In Vitro Propagation of XXY Undifferentiated Mouse Spermatogonia: Model for Fertility Preservation in Klinefelter Syndrome Patients
Abstract
1. Introduction
2. Results
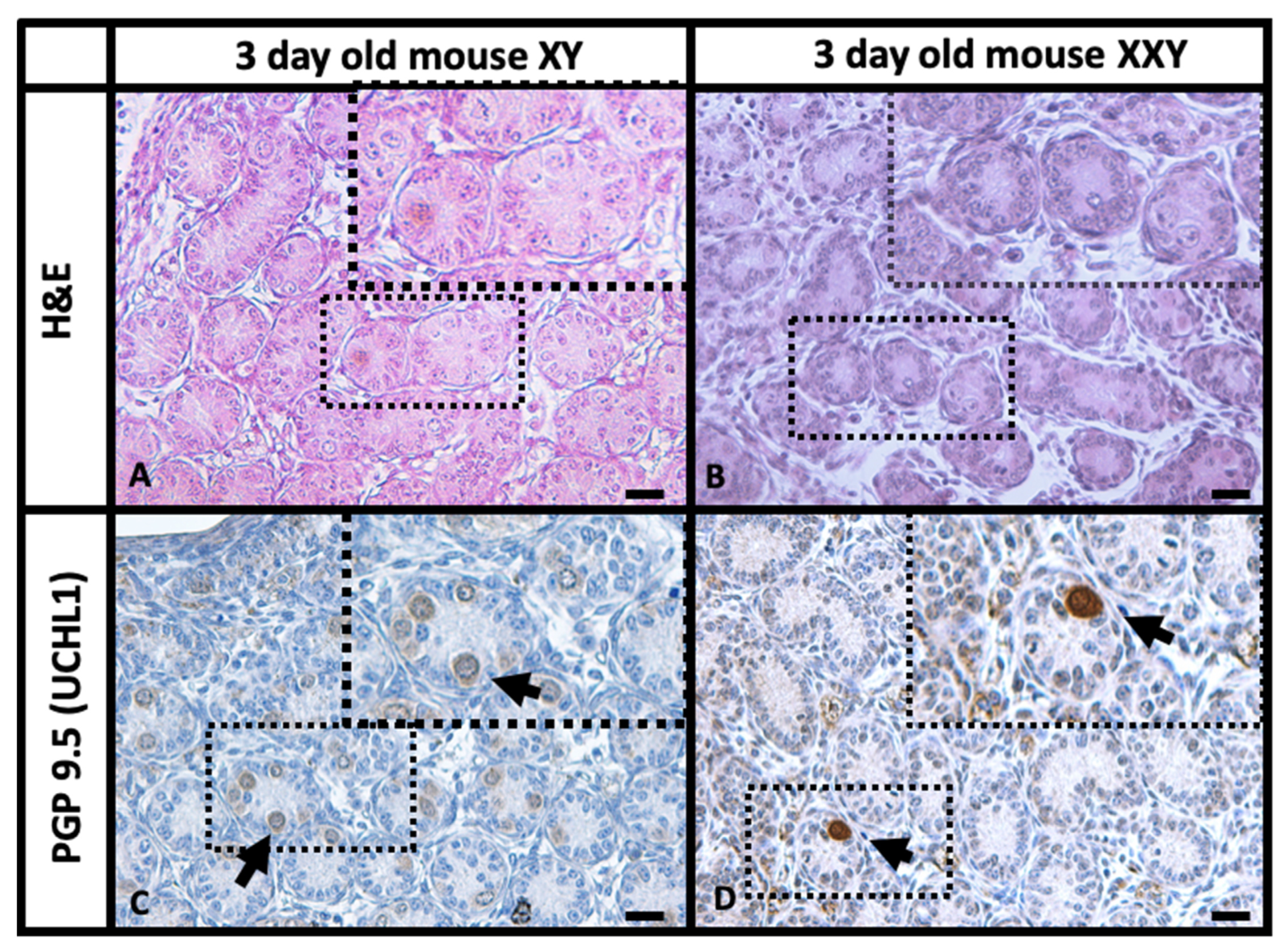
2.1. Histology and Immunohistochemistry (IHC) Comparison of XY and XXY Neonatal Mouse Testes
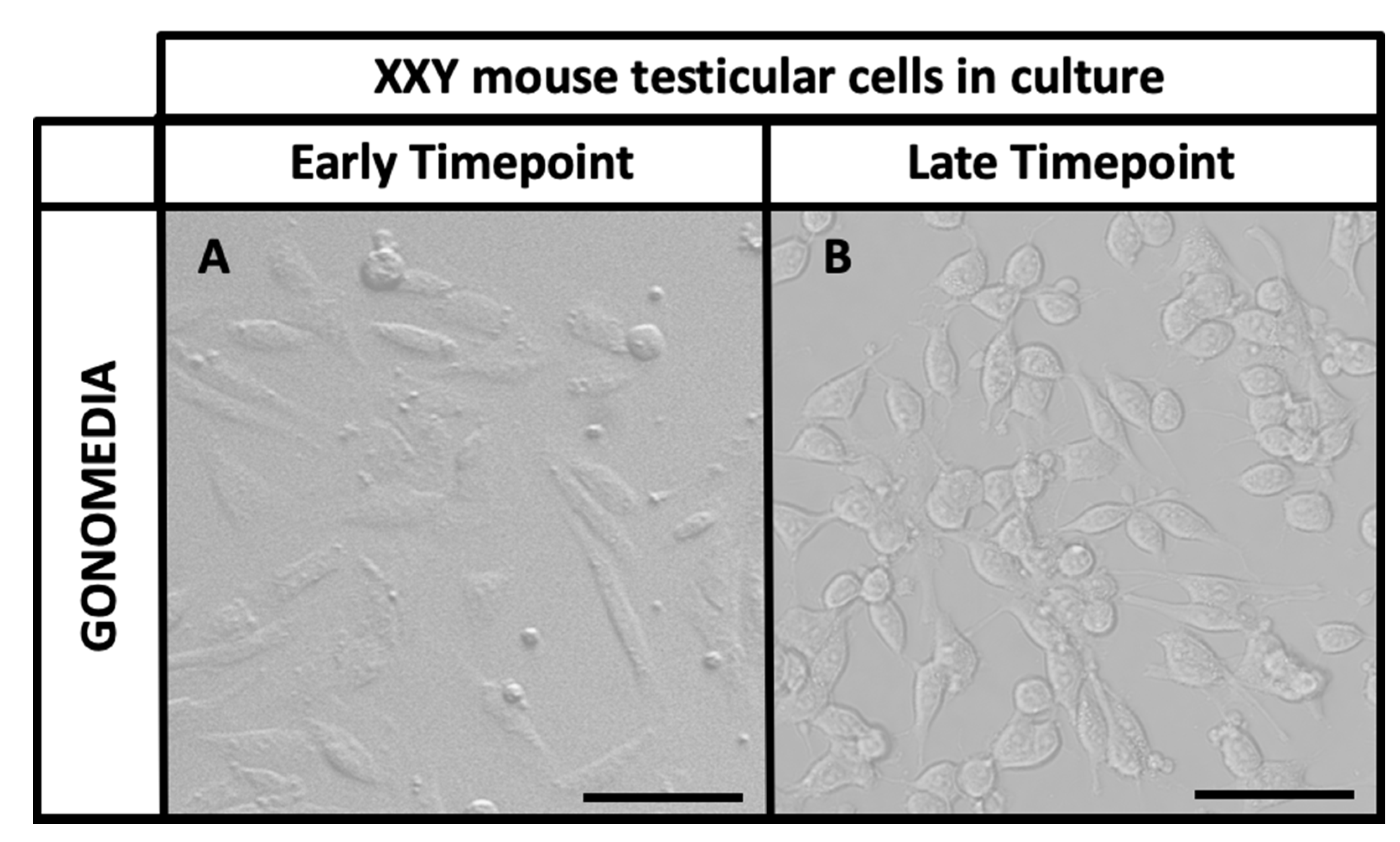
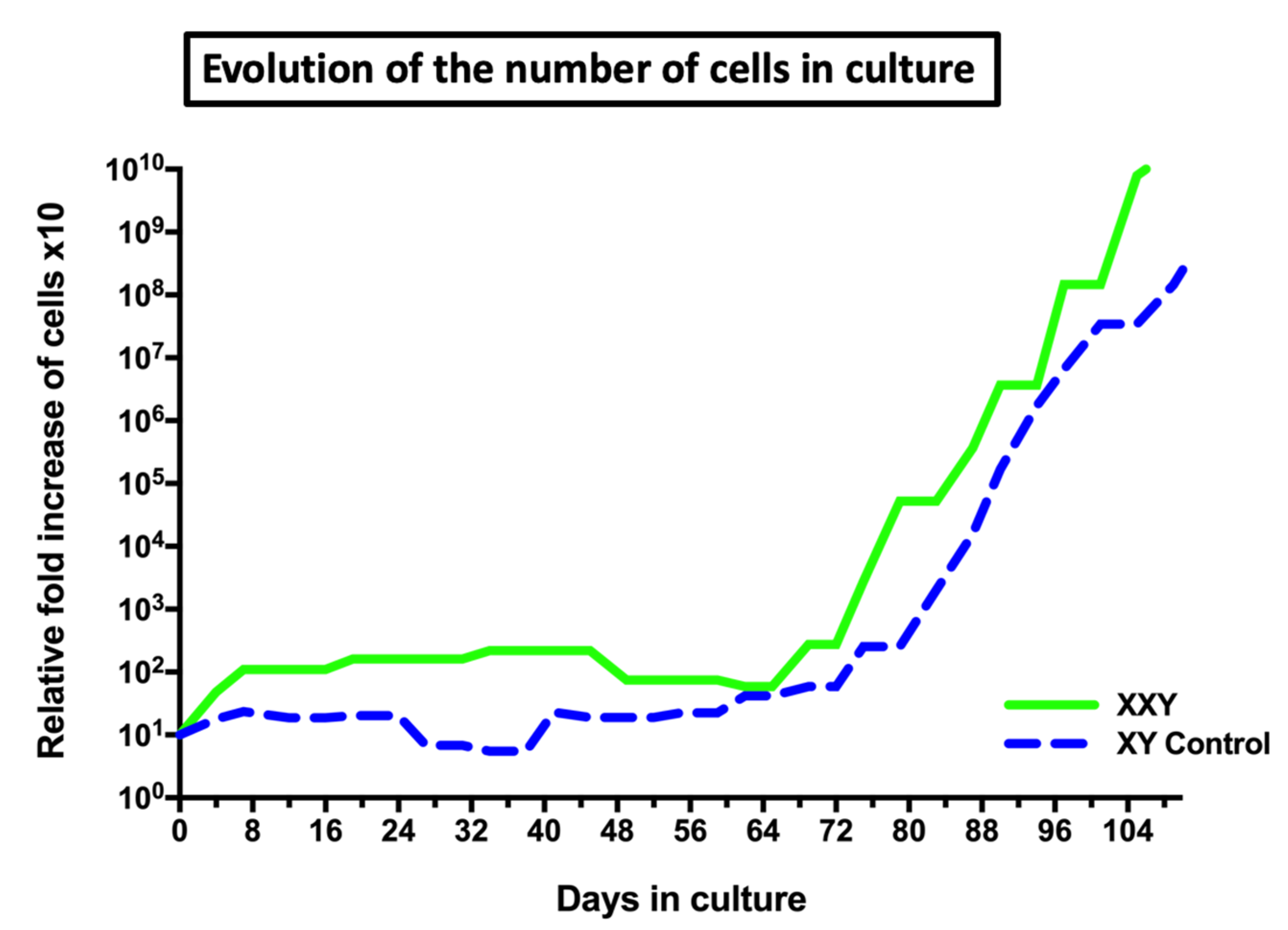
2.2. Isolation and Culture of XXY Neonatal Mouse Testicular Cells
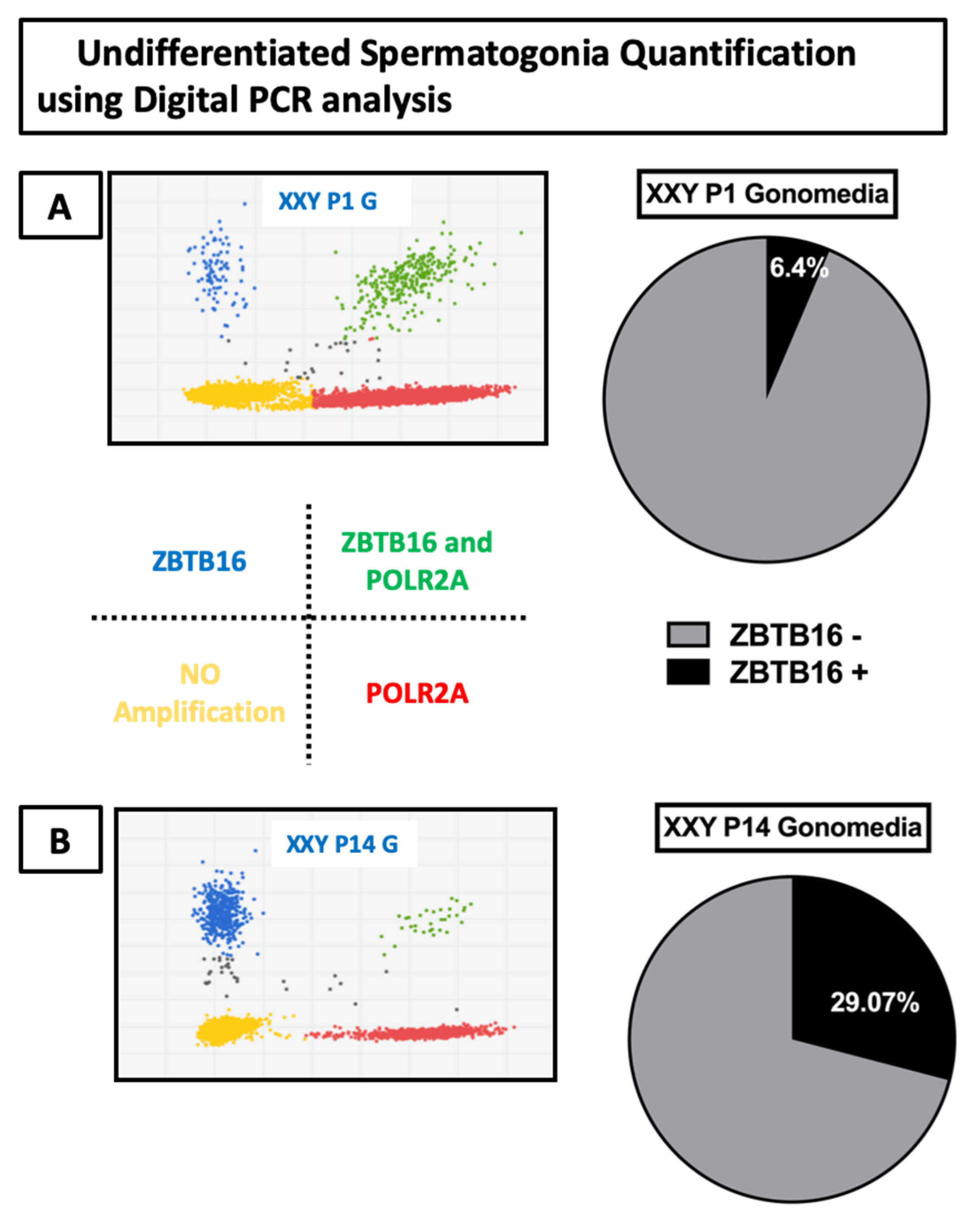
2.3. Gene Expression Analyses of Cultured Cells
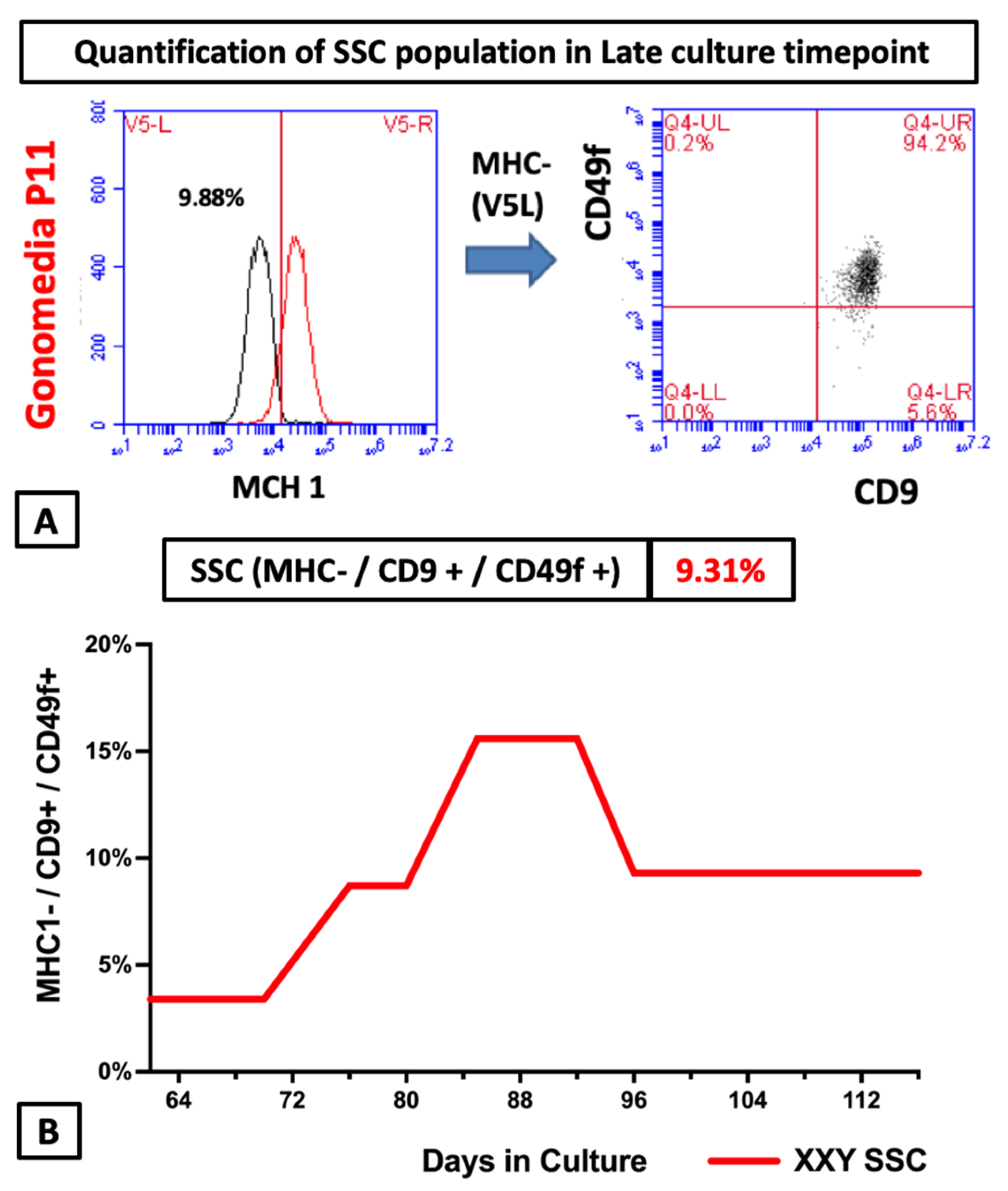
2.4. Flow Cytometry Analyses of Cultured Cells from XXY Testes
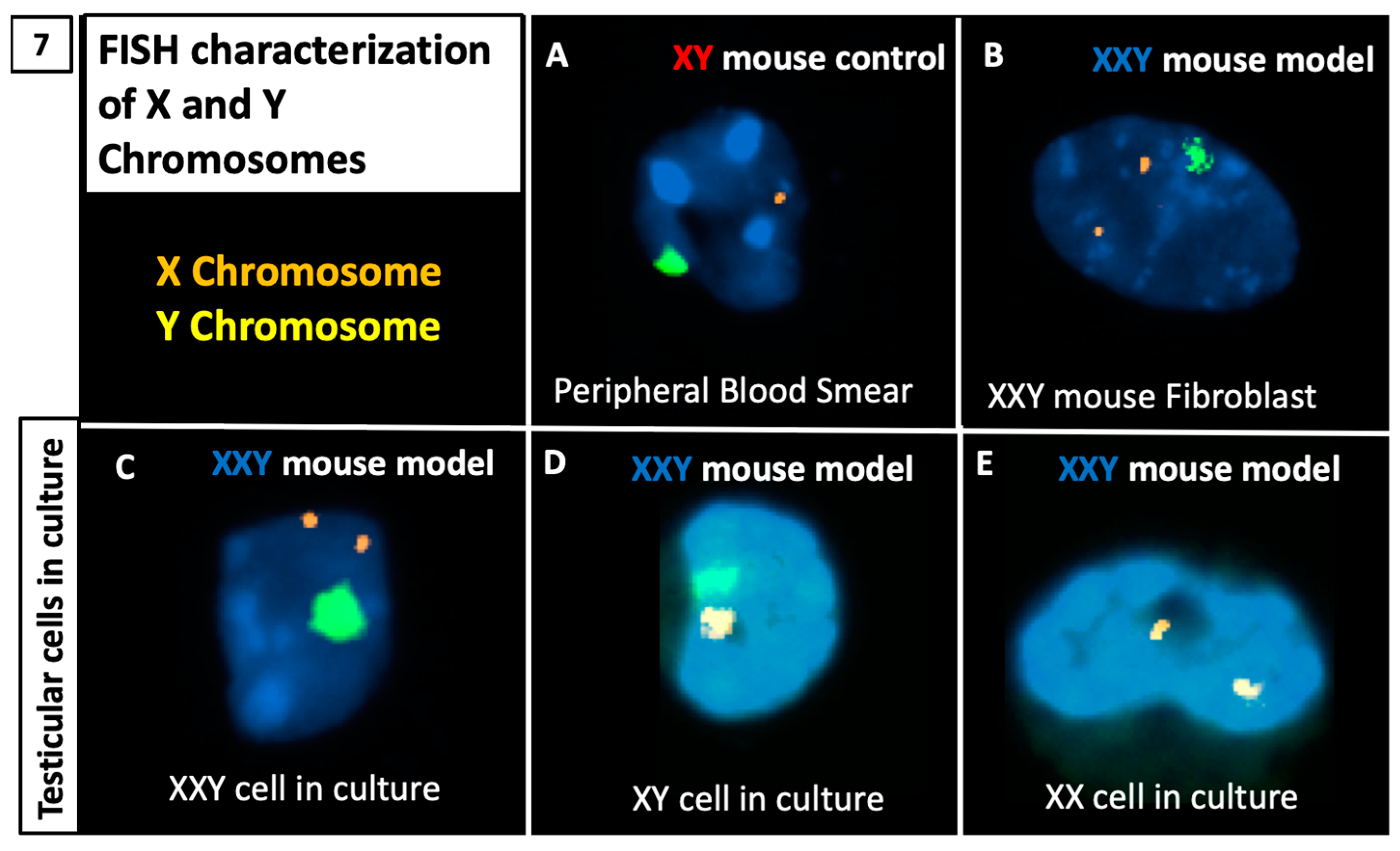
2.5. DNA FISH Analysis for X and Y Chromosomes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histology, Immunohistochemistry, and Viability Assay
4.2.1. Hematoxylin and Eosin Staining
4.2.2. Immunohistochemical Staining
4.3. Cell Isolation, Culture, and Cryopreservation
4.4. Quantitative Reverse Transcriptase Polymerase Chain Reaction (q RT-PCR)
4.5. Digital Reverse Transcriptase Polymerase Chain Reaction (d RT-PCR)
4.6. Flow Cytometry Analyses
4.7. X and Y Chromosome Fluorescent In Situ Hybridization (FISH)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanfranco, F.; Kamischke, A.; Zitzmann, M.; Nieschlag, E. Klinefelter’s Syndrome. Lancet 2004, 364, 273–283. [Google Scholar] [CrossRef]
- Nielsen, J.; Wohlert, M. Sex Chromosome Abnormalities Found among 34,910 Newborn Children: Results from a 13-Year Incidence Study in Arhus, Denmark. Birth Defects Orig. Artic. Ser. 1990, 26, 209–223. [Google Scholar]
- Bojesen, A.; Juul, S.; Gravholt, C.H. Prenatal and Postnatal Prevalence of Klinefelter Syndrome: A National Registry Study. J Clin. Endocrinol. Metab 2003, 88, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.L.; Krmpotic, E.; Thomas, W.; Gandy, H.M.; Paulsen, C.A. Pathologic Testicular Findings in Klinefelter’s Syndrome. 47,XXY vs. 46,XY-47,XXY. Arch. Intern. Med. 1972, 130, 726–729. [Google Scholar] [CrossRef]
- Autio-Harmainen, H.; Rapola, J.; Aula, P. Fetal Gonadal Histology in XXXXY, XYY and XXX Syndromes. Clin. Genet. 1980, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Coerdt, W.; Rehder, H.; Gausmann, I.; Johannisson, R.; Gropp, A. Quantitative Histology of Human Fetal Testes in Chromosomal Disease. Pediatr. Pathol. 1985, 3, 245–259. [Google Scholar] [CrossRef]
