Absolute Differential Cross-Sections for Elastic Electron Scattering from Sevoflurane Molecule in the Energy Range from 50–300 eV
Abstract
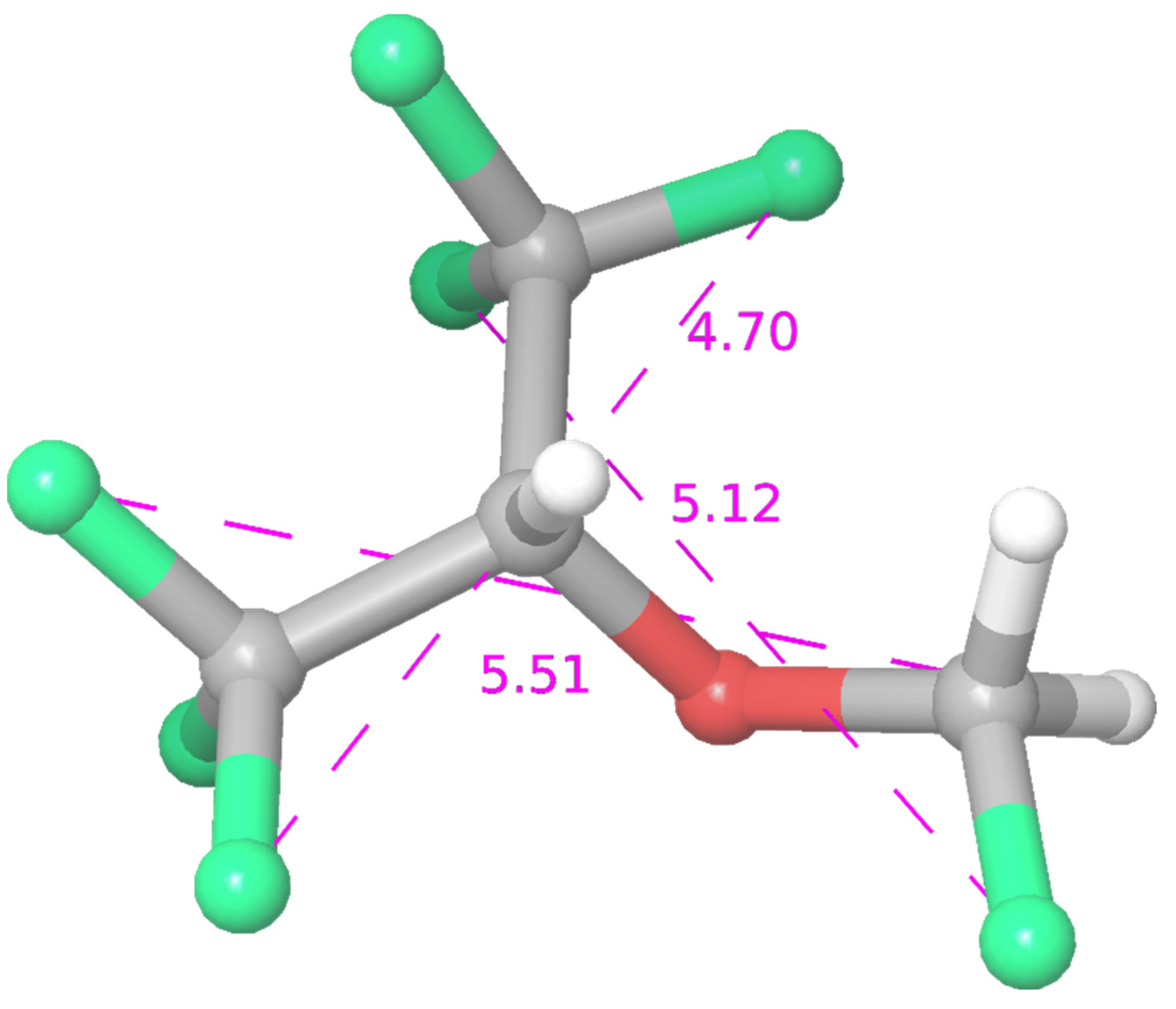
:1. Introduction
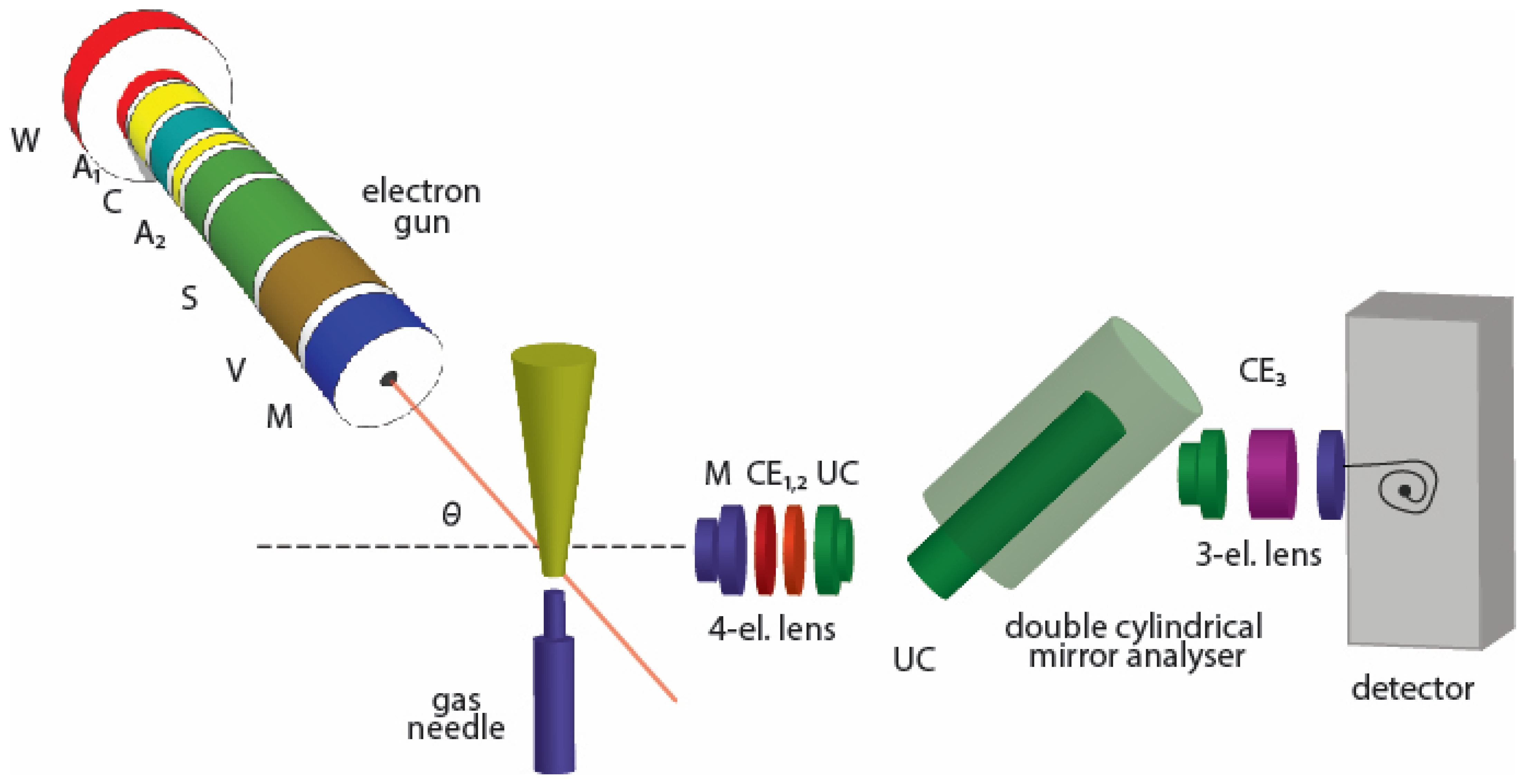
2. Experiment
- Stage 1: obtaining relative DCSs by measuring the intensity of electrons elastically scattered from SF, in the function of scattering angle (from 25° to 125° in 5° steps), for a given incident electron energy (50, 100, 150, 200, 250 and 300 eV). Electron intensities are measured at least three times for every incident energy. Background contributions are suppressed by introducing gas into the chamber, away from the interaction volume, via a side leak, and by subtracting measured intensities from the apparent signal for every angle at the given energy;
3. Theory
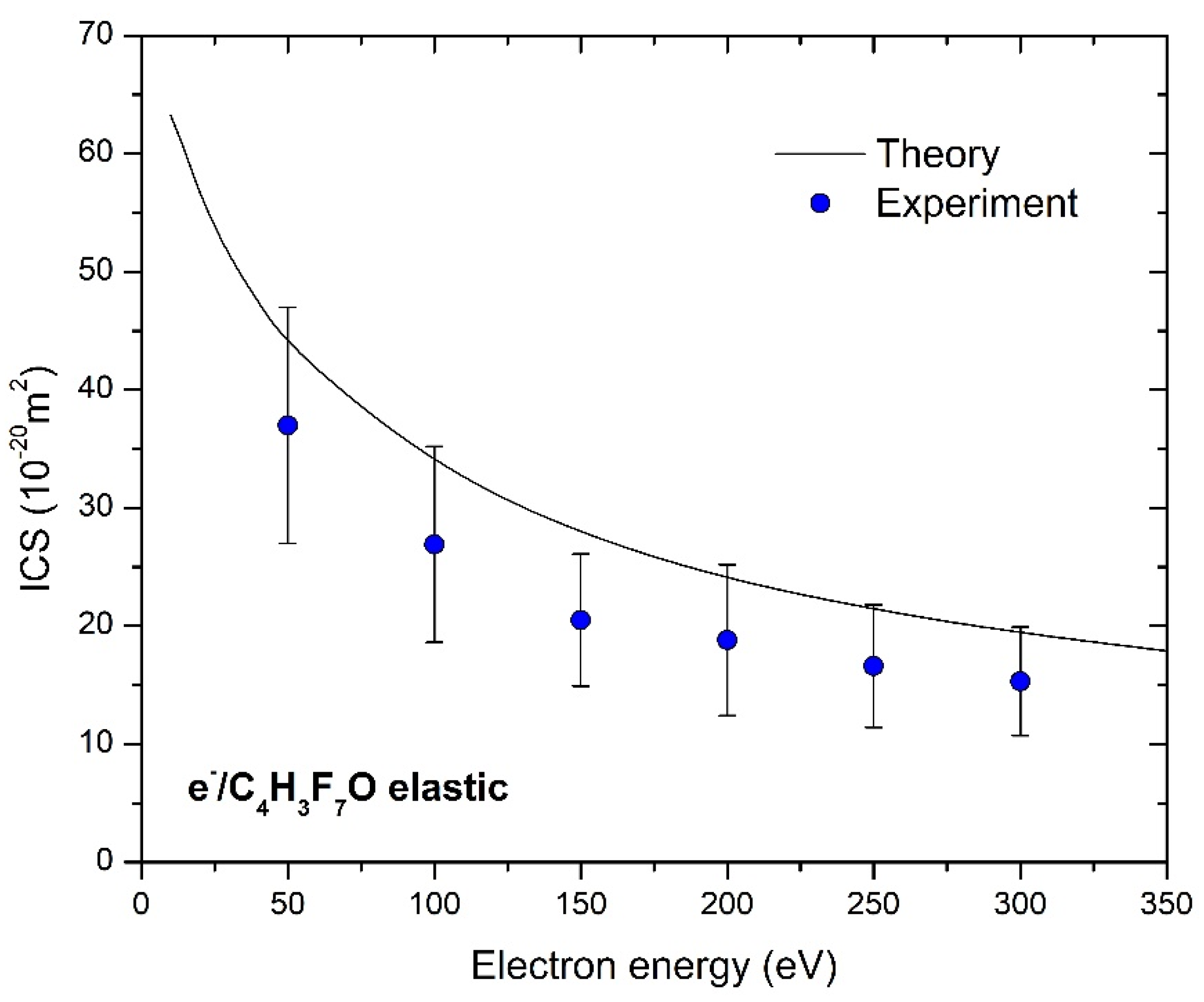
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, P.; Zubrycki, I.; Xu, Y. Ab Initio Calculation of structures and properties of halogenated general anesthetics: Halothane and sevoflurane. J. Comput. Chem. 2001, 22, 436–444. [Google Scholar] [CrossRef]
- Gaya da Costa, M.; Kalmar, A.F.; Struys, M.M.R.F. Inhaled Anesthetics: Environmental Role, Occupational Risk, and Clinical Use. J. Clin. Med. 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Langbein, T.; Sonntag, H.; Trapp, D.; Hoffmann, A.; Malms, W.; Röth, E.P.; Mörs, V.; Zellner, R. Volatile anaesthetics and the atmosphere: Atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br. J. Anaesth. 1999, 82, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lesarri, A.; Vega-Toribio, A.; Suenram, R.D.; Brughc, D.J.; Grabowd, J.U. The conformational landscape of the volatile anesthetic sevoflurane. Phys. Chem. Chem. Phys. 2010, 33, 9624–9631. [Google Scholar] [CrossRef]
