Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus aureus (MRSA) Infection
Abstract
:1. Introduction
2. Results
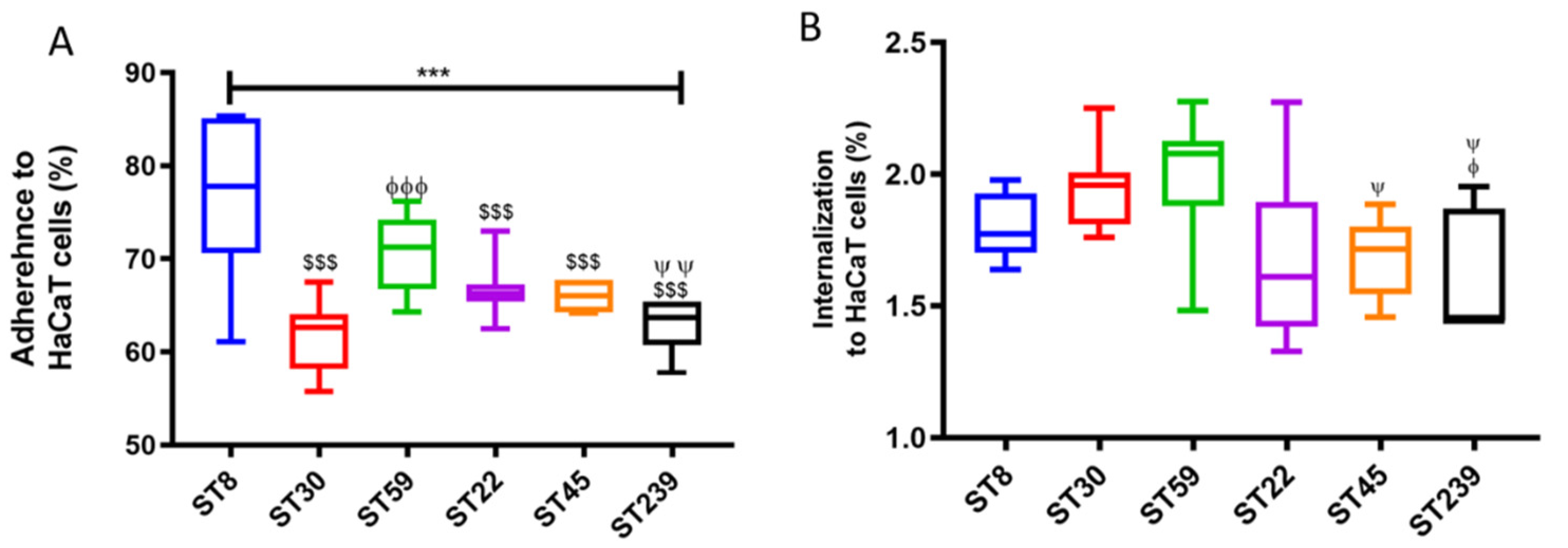
2.1. Adhesion and Internalization Assay Using HaCaT Monolayers
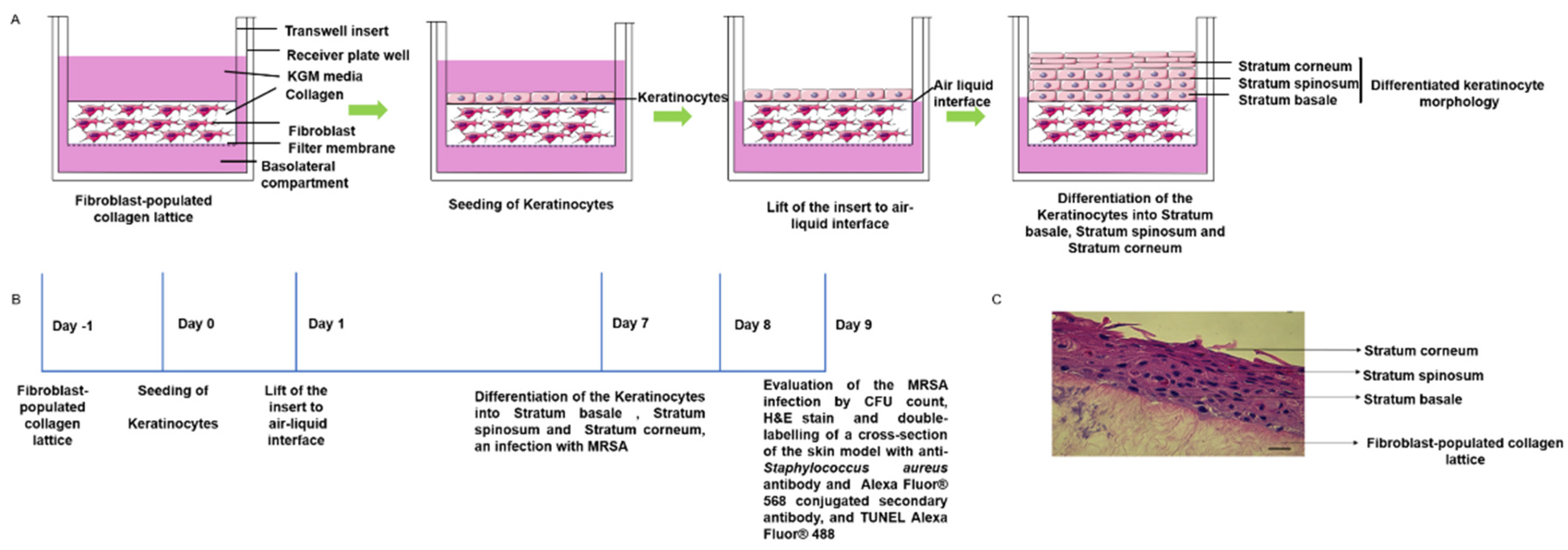
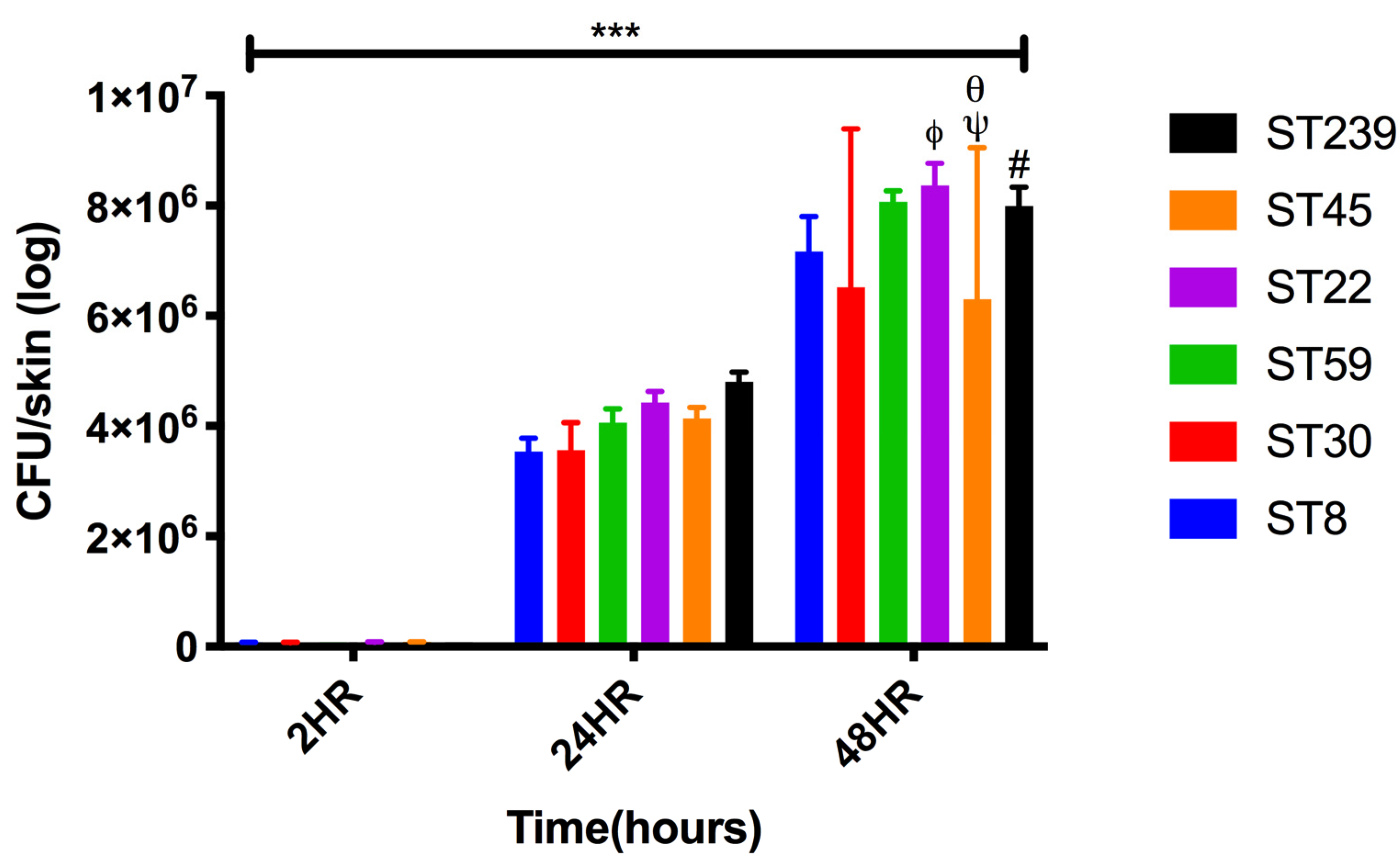
2.2. Colonization and Infection of Keratinocyte Organotypic Model
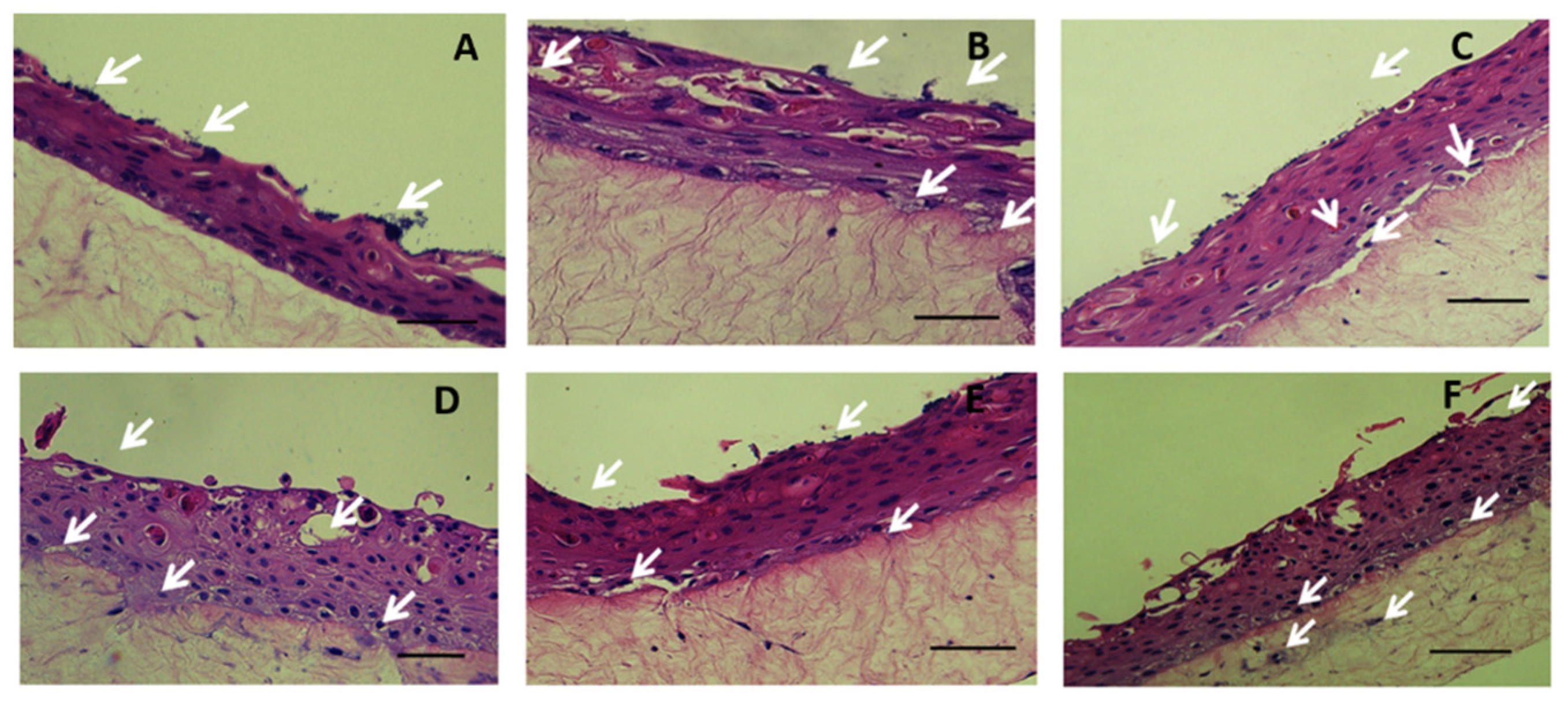
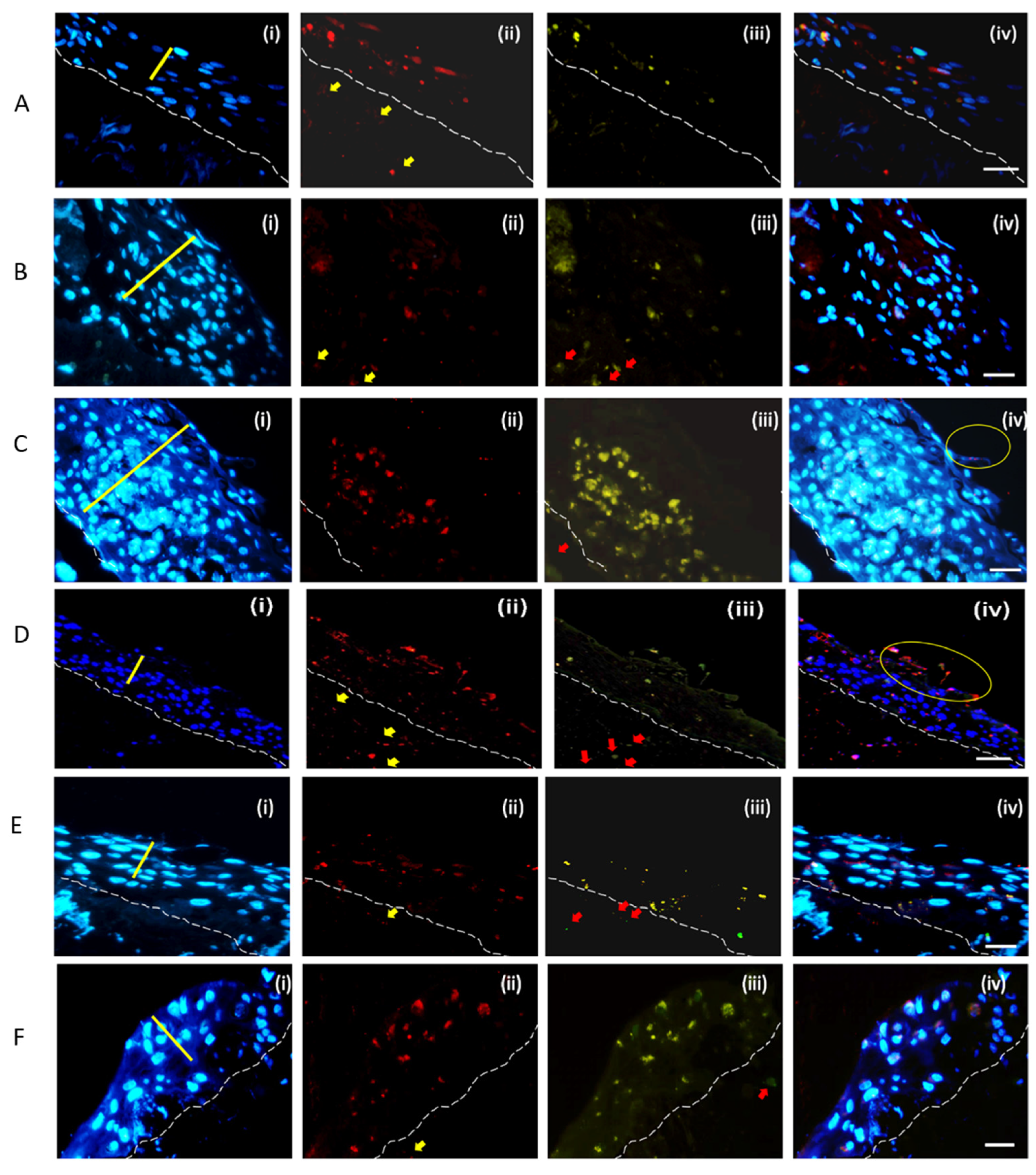
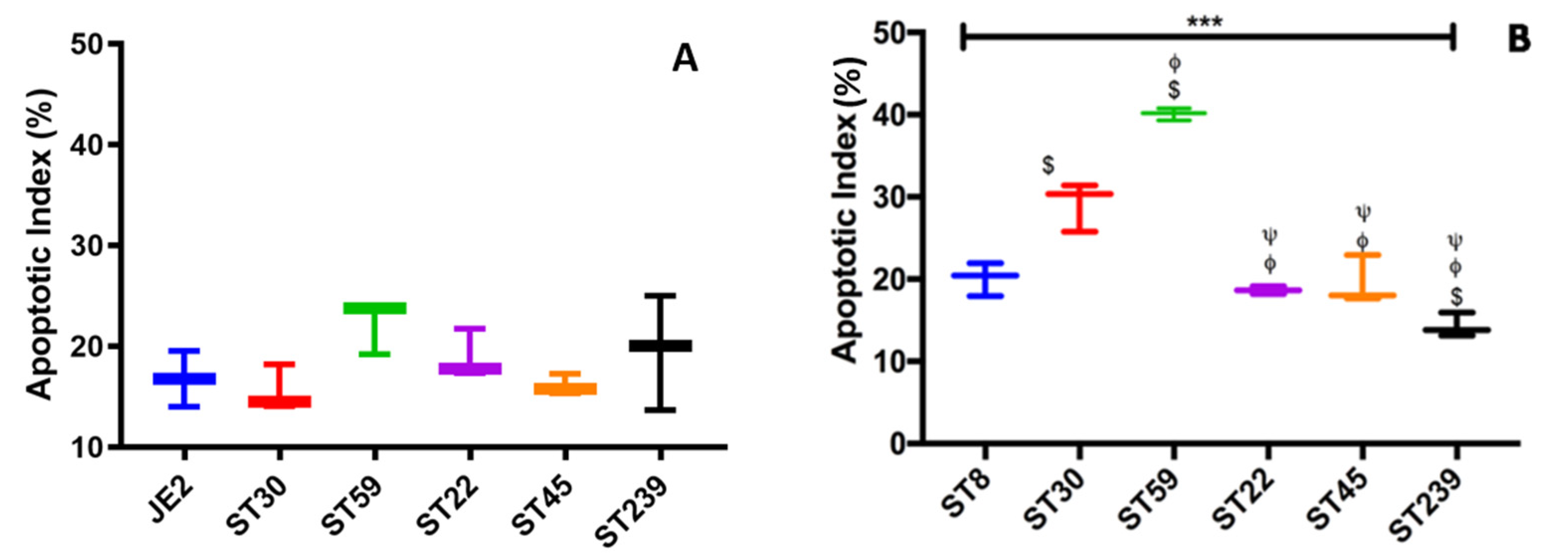
2.3. Invasion and Cell Death Modulation by the MRSA Clonal Types
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, HaCaT Cells, Primary Human Cells and Reagents
4.2. Adhesion and Internalization Assays Using HaCaT Monolayers
4.3. D Skin Model: Keratinocyte Organotypic Culture on Collagen Gel Incorporated with Fibroblast
4.4. MRSA Colonization on 3D-Skin Model
4.5. Histology
4.6. Assessment of Bacterial Dissemination and Cell Death Modulation in the Skin Model
4.7. Apoptotic Index
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.W.H.; Ip, M.; Tang, A.; Wei, V.W.I.; Wong, S.Y.S.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and risk factors of community-associated methicillin-resistant staphylococcus aureus carriage in asia-pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef] [Green Version]