- Mikamo, K.; Aguercif, M.; Hazeghi, P.; Martin-Du Pan, R. Chromatin-Positive Klinefelter’s Syndrome. A Quantitative Analysis of Spermatogonial Deficiency at 3, 4, and 12 Months of Age. Fertil. Steril. 1968, 19, 731–739. [Google Scholar] [CrossRef]
- Winge, S.B.; Dalgaard, M.D.; Jensen, J.M.; Graem, N.; Schierup, M.H.; Juul, A.; Rajpert-De Meyts, E.; Almstrup, K. Transcriptome Profiling of Fetal Klinefelter Testis Tissue Reveals a Possible Involvement of Long Non-Coding RNAs in Gonocyte Maturation. Hum. Mol. Genet. 2018, 27, 430–439. [Google Scholar] [CrossRef]
- Wikstrom, A.M.; Raivio, T.; Hadziselimovic, F.; Wikstrom, S.; Tuuri, T.; Dunkel, L. Klinefelter Syndrome in Adolescence: Onset of Puberty Is Associated with Accelerated Germ Cell Depletion. J. Clin. Endocrinol. Metab. 2004, 89, 2263–2270. [Google Scholar] [CrossRef]
- Aksglaede, L.; Wikstrom, A.M.; Rajpert-De Meyts, E.; Dunkel, L.; Skakkebaek, N.E.; Juul, A. Natural History of Seminiferous Tubule Degeneration in Klinefelter Syndrome. Hum. Reprod. Update 2006, 12, 39–48. [Google Scholar] [CrossRef]
- Groth, K.A.; Skakkebaek, A.; Host, C.; Gravholt, C.H.; Bojesen, A. Clinical Review: Klinefelter Syndrome--a Clinical Update. J. Clin. Endocrinol. Metab. 2013, 98, 20–30. [Google Scholar] [CrossRef]
- Wikstrom, A.M.; Hoei-Hansen, C.E.; Dunkel, L.; Rajpert-De Meyts, E. Immunoexpression of Androgen Receptor and Nine Markers of Maturation in the Testes of Adolescent Boys with Klinefelter Syndrome: Evidence for Degeneration of Germ Cells at the Onset of Meiosis. J. Clin. Endocrinol. Metab. 2007, 92, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sofikitis, N.; Mio, Y.; Loutradis, D.; Kaponis, A.; Miyagawa, I. Morphometric and Cytogenetic Characteristics of Testicular Germ Cells and Sertoli Cell Secretory Function in Men with Non-Mosaic Klinefelter’s Syndrome. Hum. Reprod. 2002, 17, 886–896. [Google Scholar] [CrossRef]
- Paduch, D.A.; Bolyakov, A.; Cohen, P.; Travis, A. Reproduction in Men with Klinefelter Syndrome: The Past, the Present, and the Future. Semin. Reprod. Med. 2009, 27, 137–148. [Google Scholar] [CrossRef]
- Foresta, C.; Galeazzi, C.; Bettella, A.; Marin, P.; Rossato, M.; Garolla, A.; Ferlin, A. Analysis of Meiosis in Intratesticular Germ Cells from Subjects Affected by Classic Klinefelter’s Syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 3807–3810. [Google Scholar] [CrossRef] [PubMed]
- Salbenblatt, J.A.; Bender, B.G.; Puck, M.H.; Robinson, A.; Faiman, C.; Winter, J.S. Pituitary-Gonadal Function in Klinefelter Syndrome before and during Puberty. Pediatr. Res. 1985, 19, 82–86. [Google Scholar] [CrossRef]
