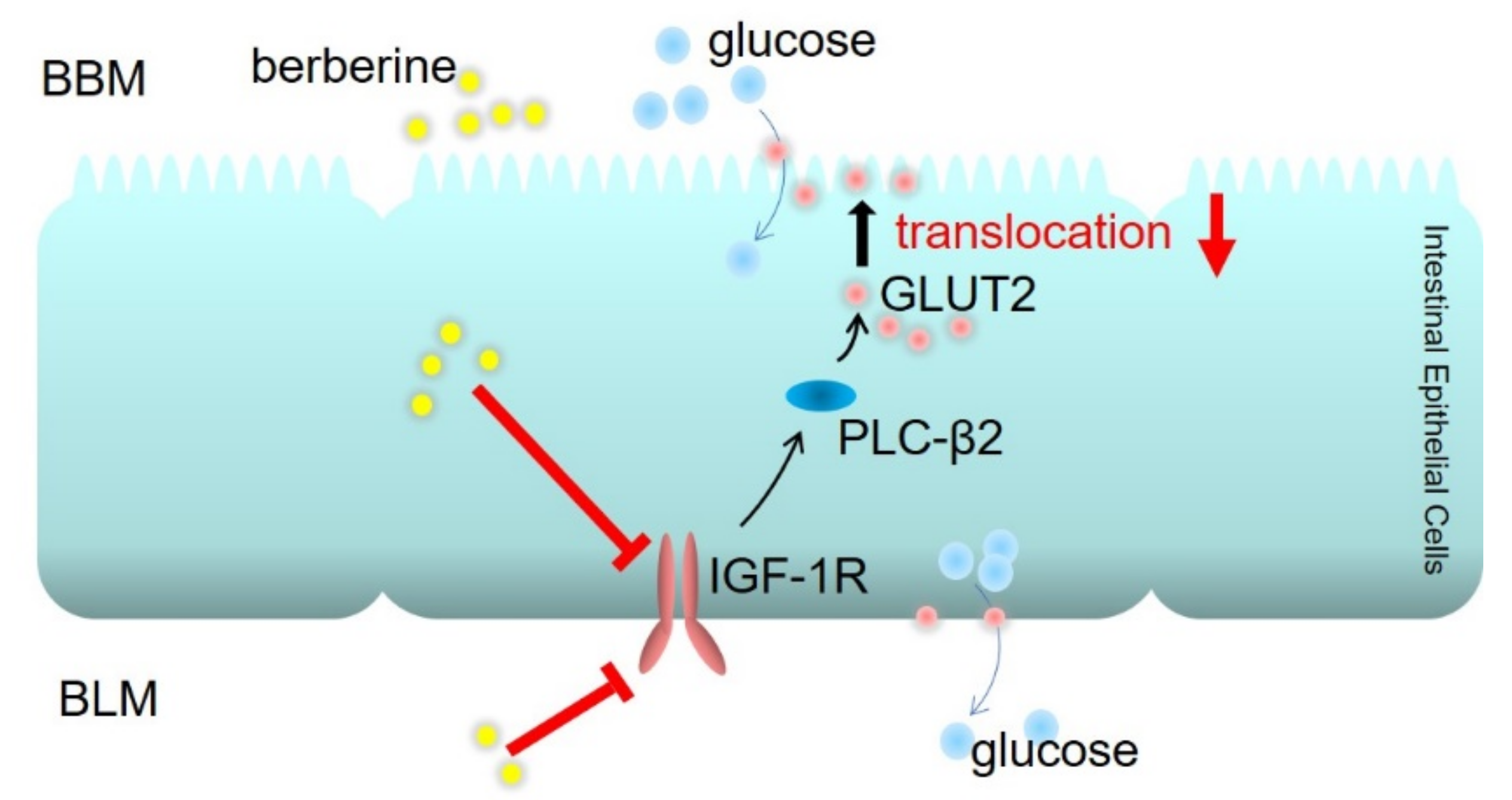
2.6. Berberine Decreased GLUT2 Translocation and Glucose Uptake through Inhibiting IGF-1R-PLC-β2-GLUT2 Signal Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Glucose Tolerance Test
4.3. Intestine Glucose Transport Test and Cellular Glucose Uptake Test
4.4. Immunofluorescence Histochemistry and Immunohistochemistry for Electron Microscopy
4.5. Western Blot Analysis
4.6. Measurement of Hormone
4.7. Small Interfering RNA Design and Transfection
4.8. Feces Glucose Test
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, T.; Rayner, C.K.; Jones, K.L.; Xie, C.; Marathe, C.; Horowitz, M. Role of intestinal glucose absorption in glucose tolerance. Curr. Opin. Pharmacol. 2020, 55, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. Eur. J. Phys. 2020, 472, 1207–1248. [Google Scholar] [CrossRef] [PubMed]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of glucose absorption in the small intestine in health and metabolic diseases and their role in appetite regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef]
- Tobin, V.; Le Gall, M.; Fioramonti, X.; Stolarczyk, E.; Blazquez, A.G.; Klein, C.; Prigent, M.; Serradas, P.; Cuif, M.; Magnan, C.; et al. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes 2008, 57, 555–562. [Google Scholar] [CrossRef]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; Le Gall, M.; Cotillard, A.; Gilet, J.; Garbin, K.; Houllier, A.; Chateau, D.; Lacombe, A.; et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: A study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 2011, 60, 2598–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of GLUT2. Annu. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef]
- Mace, O.J.; Affleck, J.; Patel, N.; Kellett, G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007, 582, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Karimian, A.E.; LaMoia, T.E.; Hussain, T.; Vargova, V.; Karolyi, K.; Veldhuis, P.P.; Arnoletti, J.P.; de la Fuente, S.G.; Pratley, R.E.; et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol. Metab. 2018, 17, 98–111. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine Pretreatment confers cardioprotection against ischemia-reperfusion injury in a rat model of Type 2 Diabetes. J Cardiovasc. Pharm. T 2016, 21, 486–494. [Google Scholar] [CrossRef]
- Tan, X.; Ma, J.; Feng, R.; Ma, C.; Chen, W.; Sun, Y.; Fu, J.; Huang, M.; He, C.; Shou, J.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xing, W.; Zhang, M.; Geng, F.; Yang, H.; Zhang, H.; Zhang, X.; Li, J.; Dong, L.; Gao, F. Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H802–H813. [Google Scholar] [CrossRef]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Wang, X.; Lei, L.; Liu, M.Z.; Li, R.C.; Sun, S.J.; Liu, S.N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020, 34, 1166–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Yu, Y.L.; Yang, J.S.; Li, Y.; Liu, Y.W.; Liang, Y.; Liu, X.D.; Xie, L.; Wang, G.J. Berberine suppresses intestinal disaccharidases with beneficial metabolic effects in diabetic states, evidences from in vivo and in vitro study. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 381, 371–381. [Google Scholar] [CrossRef]