- Dom, J.; Van der Veken, B.; Michielsen, B.; Jacobs, S.; Xue, Z.; Hesse, S.; Loritz, H.-M.; Suhmb, M.A.; Herrebout, W.A. On the weakly C–H⋯π hydrogen bonded complexes of sevoflurane and benzene. Phys. Chem. Chem. Phys. 2011, 13, 14142–14152. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Ikeda, K. Uptake and biotransformation of sevoflurane in humans: A comparative study with halothane, enflurane, and isoflurane. J. Clin. Anesth. 1990, 2, 381–386. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, F.; Huang, L.; Fang, F.; Yang, G.; Tanzi, R.E.; Zhang, Y.; Quan, Q.; Xie, Z. The anesthetic sevoflurane induces tau trafficking from neurons to microglia. Commun. Biol. 2021, 4, 560. [Google Scholar] [CrossRef]
- Lange, E.; Ferreira da Silva, F.; Jones, N.C.; Hoffmann, S.V.; Duflot, D.; Limão-Vieira, P.M. The lowest-lying electronic states of isoflurane and sevoflurane in the 5.0–10.8 eV energy range investigated by experimental and theoretical methods. Chem. Phys. Lett. 2019, 716, 42–48. [Google Scholar] [CrossRef]
- Lozano, A.I.; Ferreira da Silva, F.; Blanco, F.; Limão-Vieira, P.M.; García, G. Total electron scattering cross section from sevoflurane by 1–300 eV energy electron impact. Chem. Phys. Lett. 2018, 706, 533–537. [Google Scholar] [CrossRef]
- Tanaka, H.; Brunger, M.J.; Campbell, L.; Kato, K.; Hoshino, M.; Rau, A.R.P. Scaled plane-wave Born cross sections for atoms and molecules. Rev. Mod. Phys. 2016, 88, 025004. [Google Scholar] [CrossRef]
- Brunger, M.J. Electron scattering and transport in biofuels, biomolecules and biomass fragments. Int. Rev. Phys. Chem. 2017, 36, 333–376. [Google Scholar] [CrossRef]