- Wikstrom, A.M.; Dunkel, L. Testicular Function in Klinefelter Syndrome. Horm. Res. 2008, 69, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Jorgensen, N.; Skakkebaek, N.E.; Juul, A. Low Semen Volume in 47 Adolescents and Adults with 47,XXY Klinefelter or 46,XX Male Syndrome. Int. J. Androl. 2009, 32, 376–384. [Google Scholar] [CrossRef]
- Cruger, D.; Toft, B.; Agerholm, I.; Fedder, J.; Hald, F.; Bruun-Petersen, G. Birth of a Healthy Girl after ICSI with Ejaculated Spermatozoa from a Man with Non-Mosaic Klinefelter’s Syndrome. Hum. Reprod. 2001, 16, 1909–1911. [Google Scholar] [CrossRef]
- Kitamura, M.; Matsumiya, K.; Koga, M.; Nishimura, K.; Miura, H.; Tsuji, T.; Matsumoto, M.; Okamoto, Y.; Okuyama, A. Ejaculated Spermatozoa in Patients with Non-Mosaic Klinefelter’s Syndrome. Int. J. Urol. 2000, 7, 88–92; discussion 93–94. [Google Scholar] [PubMed]
- Akashi, T.; Fuse, H.; Kojima, Y.; Hayashi, M.; Honda, S. Birth after Intracytoplasmic Sperm Injection of Ejaculated Spermatozoa from a Man with Mosaic Klinefelter’s Syndrome. Asian J. Androl. 2005, 7, 217–220. [Google Scholar] [CrossRef]
- De Sanctis, V.; Ciccone, S. Fertility Preservation in Adolescents with Klinefelter’s Syndrome. Pediatr. Endocrinol. Rev. 2010, 8 (Suppl. 1), 178–181. [Google Scholar] [PubMed]
- Forti, G.; Corona, G.; Vignozzi, L.; Krausz, C.; Maggi, M. Klinefelter’s Syndrome: A Clinical and Therapeutical Update. Sex. Dev. 2010, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Pizzocaro, A.; Lanfranco, F.; Garolla, A.; Pelliccione, F.; Vignozzi, L.; Ferlin, A.; Foresta, C.; Jannini, E.A.; Maggi, M.; et al. Sperm Recovery and ICSI Outcomes in Klinefelter Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2017, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Sermondade, N.; Brioude, F.; Berthaut, I.; Bachelot, A.; Hamid, R.H.; Khattabi, L.E.; Prades, M.; Lévy, R.; Dupont, C. Fertility Preservation in Young Men with Klinefelter Syndrome: A Systematic Review. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102177. [Google Scholar] [CrossRef]
- Vicdan, K.; Akarsu, C.; Sozen, E.; Buluc, B.; Vicdan, A.; Yilmaz, Y.; Biberoglu, K. Outcome of Intracytoplasmic Sperm Injection Using Fresh and Cryopreserved-Thawed Testicular Spermatozoa in 83 Azoospermic Men with Klinefelter Syndrome. J. Obstet. Gynaecol. Res. 2016, 42, 1558–1566. [Google Scholar] [CrossRef]