- Yue, X.; Liang, J.; Gu, F.; Du, D.; Chen, F. Berberine activates bitter taste responses of enteroendocrine STC-1 cells. Mol. Cell Biochem. 2018, 447, 21–32. [Google Scholar] [CrossRef]
- Choi, P.M.; Sun, R.C.; Guo, J.; Erwin, C.R.; Warner, B.W. High-fat diet enhances villus growth during the adaptation response to massive proximal small bowel resection. J. Gastrointest. Surg. 2013, 18, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Vassilakos, G.; Lei, H.; Yang, Y.; Puglise, J.; Matheny, M.; Durzynska, J.; Ozery, M.; Bennett, K.; Spradlin, R.; Bonanno, H.; et al. Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice. FASEB J. 2019, 33, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takao, T.; Takahashi, K.; Suka, M.; Suzuki, N.; Yanagisawa, H. Association between postprandial hyperglycemia at clinic visits and all-cause and cancer mortality in patients with type 2 diabetes: A long-term historical cohort study in Japan. Diabetes Res. Clin. Pract. 2019, 148, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Aryangat, A.V.; Gerich, J.E. Type 2 diabetes: Postprandial hyperglycemia and increased cardiovascular risk. Vasc. Health Risk Manag. 2010, 6, 145–155. [Google Scholar]
- Merino, B.; Fernandez-Diaz, C.M.; Cozar-Castellano, I.; Perdomo, G. Intestinal fructose and glucose metabolism in health and disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.H.; Li, G.H.; Zhang, X.; Zhang, P.; Dong, M.Q.; Zhao, Z.J.; Zhang, Y.; Dong, L.; Gao, F. Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br. J. Pharmacol. 2016, 173, 1569–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaba, S.; Saini, A.; Singh, G.; Monga, V. An insight into the medicinal attributes of berberine derivatives: A review. Bioorg. Med. Chem. 2021, 38, 116143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.D.; Li, Y.; Sun, M.; Yu, C.J.; Li, J.Y.; Wang, S.H.; Yang, D.; Guo, C.L.; Du, X.; Zhang, W.J.; et al. Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J. Gastroenterol. 2021, 27, 708–724. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tuo, B.; Dong, H. Regulation of intestinal glucose absorption by ion channels and transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, D.Y. Insulin-like growth factor (IGF)-I and IGF binding proteins axis in diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Drogan, D.; Schulze, M.B.; Boeing, H.; Pischon, T. Insulin-Like growth factor 1 and insulin-like growth factor-binding protein 3 in relation to the risk of Type 2 diabetes mellitus: Results from the EPIC-Potsdam study. Am. J. Epidemiol. 2016, 183, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Lewitt, M.S.; Dent, M.S.; Hall, K. The insulin-like growth factor system in obesity, insulin resistance and Type 2 diabetes mellitus. J. Clin. Med. 2014, 3, 1561–1574. [Google Scholar] [CrossRef]