- Vukalović, J.; Maljković, J.B.; Tökési, K.; Predojević, B.; Marinković, B.P. Elastic Electron Scattering from Methane Molecule in the Energy Range from 50–300 eV. Int. J. Mol. Sci. 2021, 22, 647. [Google Scholar] [CrossRef] [PubMed]
- Maljković, J.B.; Vuković, J.; Tökési, K.; Predojević, B.; Marinković, B.P. Elastic electron scattering cross sections for triethyl phosphate molecule at intermediate electron energies from 50 to 250 eV. Eur. Phys. J. D 2019, 73, 27. [Google Scholar] [CrossRef]
- Ranković, M.; Maljković, J.B.; Tökési, K.; Marinković, B.P. Elastic electron differential cross sections for argon atom in the intermediate energy range from 40 eV to 300 eV. Eur. Phys. J. D 2018, 72, 30. [Google Scholar] [CrossRef]
- Lucas, B.C. Atomic and Molecular Beams Production and Collimation; CRC Press: Boca Raton, FL, USA, 2012; pp. 217–223. [Google Scholar]
- Milosavljević, A.R.; Giuliani, A.; Šević, D.; Hubin-Franskin, M.J.; Marinković, B.P. Elastic scattering of electrons from tetrahydrofuran molecule. Eur. Phys. J. D 2005, 35, 411–416. [Google Scholar]
- Allan, M. Absolute angle-differential elastic and vibrational excitation cross sections for electron collisions with tetrahydrofuran. J. Phys. B 2007, 40, 3531–3544. [Google Scholar] [CrossRef] [Green Version]
- Homem, M.G.P.; Iga, I.; Sugohara, R.T.; Sanches, I.P.; Lee, M. Role of adsorption effects on absolute electron-molecule cross-section calibration using the relative flow technique. Rev. Sci. Instrum. 2011, 82, 013109. [Google Scholar] [CrossRef]
- Nickel, J.C.; Mott, C.; Kanik, I.; McCollum, D.C. Absolute elastic differential electron scattering cross sections for carbon monoxide and molecular nitrogen in the intermediate energy region. J. Phys. B 1988, 21, 1867–1877. [Google Scholar] [CrossRef]
