Relevance of AIF/CypA Lethal Pathway in SH-SY5Y Cells Treated with Staurosporine
Abstract
:1. Introduction
2. Results
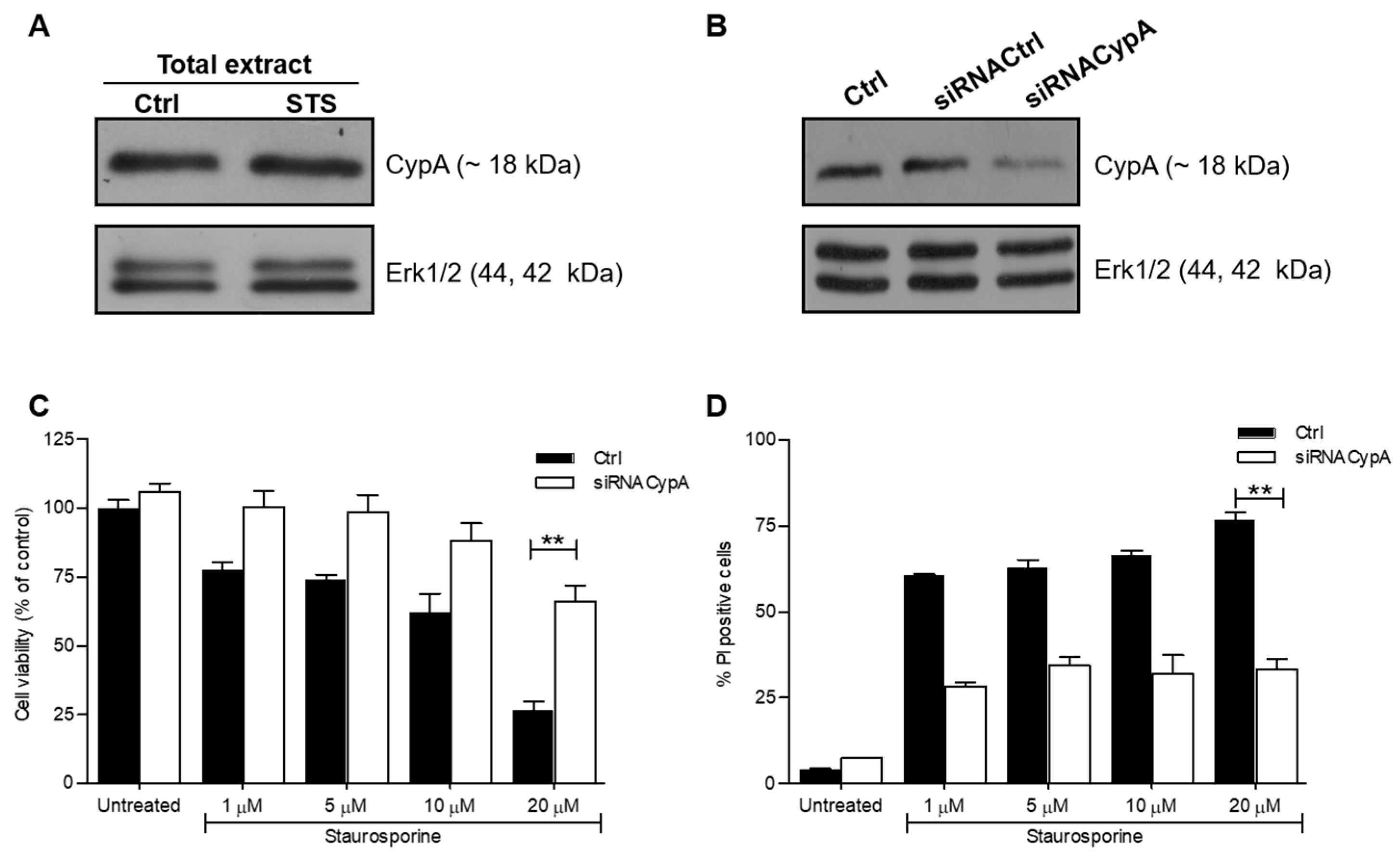
2.1. Down-Regulation of CypA Protects SH-SY5Y Cells from Death Induced by STS
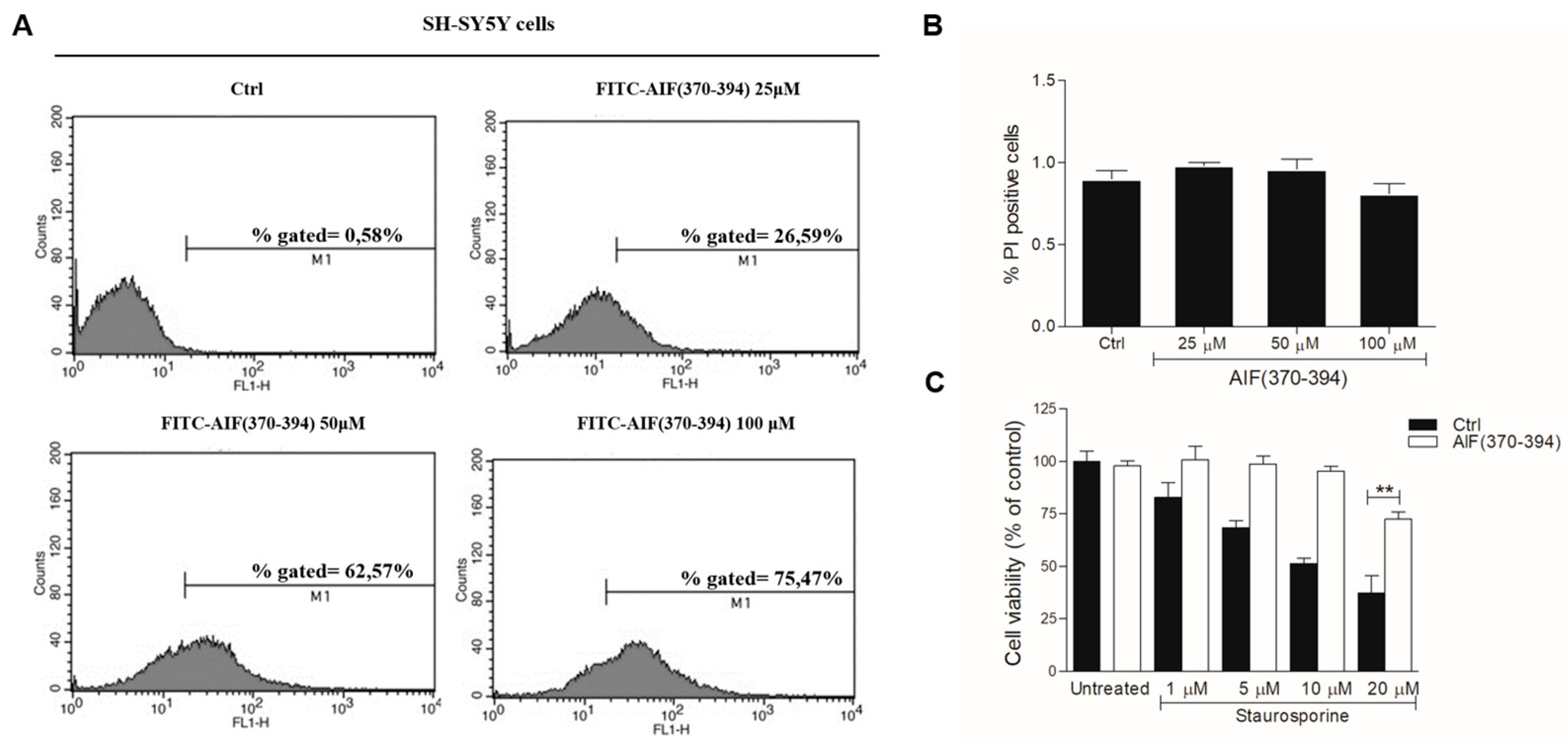
2.2. STS-Induced Cell Death Is Counteracted in AIF(370-394)-Treated Cells
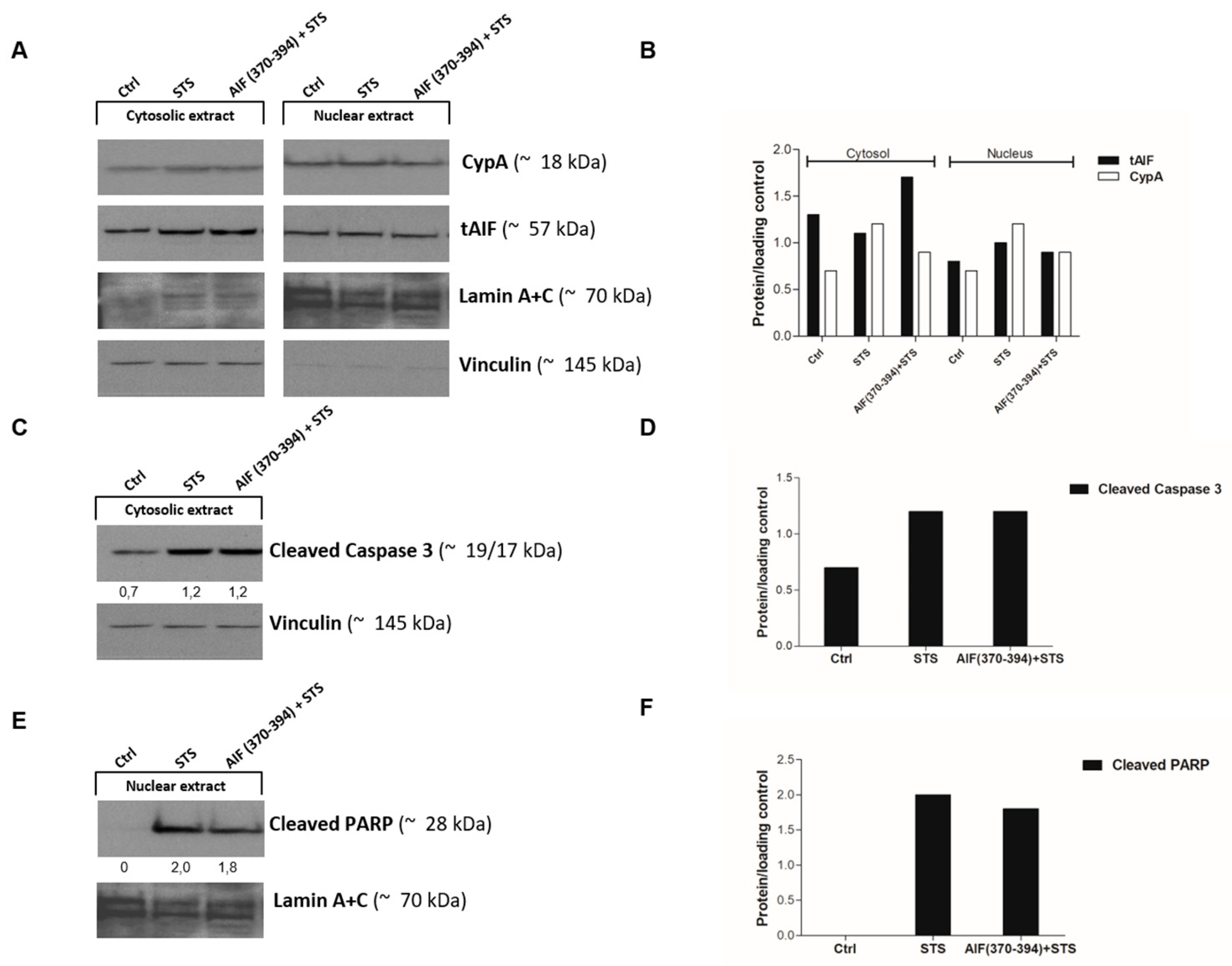
2.3. AIF(370-394) Influences the AIF/CypA Nuclear Translocation Induced by STS, without Affecting Caspase-3 Activation and PARP
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. siRNA
4.3. Peptide Synthesis and Characterization
4.4. Neuroblastoma SH-SY5Y Cell Culture and Transfection
4.5. Cell Treatment
4.6. Cell Viability Assay (MTT)
4.7. Cell Death Assay by Propidium Iodide (PI)
4.8. Subcellular Fractionation and Western Blot Analysis
4.9. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmokhtar, C.A.; Hillion, J.; Segal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boix, J.; Llecha, N.; Yuste, V.J.; Comella, J.X. Characterization of the cell death process induced by staurosporine in human neuroblastoma cell lines. Neuropharmacology 1997, 36, 811–821. [Google Scholar] [CrossRef]
- Lopez, E.; Ferrer, I. Staurosporine- and H-7-induced cell death in SH-SY5Y neuroblastoma cells is associated with caspase-2 and caspase-3 activation, but not with activation of the FAS/FAS-L-caspase-8 signaling pathway. Brain Res. Mol. Brain Res. 2000, 85, 61–67. [Google Scholar] [CrossRef]
- Yuste, V.J.; Sanchez-Lopez, I.; Sole, C.; Encinas, M.; Bayascas, J.R.; Boix, J.; Comella, J.X. The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J. Neurochem. 2002, 80, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, B.R.; Walker, T.; Rasquinha, I.; Hill, I.E.; MacManus, J.P. Activation of DNA-dependent protein kinase may play a role in apoptosis of human neuroblastoma cells. J. Neurochem. 1999, 72, 933–942. [Google Scholar] [CrossRef]