- Sapugahawatte, D.N.; Li, C.; Yeoh, Y.K.; Dharmaratne, P.; Zhu, C.; Ip, M. Swine methicillin-resistant Staphylococcus aureus carrying toxic-shock syndrome toxin gene in Hong Kong, China. Emerg. Microbes Infect. 2020, 9, 1534–1536. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Talan, D.A.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; Limbago, B.; Albrecht, V.; Moran, G.J. Comparison of staphylococcus aureus from skin and soft-tissue infections in us emergency Department patients, 2004 and 2008. Clin. Infect. Dis. 2011, 53, 144–149. [Google Scholar] [CrossRef]
- Soong, G.; Chun, J.; Parker, D.; Prince, A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J. Infect. Dis. 2012, 205, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Ding, Y.; Wang, J.; Wang, F. Staphylococcus aureus induces TGF-β1 and bFGF expression through the activation of AP-1 and NF-κB transcription factors in bovine mammary epithelial cells. Microb. Pathog. 2018, 117, 276–284. [Google Scholar] [CrossRef]
- Josse, J.; Velard, F.; Gangloff, S.C. Staphylococcus aureus vs. osteoblast: Relationship and consequences in osteomyelitis. Front. Cell. Infect. Microbiol. 2015, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef] [Green Version]
- Recker, M.; Laabei, M.; Toleman, M.S.; Reuter, S.; Saunderson, R.B.; Blane, B.; Török, M.E.; Ouadi, K.; Stevens, E.; Yokoyama, M.; et al. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat. Microbiol. 2017, 2, 1381–1388. [Google Scholar] [CrossRef]