- Nickel, J.C.; Zetner, P.V.; Shen, G.; Trajmar, S. Principles and procedures for determining absolute differential electron-molecule (atom) scattering cross sections. J. Phys. E 1989, 22, 730–738. [Google Scholar] [CrossRef]
- Williams, J.F.; Willis, B.A. The scattering of electrons from inert gases I. Absolute differential elastic cross sections for argon atoms. J. Phys. B 1975, 8, 1670–1682. [Google Scholar] [CrossRef]
- Maljković, J.B.; Blanco, F.; García, G.; Marinković, B.P.; Milosavljević, A.R. Absolute cross sections for elastic electron scattering from methylformamide. Phys. Rev. A 2012, 85, 042723. [Google Scholar] [CrossRef] [Green Version]
- Olander, D.R.; Kruger, V. Molecular Beam Sources Fabricated from Multichannel Arrays. III. The Exit Density Problem. J. Appl. Phys. 1970, 41, 2769–2776. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.; Rosado, J.; Illana, A.; García, G. Comparison of two screening corrections to the additivity rule for the calculation of electron scattering from polyatomic molecules. Phys. Lett. A 2010, 374, 4420–4424. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.; Ellis-Gibbings, L.; García, G. Screening corrections for the interference contributions to the electron and positron scattering cross sections from polyatomic molecules. Chem. Phys. Lett. 2016, 645, 71–75. [Google Scholar] [CrossRef]
- Traore Dubuis, A.; Verkhovtsev, A.; Ellis-Gibbings, L.; Krupa, K.; Blanco, F.; Jones, D.B.; Brunger, M.J.; García, G. Total cross section of furfural by electron impact: Experiment and theory. J. Chem. Phys. 2017, 147, 054301. [Google Scholar] [CrossRef]