- Deebel, N.A.; Galdon, G.; Zarandi, N.P.; Stogner-Underwood, K.; Howards, S.; Lovato, J.; Kogan, S.; Atala, A.; Lue, Y.; Sadri-Ardekani, H. Spermatogonia Stem Cell Technology: A New Avenue for All Age Klinefelter Patients. Hum. Reprod. Update 2021, 15, dmab025. [Google Scholar] [CrossRef]
- Van Saen, D.; Gies, I.; De Schepper, J.; Tournaye, H.; Goossens, E. Can Pubertal Boys with Klinefelter Syndrome Benefit from Spermatogonial Stem Cell Banking? Hum. Reprod. 2012, 27, 323–330. [Google Scholar] [CrossRef]
- Damani, M.N.; Mittal, R.; Oates, R.D. Testicular Tissue Extraction in a Young Male with 47,XXY Klinefelter’s Syndrome: Potential Strategy for Preservation of Fertility. Fertil. Steril. 2001, 76, 1054–1056. [Google Scholar] [CrossRef]
- Gies, I.; De Schepper, J.; Goossens, E.; Van Saen, D.; Pennings, G.; Tournaye, H. Spermatogonial Stem Cell Preservation in Boys with Klinefelter Syndrome: To Bank or Not to Bank, That’s the Question. Fertil. Steril. 2012, 98, 284–289. [Google Scholar] [CrossRef]
- Gies, I.; Oates, R.; De Schepper, J.; Tournaye, H. Testicular Biopsy and Cryopreservation for Fertility Preservation of Prepubertal Boys with Klinefelter Syndrome: A pro/Con Debate. Fertil. Steril. 2016, 105, 249–255. [Google Scholar] [CrossRef]
- Van Saen, D.; Vloeberghs, V.; Gies, I.; Mateizel, I.; Sermon, K.; De Schepper, J.; Tournaye, H.; Goossens, E. When Does Germ Cell Loss and Fibrosis Occur in Patients with Klinefelter Syndrome? Hum. Reprod. 2018, 33, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Paduch, D.A. Klinefelter Syndrome: An Argument for Early Aggressive Hormonal and Fertility Management. Fertil. Steril. 2012, 98, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Deebel, N.A.; Galdon, G.; Zarandi, N.P.; Stogner-Underwood, K.; Howards, S.; Lovato, J.; Kogan, S.; Atala, A.; Lue, Y.; Sadri-Ardekani, H. Age-Related Presence of Spermatogonia in Patients with Klinefelter Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2020, 26, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Lue, Y.; Jentsch, J.D.; Wang, C.; Rao, P.N.; Hikim, A.P.; Salameh, W.; Swerdloff, R.S. XXY Mice Exhibit Gonadal and Behavioral Phenotypes Similar to Klinefelter Syndrome. Endocrinology 2005, 146, 4148–4154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lue, Y.; Liu, P.Y.; Erkkila, K.; Ma, K.; Schwarcz, M.; Wang, C.; Swerdloff, R.S. Transplanted XY Germ Cells Produce Spermatozoa in Testes of XXY Mice. Int. J. Androl. 2010, 33, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lue, Y.; Rao, P.N.; Sinha Hikim, A.P.; Im, M.; Salameh, W.A.; Yen, P.H.; Wang, C.; Swerdloff, R.S. XXY Male Mice: An Experimental Model for Klinefelter Syndrome. Endocrinology 2001, 142, 1461–1470. [Google Scholar] [CrossRef]