- Jantas, D.; Pytel, M.; Mozrzymas, J.W.; Leskiewicz, M.; Regulska, M.; Antkiewicz-Michaluk, L.; Lason, W. The attenuating effect of memantine on staurosporine-, salsolinol- and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2008, 52, 864–877. [Google Scholar] [CrossRef]
- Posmantur, R.; McGinnis, K.; Nadimpalli, R.; Gilbertsen, R.B.; Wang, K.K. Characterization of CPP32-like protease activity following apoptotic challenge in SH-SY5Y neuroblastoma cells. J. Neurochem. 1997, 68, 2328–2337. [Google Scholar] [CrossRef] [Green Version]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prevost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Cheung, E.C.; Joza, N.; Steenaart, N.A.; McClellan, K.A.; Neuspiel, M.; McNamara, S.; MacLaurin, J.G.; Rippstein, P.; Park, D.S.; Shore, G.C.; et al. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J. 2006, 25, 4061–4073. [Google Scholar] [CrossRef] [Green Version]
- Hangen, E.; Feraud, O.; Lachkar, S.; Mou, H.; Doti, N.; Fimia, G.M.; Lam, N.V.; Zhu, C.; Godin, I.; Muller, K.; et al. Interaction between AIF and CHCHD4 Regulates Respiratory Chain Biogenesis. Mol. Cell 2015, 58, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Sevrioukova, I.F. Apoptosis-inducing factor: Structure, function, and redox regulation. Antioxid. Redox Signal. 2011, 14, 2545–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, G.; Clark, R.S.; Pei, W.; Yin, W.; Zhang, F.; Sun, F.Y.; Graham, S.H.; Chen, J. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J. Cereb. Blood Flow. Metab. 2003, 23, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otera, H.; Ohsakaya, S.; Nagaura, Z.; Ishihara, N.; Mihara, K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005, 24, 1375–1386. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Graham, S.H.; Du, L.; Kochanek, P.M.; Draviam, R.; Guo, F.; Nathaniel, P.D.; Szabo, C.; Watkins, S.C.; et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 2002, 82, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Longo-Guess, C.M.; Rossmann, M.P.; Seburn, K.L.; Hurd, R.E.; Frankel, W.N.; Bronson, R.T.; Ackerman, S.L. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 2002, 419, 367–374. [Google Scholar] [CrossRef]
- Piao, C.S.; Loane, D.J.; Stoica, B.A.; Li, S.; Hanscom, M.; Cabatbat, R.; Blomgren, K.; Faden, A.I. Combined inhibition of cell death induced by apoptosis inducing factor and caspases provides additive neuroprotection in experimental traumatic brain injury. Neurobiol. Dis. 2012, 46, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Ravagnan, L.; Gurbuxani, S.; Susin, S.A.; Maisse, C.; Daugas, E.; Zamzami, N.; Mak, T.; Jaattela, M.; Penninger, J.M.; Garrido, C.; et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001, 3, 839–843. [Google Scholar] [CrossRef]
- Susin, S.A.; Daugas, E.; Ravagnan, L.; Samejima, K.; Zamzami, N.; Loeffler, M.; Costantini, P.; Ferri, K.F.; Irinopoulou, T.; Prevost, M.C.; et al. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 2000, 192, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Zamzami, N.; Castedo, M.; Hirsch, T.; Marchetti, P.; Macho, A.; Daugas, E.; Geuskens, M.; Kroemer, G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 1996, 184, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Burguillos, M.A.; Hajji, N.; Englund, E.; Persson, A.; Cenci, A.M.; Machado, A.; Cano, J.; Joseph, B.; Venero, J.L. Apoptosis-inducing factor mediates dopaminergic cell death in response to LPS-induced inflammatory stimulus: Evidence in Parkinson’s disease patients. Neurobiol. Dis. 2011, 41, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalcinkaya, N.; Haytural, H.; Bilgic, B.; Ozdemir, O.; Hanagasi, H.; Kucukali, C.I.; Ozbek, Z.; Akcan, U.; Idrisoglu, H.A.; Gurvit, H.; et al. Expression changes of genes associated with apoptosis and survival processes in Parkinson’s disease. Neurosci. Lett. 2016, 615, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Artus, C.; Boujrad, H.; Bouharrour, A.; Brunelle, M.N.; Hoos, S.; Yuste, V.J.; Lenormand, P.; Rousselle, J.C.; Namane, A.; England, P.; et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010, 29, 1585–1599. [Google Scholar] [CrossRef] [Green Version]
- Doti, N.; Reuther, C.; Scognamiglio, P.L.; Dolga, A.M.; Plesnila, N.; Ruvo, M.; Culmsee, C. Inhibition of the AIF/CypA complex protects against intrinsic death pathways induced by oxidative stress. Cell Death Dis. 2014, 5, e993. [Google Scholar] [CrossRef]
- Rodriguez, J.; Xie, C.; Li, T.; Sun, Y.; Wang, Y.; Xu, Y.; Li, K.; Zhang, S.; Zhou, K.; Wang, Y.; et al. Inhibiting the interaction between apoptosis-inducing factor and cyclophilin A prevents brain injury in neonatal mice after hypoxia-ischemia. Neuropharmacology 2020, 171, 108088. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, B.; Di Sorbo, G.; Chambery, A.; Caporale, A.; Leoni, G.; Russo, R.; Mascanzoni, F.; Raimondo, D.; Fattorusso, R.; Ruvo, M.; et al. Structural and biochemical insights of CypA and AIF interaction. Sci. Rep. 2017, 7, 1138. [Google Scholar] [CrossRef]
- Farina, B.; Sturlese, M.; Mascanzoni, F.; Caporale, A.; Monti, A.; Di Sorbo, G.; Fattorusso, R.; Ruvo, M.; Doti, N. Binding mode of AIF(370-394) peptide to CypA: Insights from NMR, label-free and molecular docking studies. Biochem. J. 2018, 475, 2377–2393. [Google Scholar] [CrossRef] [Green Version]