- Fuss, M.C.; Sanz, A.G.; Blanco, F.; Oller, J.C.; Limão-Vieira, P.; Brunger, M.J.; García, G. Total electron-scattering cross sections from pyrimidine as measured using a magnetically confined experimental system. Phys. Rev. A 2013, 88, 042702. [Google Scholar] [CrossRef] [Green Version]
- Lozano, A.I.; Oller, J.C.; Jones, D.B.; da Costa, R.F.; do, N.; Varella, M.T.; Bettega, M.H.F.; Ferreira da Silva, F.; Limão-Vieira, P.; Lima, M.A.P.; et al. Total electron scattering cross sections from para-benzoquinone in the energy range 1–200 eV. Phys. Chem. Chem. Phys. 2018, 20, 22368–22378. [Google Scholar] [CrossRef] [PubMed]
- Staszewska, G.; Schwenke, D.W.; Thirumalai, D.; Truhlar, D.G. Quasifree-scattering model for the imaginary part of the optical potential for electron scattering. Phys. Rev. A 1983, 28, 2740–2751. [Google Scholar] [CrossRef]
- Cowan, R.D. The Theory of Atomic Structure and Spectra; University of California Press: Berkeley, CA, USA, 1981. [Google Scholar]
- Riley, M.E.; Truhlar, D.G. Approximations for the exchange potential in electron scattering. J. Chem. Phys. 1975, 63, 2182–2191. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Liu, Y. A new approach to the correlation polarization potential-low-energy electron elastic scattering by He atoms. J. Phys. B At. Mol. Opt. Phys. 1992, 25, 1893–1897. [Google Scholar] [CrossRef]
- Blanco, F.; García, G. Screening corrections for calculation of electron scattering differential cross sections from polyatomic molecules. Phys. Lett. A 2004, 330, 230–237. [Google Scholar] [CrossRef]
- Sanz, A.G.; Fuss, M.C.; Blanco, F.; Sebastianelli, F.; Gianturco, F.A.; García, G. Electron scattering cross sections from HCN over a broad energy range (0.1–10000 eV): Influence of the permanent dipole moment on the scattering process. J. Chem. Phys. 2012, 137, 124103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