- Hirota, T.; Ohta, H.; Powell, B.E.; Mahadevaiah, S.K.; Ojarikre, O.A.; Saitou, M.; Turner, J.M.A. Fertile Offspring from Sterile Sex Chromosome Trisomic Mice. Science 2017, 357, 932–935. [Google Scholar] [CrossRef]
- Joerg, H.; Janett, F.; Schlatt, S.; Mueller, S.; Graphodatskaya, D.; Suwattana, D.; Asai, M.; Stranzinger, G. Germ Cell Transplantation in an Azoospermic Klinefelter Bull. Biol. Reprod. 2003, 69, 1940–1944. [Google Scholar] [CrossRef][Green Version]
- Wistuba, J.; Luetjens, C.M.; Stukenborg, J.B.; Poplinski, A.; Werler, S.; Dittmann, M.; Damm, O.S.; Hamalainen, T.; Simoni, M.; Gromoll, J. Male 41, XXY* Mice as a Model for Klinefelter Syndrome: Hyperactivation of Leydig Cells. Endocrinology 2010, 151, 2898–2910. [Google Scholar] [CrossRef][Green Version]
- Werler, S.; Demond, H.; Damm, O.S.; Ehmcke, J.; Middendorff, R.; Gromoll, J.; Wistuba, J. Germ Cell Loss Is Associated with Fading Lin28a Expression in a Mouse Model for Klinefelter’s Syndrome. Reproduction 2014, 147, 253–264. [Google Scholar] [CrossRef]
- Werler, S.; Poplinski, A.; Gromoll, J.; Wistuba, J. Expression of Selected Genes Escaping from X Inactivation in the 41, XX(Y)* Mouse Model for Klinefelter’s Syndrome. Acta Paediatr. 2011, 100, 885–891. [Google Scholar] [CrossRef]
- Hunt, P.A.; Worthman, C.; Levinson, H.; Stallings, J.; LeMaire, R.; Mroz, K.; Park, C.; Handel, M.A. Germ Cell Loss in the XXY Male Mouse: Altered X-Chromosome Dosage Affects Prenatal Development. Mol. Reprod. Dev. 1998, 49, 101–111. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Atala, A. Testicular Tissue Cryopreservation and Spermatogonial Stem Cell Transplantation to Restore Fertility: From Bench to Bedside. Stem Cell Res. Ther. 2014, 5, 68. [Google Scholar] [CrossRef]
- Galdon, G.; Atala, A.; Sadri-Ardekani, H. In Vitro Sperm.matogenesis: How Far from Clinical Application? Curr. Urol. Rep. 2016, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Morimoto, H.; Ogura, A.; Shinohara, T. Serum- and Feeder-Free Culture of Mouse Germline Stem Cells. Biol. Reprod. 2011, 84, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-Term Culture of Mouse Male Germline Stem Cells under Serum-or Feeder-Free Conditions. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Matoba, S.; Morimoto, H.; Ogura, A.; Shinohara, T. Improved Serum- and Feeder-Free Culture of Mouse Germline Stem Cells. Biol. Reprod. 2014, 91, 88. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.; van der Veen, F.; et al. Propagation of Human Spermatogonial Stem Cells in Vitro. JAMA 2009, 302, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Sadri-Ardekani, H.; Akhondi, M.A.; van der Veen, F.; Repping, S.; van Pelt, A.M. In Vitro Propagation of Human Prepubertal Spermatogonial Stem Cells. JAMA 2011, 305, 2416–2418. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.O.; Barber, P.C.; Hamid, Q.A.; Power, B.F.; Dhillon, A.P.; Rode, J.; Day, I.N.; Thompson, R.J.; Polak, J.M. The Immunolocalization of Protein Gene Product 9.5 Using Rabbit Polyclonal and Mouse Monoclonal Antibodies. Br. J. Exp. Pathol. 1988, 69, 91–104. [Google Scholar]
- Marini, M.; Rosa, I.; Guasti, D.; Gacci, M.; Sgambati, E.; Ibba-Manneschi, L.; Manetti, M. Reappraising the Microscopic Anatomy of Human Testis: Identification of Telocyte Networks in the Peritubular and Intertubular Stromal Space. Sci. Rep. 2018, 8, 14780. [Google Scholar] [CrossRef] [PubMed]
- Nickkholgh, B.; Mizrak, S.C.; Korver, C.M.; van Daalen, S.K.; Meissner, A.; Repping, S.; van Pelt, A.M. Enrichment of Spermatogonial Stem Cells from Long-Term Cultured Human Testicular Cells. Fertil. Steril. 2014, 102, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Zohni, K.; Zhang, X.; Tan, S.L.; Chan, P.; Nagano, M. CD9 Is Expressed on Human Male Germ Cells That Have a Long-Term Repopulation Potential after Transplantation into Mouse Testes. Biol. Reprod. 2012, 87, 27. [Google Scholar] [CrossRef]
- Guo, Y.; Hai, Y.; Gong, Y.; Li, Z.; He, Z. Characterization, Isolation, and Culture of Mouse and Human Spermatogonial Stem Cells. J. Cell Physiol. 2014, 229, 407–413. [Google Scholar] [CrossRef]
- Hutter, H.; Dohr, G. HLA Expression on Immature and Mature Human Germ Cells. J. Reprod. Immunol. 1998, 38, 101–122. [Google Scholar] [CrossRef]
- Harichandan, A.; Sivasubramaniyan, K.; Hennenlotter, J.; Poths, S.; Bedke, J.; Kruck, S.; Stenzl, A.; Buhring, H.J. Molecular Signatures of Primary Human Spermatogonial Progenitors and Its Neighboring Peritubular Stromal Compartment. Stem Cells Dev. 2017, 26, 263–273. [Google Scholar] [CrossRef]
- Shinohara, T.; Avarbock, M.R.; Brinster, R.L. Beta1- and Alpha6-Integrin Are Surface Markers on Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 5504–5509. [Google Scholar] [CrossRef]