- Monti, A.; Sturlese, M.; Caporale, A.; Roger, J.A.; Mascanzoni, F.; Ruvo, M.; Doti, N. Design, synthesis, structural analysis and biochemical studies of stapled AIF(370–394) analogues as ligand of CypA. Biochim. Biophys Acta Gen. Subj. 2020, 1864, 129717. [Google Scholar] [CrossRef]
- Russo, L.; Mascanzoni, F.; Farina, B.; Dolga, A.M.; Monti, A.; Caporale, A.; Culmsee, C.; Fattorusso, R.; Ruvo, M.; Doti, N. Design, Optimization, and Structural Characterization of an Apoptosis-Inducing Factor Peptide Targeting Human Cyclophilin A to Inhibit Apoptosis Inducing Factor-Mediated Cell Death. J. Med. Chem. 2021, 64, 11445–11459. [Google Scholar] [CrossRef]
- Chelko, S.P.; Keceli, G.; Carpi, A.; Doti, N.; Agrimi, J.; Asimaki, A.; Beti, C.B.; Miyamoto, M.; Amat-Codina, N.; Bedja, D.; et al. Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2021, 13, eabf0891. [Google Scholar] [CrossRef]
- Curci, A.; Mele, A.; Camerino, G.M.; Dinardo, M.M.; Tricarico, D. The large conductance Ca(2+) -activated K(+) (BKCa) channel regulates cell proliferation in SH-SY5Y neuroblastoma cells by activating the staurosporine-sensitive protein kinases. Front. Physiol. 2014, 5, 476. [Google Scholar] [CrossRef] [Green Version]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef]
- Mookherjee, P.; Quintanilla, R.; Roh, M.S.; Zmijewska, A.A.; Jope, R.S.; Johnson, G.V. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J. Cell Biochem. 2007, 102, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.E.; Du, F.; Fang, M.; Wang, X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc. Natl. Acad. Sci. USA 2005, 102, 17545–17550. [Google Scholar] [CrossRef] [Green Version]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sisson, E.M. Liraglutide: Clinical pharmacology and considerations for therapy. Pharmacotherapy 2011, 31, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Chaturvedi, R.K. Peptide therapeutics in neurodegenerative disorders. Curr. Med. Chem. 2014, 21, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019, 9, 6298. [Google Scholar] [CrossRef]