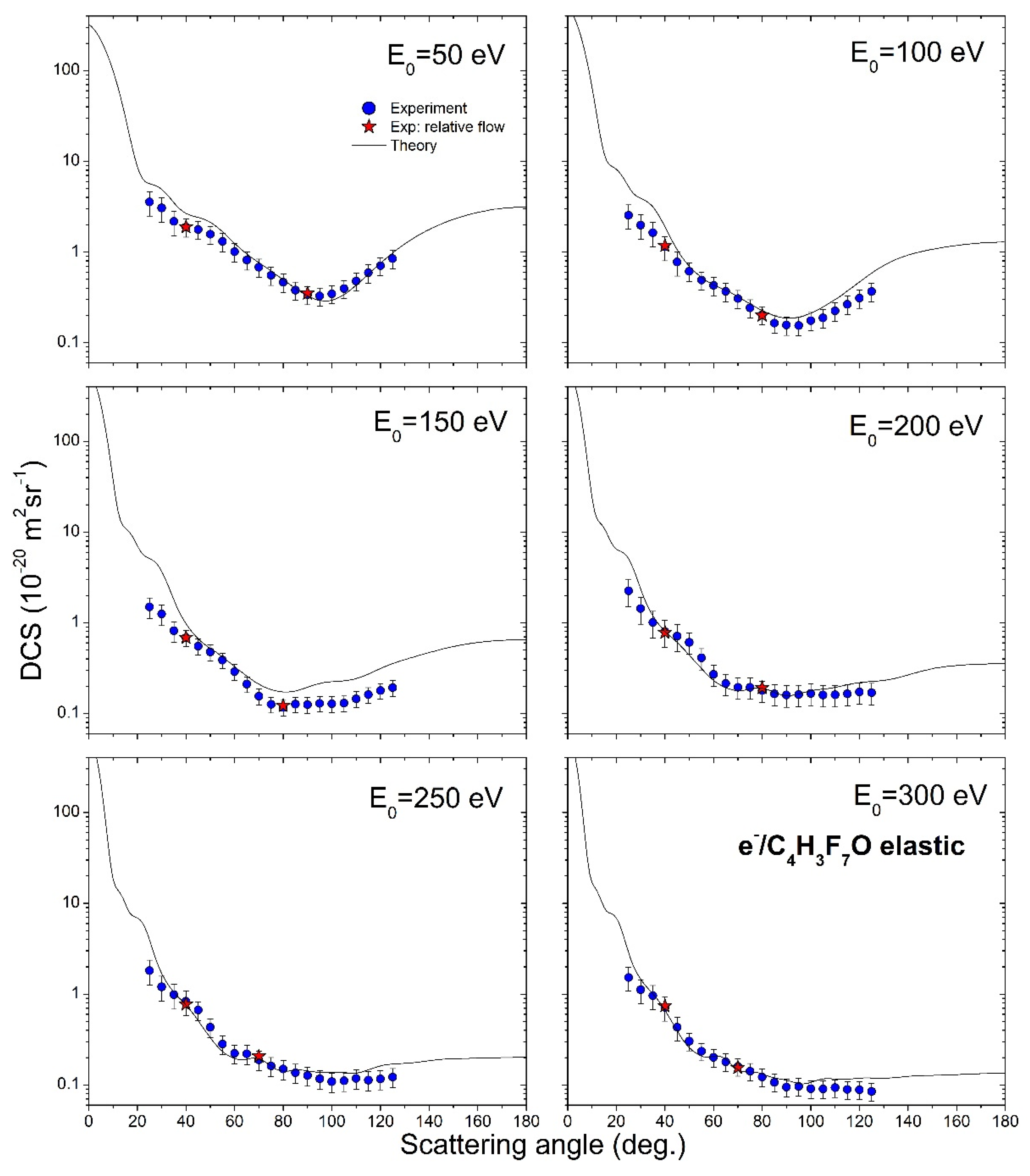
| θ (°) | DCS (10−20 m2 sr−1) | |||||
|---|---|---|---|---|---|---|
| 50 (eV) | 100 (eV) | 150 (eV) | 200 (eV) | 250 (eV) | 300 (eV) | |
| 25 | 3.6(1.1) | 2.55(76) | 1.50(39) | 2.25(74) | 1.82(56) | 1.53(45) |
| 30 | 3.07(93) | 1.98(59) | 1.25(32) | 1.44(48) | 1.20(37) | 1.12(33) |
| 35 | 2.17(66) | 1.64(49) | 0.82(21) | 1.01(33) | 0.99(30) | 0.96(28) |
| 40 | 1.89(43) | 1.14(34) | 0.69(14) | 0.80(26) | 0.84(26) | 0.72(21) |
| 45 | 1.77(40) | 0.78(23) | 0.55(11) | 0.71(24) | 0.67(16) | 0.43(13) |
| 50 | 1.57(36) | 0.61(14) | 0.476(94) | 0.61(16) | 0.43(10) | 0.304(66) |
| 55 | 1.31(30) | 0.49(11) | 0.386(77) | 0.41(11) | 0.282(67) | 0.235(51) |
| 60 | 1.01(23) | 0.428(98) | 0.289(57) | 0.270(71) | 0.223(53) | 0.202(44) |
| 65 | 0.82(18) | 0.369(84) | 0.211(42) | 0.214(57) | 0.220(52) | 0.180(39) |
| 70 | 0.68(15) | 0.307(70) | 0.155(31) | 0.194(51) | 0.189(45) | 0.160(35) |
| 75 | 0.55(13) | 0.243(56) | 0.126(25) | 0.195(51) | 0.162(38) | 0.141(31) |
| 80 | 0.47(11) | 0.203(47) | 0.117(23) | 0.179(47) | 0.150(36) | 0.122(27) |
| 85 | 0.380(86) | 0.165(38) | 0.127(25) | 0.165(44) | 0.139(33) | 0.107(24) |
| 90 | 0.339(77) | 0.157(36) | 0.125(25) | 0.160(43) | 0.127(30) | 0.095(21) |
| 95 | 0.327(74) | 0.154(35) | 0.129(26) | 0.162(44) | 0.117(28) | 0.096(21) |
| 100 | 0.347(79) | 0.175(40) | 0.128(25) | 0.167(44) | 0.109(26) | 0.091(20) |
| 105 | 0.396(90) | 0.188(43) | 0.130(26) | 0.160(42) | 0.111(27) | 0.090(21) |
| 110 | 0.48(11) | 0.225(51) | 0.146(29) | 0.160(42) | 0.118(28) | 0.093(20) |