- Deebel, N.A.; Bradshaw, A.W.; Sadri-Ardekani, H. Infertility Considerations in Klinefelter Syndrome: From Origin to Management. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101480. [Google Scholar] [CrossRef] [PubMed]
- Culty, M. Gonocytes, the Forgotten Cells of the Germ Cell Lineage. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 1–26. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth Factors Essential for Self-Renewal and Expansion of Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- Meehan, T.; Schlatt, S.; O’Bryan, M.K.; de Kretser, D.M.; Loveland, K.L. Regulation of Germ Cell and Sertoli Cell Development by Activin, Follistatin, and FSH. Dev. Biol. 2000, 220, 225–237. [Google Scholar] [CrossRef]
- Hasthorpe, S. Clonogenic Culture of Normal Spermatogonia: In Vitro Regulation of Postnatal Germ Cell Proliferation. Biol. Reprod. 2003, 68, 1354–1360. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Iwano, T.; Lee, J.; Kazuki, Y.; Inoue, K.; Miki, H.; Takehashi, M.; Toyokuni, S.; Shinkai, Y.; et al. Genetic and Epigenetic Properties of Mouse Male Germline Stem Cells during Long-Term Culture. Dev. Camb. Engl. 2005, 132, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Braye, A.; Struijk, R.B.; van Pelt, A.M.; Goossens, E. Cryopreservation of Testicular Tissue before Long-Term Testicular Cell Culture Does Not Alter in Vitro Cell Dynamics. Fertil. Steril. 2015, 104, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.V.; Kanaan, S.B.; Hemon, M.F.; Azzouz, D.F.; El Haddad, M.; Balandraud, N.; Mignon-Ravix, C.; Picard, C.; Arnoux, F.; Martin, M.; et al. Mosaicism of XX and XXY Cells Accounts for High Copy Number of Toll like Receptor 7 and 8 Genes in Peripheral Blood of Men with Rheumatoid Arthritis. Sci. Rep. 2019, 9, 12880. [Google Scholar] [CrossRef]
- Radonic, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to Reference Gene Selection for Quantitative Real-Time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef] [PubMed]


| Reagent | Company | Catalog # | Final Concentration |
|---|---|---|---|
| Stem Pro-34 SFM | Invitrogen | 10639-011 | Base Medium |
| Stem Pro Supplement | Invitrogen | 10639-011 | 26 µL/mL |
| Bovine Albumin | Roche | 10735094001 | 5 mg/mL |
| D(+) Glucose | Sigma | G7021 | 6 mg/mL |
| Ascorbic acid | Sigma | A4544 | 1 × 10−4 M |
| Transferrin | Sigma | T1147 | 100 µg/mL |
| Pyruvic acid | Sigma | P2256 | 30 mg/mL |
| d-Biotin | Sigma | B4501 | 10 µg/mL |