- Popov, L.; Kovalski, J.; Grandi, G.; Bagnoli, F.; Amieva, M.R. Three-dimensional human skin models to understand Staphylococcus aureus skin colonization and infection. Front. Immunol. 2014, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Palankar, R.; Binsker, U.; Haracska, B.; Wesche, J.; Greinacher, A.; Hammerschmidt, S. Interaction between the Staphylococcus aureus extracellular adherence protein Eap and its subdomains with platelets. Int. J. Med. Microbiol. 2018, 308, 683–691. [Google Scholar] [CrossRef]
- Cho, S.H.; Strickland, I.; Boguniewicz, M.; Leung, D.Y.M. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J. Allergy Clin. Immunol. 2001, 108, 269–274. [Google Scholar] [CrossRef]
- Dziewanowska, K.; Carson, A.R.; Patti, J.M.; Deobald, C.F.; Bayles, K.W.; Bohach, G.A. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: Role in internalization by epithelial cells. Infect. Immun. 2000, 68, 6321–6328. [Google Scholar] [CrossRef]
- Vanassche, T.; Verhaegen, J.; Peetermans, W.E.; Van Ryn, J.; Cheng, A.; Schneewind, O.; Hoylaerts, M.F.; Verhamme, P. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. J. Thromb. Haemost. 2011, 9, 2436–2446. [Google Scholar] [CrossRef]
- Ko, Y.P.; Flick, M.J. Fibrinogen Is at the Interface of Host Defense and Pathogen Virulence in Staphylococcus aureus Infection. Semin. Thromb. Hemost. 2016, 42, 408–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mempel, M.; Schnopp, C.; Hojka, M.; Fesq, H.; Weidinger, S.; Schaller, M.; Korting, H.C.; Ring, J.; Abeck, D. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 2002, 146, 943–951. [Google Scholar] [CrossRef]