| 115 | 0.59(13) | 0.265(61) | 0.161(32) | 0.165(44) | 0.113(27) | 0.089(20) |
| 120 | 0.71(16) | 0.311(71) | 0.179(35) | 0.173(46) | 0.116(28) | 0.088(20) |
| 125 | 0.85(19) | 0.367(84) | 0.193(38) | 0.170(45) | 0.122(29) | 0.085(19) |
| ICS’s | 37(10) | 26.9(8.3) | 20.5(5.6) | 18.8(6.4) | 16.6(5.2) | 15.3(4.6) |
| MTCS’s | 13.5(4.3) | 5.8(1.5) | 2.94(68) | 2.90(85) | 2.14(58) | 1.66(43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukalović, J.; Maljković, J.B.; Blanco, F.; García, G.; Predojević, B.; Marinković, B.P. Absolute Differential Cross-Sections for Elastic Electron Scattering from Sevoflurane Molecule in the Energy Range from 50–300 eV. Int. J. Mol. Sci. 2022, 23, 21. https://doi.org/10.3390/ijms23010021
Vukalović J, Maljković JB, Blanco F, García G, Predojević B, Marinković BP. Absolute Differential Cross-Sections for Elastic Electron Scattering from Sevoflurane Molecule in the Energy Range from 50–300 eV. International Journal of Molecular Sciences. 2022; 23(1):21. https://doi.org/10.3390/ijms23010021
Chicago/Turabian StyleVukalović, Jelena, Jelena B. Maljković, Francisco Blanco, Gustavo García, Branko Predojević, and Bratislav P. Marinković. 2022. "Absolute Differential Cross-Sections for Elastic Electron Scattering from Sevoflurane Molecule in the Energy Range from 50–300 eV" International Journal of Molecular Sciences 23, no. 1: 21. https://doi.org/10.3390/ijms23010021
APA StyleVukalović, J., Maljković, J. B., Blanco, F., García, G., Predojević, B., & Marinković, B. P. (2022). Absolute Differential Cross-Sections for Elastic Electron Scattering from Sevoflurane Molecule in the Energy Range from 50–300 eV. International Journal of Molecular Sciences, 23(1), 21. https://doi.org/10.3390/ijms23010021