| 2-beta Mercatoethanol | Sigma | M7522 | 5 × 10−5 M |
| DL-lactic acid | Sigma | L4263 | 1 µL/mL |
| MEM-non essential aa | Invitrogen | 11140-035 | 10 µL/mL |
| Insulin | Sigma | I1882 | 25 µg/mL |
| Sodium Selenite | Sigma | S1382 | 30 nM |
| Putrescine | Sigma | P7505 | 60 µM |
| L-Glutamine | Invitrogen | 25030-024 | 2 mM |
| MEM Vitamine solution | Invitrogen | 11120-037 | 10 µL/mL |
| b-Estradiol | Sigma | E2758 | 30 ng/mL |
| Progesterone | Sigma | P8783 | 60 ng/mL |
| Human EGF | Sigma | E9644 | 20 ng/mL |
| Human bFGF | Sigma | F0291 | 10 ng/mL |
| Human LIF | Chemicon | LIF1010 | 10 ng/mL |
| GDNF | Sigma | G1777 | 10 ng/mL |
| FCS | Invitrogen | 10106-169 | 20% |
| Pen/Strep | Invitrogen | 15140122 | 0.5% |
| Platelet-Derived Growth Factor (PDGF) | Sigma | SRP3228-10UG | 10 ng/mL |
| Follistatin (Ft) | Sigma | F1175-25UG | 100 ng/mL |
| Follicle Stimulant Hormone (FSH) | Sigma | F8174-1VL | 200 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdon, G.; Deebel, N.A.; Zarandi, N.P.; Pettenati, M.J.; Kogan, S.; Wang, C.; Swerdloff, R.S.; Atala, A.; Lue, Y.; Sadri-Ardekani, H. In Vitro Propagation of XXY Undifferentiated Mouse Spermatogonia: Model for Fertility Preservation in Klinefelter Syndrome Patients. Int. J. Mol. Sci. 2022, 23, 173. https://doi.org/10.3390/ijms23010173
Galdon G, Deebel NA, Zarandi NP, Pettenati MJ, Kogan S, Wang C, Swerdloff RS, Atala A, Lue Y, Sadri-Ardekani H. In Vitro Propagation of XXY Undifferentiated Mouse Spermatogonia: Model for Fertility Preservation in Klinefelter Syndrome Patients. International Journal of Molecular Sciences. 2022; 23(1):173. https://doi.org/10.3390/ijms23010173
Chicago/Turabian StyleGaldon, Guillermo, Nicholas A. Deebel, Nima Pourhabibi Zarandi, Mark J. Pettenati, Stanley Kogan, Christina Wang, Ronald S. Swerdloff, Anthony Atala, Yanhe Lue, and Hooman Sadri-Ardekani. 2022. "In Vitro Propagation of XXY Undifferentiated Mouse Spermatogonia: Model for Fertility Preservation in Klinefelter Syndrome Patients" International Journal of Molecular Sciences 23, no. 1: 173. https://doi.org/10.3390/ijms23010173
APA StyleGaldon, G., Deebel, N. A., Zarandi, N. P., Pettenati, M. J., Kogan, S., Wang, C., Swerdloff, R. S., Atala, A., Lue, Y., & Sadri-Ardekani, H. (2022). In Vitro Propagation of XXY Undifferentiated Mouse Spermatogonia: Model for Fertility Preservation in Klinefelter Syndrome Patients. International Journal of Molecular Sciences, 23(1), 173. https://doi.org/10.3390/ijms23010173