- Kintarak, S.; Whawell, S.A.; Speight, P.M.; Packer, S.; Nair, S.P. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 2004, 72, 5668–5675. [Google Scholar] [CrossRef] [Green Version]
- Wesson, C.A.; Deringer, J.; Liou, L.E.; Bayles, K.W.; Bohach, G.A.; Trumble, W.R. Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect. Immun. 2000, 68, 2998–3001. [Google Scholar] [CrossRef] [Green Version]
- Bur, S.; Preissner, K.T.; Herrmann, M.; Bischoff, M. The staphylococcus aureus extracellular adherence protein promotes bacterial internalization by keratinocytes independent of fibronectin-binding proteins. J. Investig. Dermatol. 2013, 133, 2004–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, S.; Miller, L.S. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.M.; Walsh, E.J.; Massey, R.C.; Peacock, S.J.; Foster, T.J. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: Implications for nasal colonization. Cell. Microbiol. 2002, 4, 759–770. [Google Scholar] [CrossRef]
- Prabhakara, R.; Foreman, O.; De Pascalis, R.; Lee, G.M.; Plaut, R.D.; Kim, S.Y.; Stibitz, S.; Elkins, K.L.; Merkel, T.J. Epicutaneous model of community-acquired Staphylococcus aureus skin infections. Infect. Immun. 2013, 81, 1306–1315. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.S.; Zussman, J.; Donegan, N.P.; Ramos, R.I.; Garcia, N.C.; Uslan, D.Z.; Iwakura, Y.; Simon, S.I.; Cheung, A.L.; Modlin, R.L.; et al. Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against staphylococcus aureus and USA300 MRSA skin infections. J. Investig. Dermatol. 2011, 131, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.D.; Malachowa, N.; Whitney, A.R.; Braughton, K.R.; Gardner, D.J.; Long, D.; Wardenburg, J.B.; Schneewind, O.; Otto, M.; DeLeo, F.R. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 2011, 204, 937–941. [Google Scholar] [CrossRef]
- Nippe, N.; Varga, G.; Holzinger, D.; Löffler, B.; Medina, E.; Becker, K.; Roth, J.; Ehrchen, J.M.; Sunderkötter, C. Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J. Investig. Dermatol. 2011, 131, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangatirkar, P.; Paquet-Fifield, S.; Li, A.; Rossi, R.; Kaur, P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2007, 2, 178–186. [Google Scholar] [CrossRef]
- Stark, H.J.; Szabowski, A.; Fusenig, N.E.; Maas-Szabowski, N. Organotypic co-cultures as skin equivalents: A complex and sophisticated in vitro system. Biol. Proced. Online 2004, 6, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Auxenfans, C.; Fradette, J.; Lequeux, C.; Germain, L.; Kinikoglu, B.; Bechetoille, N.; Braye, F.; Auger, F.A.; Damour, O. Evolution of three dimensional skin equivalent models reconstructed in vitro by tissue engineering. Eur. J. Dermatol. 2009, 19, 107–113. [Google Scholar] [CrossRef]