- Caporale, A.; Doti, N.; Monti, A.; Sandomenico, A.; Ruvo, M. Automatic procedures for the synthesis of difficult peptides using oxyma as activating reagent: A comparative study on the use of bases and on different deprotection and agitation conditions. Peptides 2018, 102, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.; Doti, N.; Sandomenico, A.; Ruvo, M. Evaluation of combined use of Oxyma and HATU in aggregating peptide sequences. J. Pept. Sci. 2017, 23, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, M.; Palumbo, R.; Monti, A.; Fontana, E.; Nebbioso, A.; Ruvo, M.; Altucci, L.; Doti, N. Relevance of AIF/CypA Lethal Pathway in SH-SY5Y Cells Treated with Staurosporine. Int. J. Mol. Sci. 2022, 23, 265. https://doi.org/10.3390/ijms23010265
Conte M, Palumbo R, Monti A, Fontana E, Nebbioso A, Ruvo M, Altucci L, Doti N. Relevance of AIF/CypA Lethal Pathway in SH-SY5Y Cells Treated with Staurosporine. International Journal of Molecular Sciences. 2022; 23(1):265. https://doi.org/10.3390/ijms23010265
Chicago/Turabian StyleConte, Mariarosaria, Rosanna Palumbo, Alessandra Monti, Elisabetta Fontana, Angela Nebbioso, Menotti Ruvo, Lucia Altucci, and Nunzianna Doti. 2022. "Relevance of AIF/CypA Lethal Pathway in SH-SY5Y Cells Treated with Staurosporine" International Journal of Molecular Sciences 23, no. 1: 265. https://doi.org/10.3390/ijms23010265
APA StyleConte, M., Palumbo, R., Monti, A., Fontana, E., Nebbioso, A., Ruvo, M., Altucci, L., & Doti, N. (2022). Relevance of AIF/CypA Lethal Pathway in SH-SY5Y Cells Treated with Staurosporine. International Journal of Molecular Sciences, 23(1), 265. https://doi.org/10.3390/ijms23010265