- Assefa, B.; Mahmoud, A.M.; Pfeiffer, A.; Birkenfeld, A.L.; Spranger, J.; Arafat, A.M. Insulin-like growth factor (IGF) binding protein-2, independently of IGF-1, induces GLUT-4 translocation and glucose uptake in 3T3-L1 adipocytes. Oxid. Med. Cell. Longev. 2017, 2017, 3035184. [Google Scholar] [CrossRef]
- De Munnik, S.M.; van der Lee, R.; Velders, D.M.; van Offenbeek, J.; Smits-de, V.L.; Leurs, R.; Smit, M.J.; Vischer, H.F. The viral G protein-coupled receptor ORF74 unmasks phospholipase C signaling of the receptor tyrosine kinase IGF-1R. Cell Signal. 2016, 28, 595–605. [Google Scholar] [CrossRef]
- Crudden, C.; Ilic, M.; Suleymanova, N.; Worrall, C.; Girnita, A.; Girnita, L. The dichotomy of the Insulin-like growth factor 1 receptor: RTK and GPCR: Friend or foe for cancer treatment? Growth Horm. IGF Res. 2015, 25, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gleichmann, H. GLUT2 in pancreatic islets: Crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes 1998, 47, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Krimi, R.B.; Letteron, P.; Chedid, P.; Nazaret, C.; Ducroc, R.; Marie, J.C. Resistin-like molecule-beta inhibits SGLT-1 activity and enhances GLUT2-dependent jejunal glucose transport. Diabetes 2009, 58, 2032–2038. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods 2005, 64, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Liang, W.H.; Lam, C.S.; Huang, X.F.; Yang, S.J.; Wong-Riley, M.T.; Fung, M.L.; Liu, Y.Y. Catecholaminergic neurons in synaptic connections with pre-Botzinger complex neurons in the rostral ventrolateral medulla in normoxic and daily acute intermittent hypoxic rats. Exp. Neurol. 2017, 287, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Cornelius, R.J.; Yuan, Y.; Sansom, S.C. Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. Am. J. Physiol. Renal. Physiol. 2013, 305, F463–F476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yang, H.; Yang, E.; Li, J.; Dong, L. Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice. Int. J. Mol. Sci. 2022, 23, 327. https://doi.org/10.3390/ijms23010327
Zhang M, Yang H, Yang E, Li J, Dong L. Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice. International Journal of Molecular Sciences. 2022; 23(1):327. https://doi.org/10.3390/ijms23010327
Chicago/Turabian StyleZhang, Min, Hongyan Yang, Erwan Yang, Jia Li, and Ling Dong. 2022. "Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice" International Journal of Molecular Sciences 23, no. 1: 327. https://doi.org/10.3390/ijms23010327
APA StyleZhang, M., Yang, H., Yang, E., Li, J., & Dong, L. (2022). Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice. International Journal of Molecular Sciences, 23(1), 327. https://doi.org/10.3390/ijms23010327