- Botchkareva, N.V.; Westgate, G.E. (Eds.) Molecular Dermatology: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2020; Volume 2154, ISBN 978-1-0716-0647-6. [Google Scholar]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin bioprinting: Impending reality or fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Lee, W.; Bae, I.H.; Lee, T.R.; Croce, P.; Yoo, S.S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Enhejirigala, E.; Yao, B.; Li, Z.; Song, W.; Li, J.; Zhu, D.; Wang, Y.; Duan, X.; Yuan, X.; et al. Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles. Burns Trauma 2021, 9, tkab013. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.E.; Kourteva, I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 1998, 66, 5994–5998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nübel, U.; Roumagnac, P.; Feldkamp, M.; Song, J.H.; Ko, K.S.; Huang, Y.C.; Coombs, G.; Ip, M.; Westh, H.; Skov, R.; et al. Frequent emergence and limited geographic dispersal of methicillin- resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2008, 105, 14130–14135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufiero, B.; Duanmu, Z.; Guo, M.; Meduri, N.B.; Falkow, S.; Murakawa, G.J. Staphylococcus aureus infection of human primary keratinocytes. J. Dermatol. Sci. 2004, 36, 173–175. [Google Scholar] [CrossRef]
- Gu, H.; Huang, L.; Wong, Y.P.; Burd, A. HA modulation of epidermal morphogenesis in an organotypic keratinocyte-fibroblast co-culture model. Exp. Dermatol. 2010, 19, 336–339. [Google Scholar] [CrossRef]
- Den Reijer, P.M.; Haisma, E.M.; Lemmens-Den Toom, N.A.; Willemse, J.; Koning, R.A.; Demmers, J.A.A.; Dekkers, D.H.W.; Rijkers, E.; El Ghalbzouri, A.; Nibbering, P.H.; et al. Detection of alpha-toxin and other virulence factors in biofilms of staphylococcus aureus on polystyrene and a human epidermalmodel. PLoS ONE 2016, 11, e0145722. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, N.; Huang, L.; Li, C.; Yang, Y.; Luo, M.; Wei, W.I.; Wong, K.T.; Lo, N.W.S.; Kwok, K.O.; Ip, M. Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus aureus (MRSA) Infection. Int. J. Mol. Sci. 2022, 23, 299. https://doi.org/10.3390/ijms23010299
Barua N, Huang L, Li C, Yang Y, Luo M, Wei WI, Wong KT, Lo NWS, Kwok KO, Ip M. Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus aureus (MRSA) Infection. International Journal of Molecular Sciences. 2022; 23(1):299. https://doi.org/10.3390/ijms23010299
Chicago/Turabian StyleBarua, Nilakshi, Lin Huang, Carmen Li, Ying Yang, Mingjing Luo, Wan In Wei, Kam Tak Wong, Norman Wai Sing Lo, Kin On Kwok, and Margaret Ip. 2022. "Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus aureus (MRSA) Infection" International Journal of Molecular Sciences 23, no. 1: 299. https://doi.org/10.3390/ijms23010299
APA StyleBarua, N., Huang, L., Li, C., Yang, Y., Luo, M., Wei, W. I., Wong, K. T., Lo, N. W. S., Kwok, K. O., & Ip, M. (2022). Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus aureus (MRSA) Infection. International Journal of Molecular Sciences, 23(1), 299. https://doi.org/10.3390/ijms23010299





