The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway
Abstract
1. Introduction
2. Results
2.1. Rotation Speed of 120 rpm (3.9 dyne/cm2) Supports the Highest Level of Spermatogonia Proliferation
2.2. In Suspension Culture, Porcine Spermatogonia Proliferate More in Ambient O2 Tension but Contain Fewer Undifferentiated Spermatogonia
2.3. Shear Forces Generated in SSB Culture Activate the Wnt/β-Catenin Pathway
2.4. Activation of the Wnt/β-Catenin Pathway in Static Culture Does Not Fully Mimic the Effect of Suspension Culture
3. Discussion
4. Materials and Methods
4.1. Spermatogonia Isolation and Enrichment
4.2. Spermatogonia Culture
4.3. Oxygen Measurement
4.4. Mouse Spermatogonia Culture
4.5. CHIR Treatment
4.6. EdU Incorporation Assay
4.7. Immunofluorescence
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Maximum Shear Stress Calculation
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility Preservation for Male Patients with Childhood, Adolescent, and Young Adult Cancer: Recommendations from the Pancarelife Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; de Moor, J.S.; Hartigan, D.B.; et al. Survivors of Childhood Cancer in the United States: Prevalence and Burden of Morbidity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Male Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 332–339. [Google Scholar] [CrossRef]
- Basaria, S. Male Hypogonadism. Lancet 2014, 383, 1250–1263. [Google Scholar] [CrossRef]
- Forbes, C.M.; Flannigan, R.; Schlegel, P.N. Spermatogonial Stem Cell Transplantation and Male Infertility: Current Status and Future Directions. Arab J. Urol. 2018, 16, 171–180. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef]
- Izadyar, F.; den Ouden, K.; Creemers, L.B.; Posthuma, G.; Parvinen, M.; de Rooij, D.G. Proliferation and Differentiation of Bovine Type a Spermatogonia During Long-Term Culture. Biol. Reprod. 2003, 68, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Bahadorani, M.; Hosseini, S.M.; Abedi, P.; Abbasi, H.; Nasr-Esfahani, M.H. Glial Cell Line-Derived Neurotrophic Factor in Combination with Insulin-Like Growth Factor 1 and Basic Fibroblast Growth Factor Promote in Vitro Culture of Goat Spermatogonial Stem Cells. Growth Factors 2015, 33, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wu, J.Y.; An, X.L.; Zhao, X.X.; Wang, Z.Z.; Tang, B.; Yue, Z.P.; Li, Z.Y.; Zhang, X.M. Enrichment and Culture of Spermatogonia from Cryopreserved Adult Bovine Testis Tissue. Anim. Reprod. Sci. 2016, 166, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Oatley, M.J.; Kaucher, A.V.; Yang, Q.E.; Waqas, M.S.; Oatley, J.M. Conditions for Long-Term Culture of Cattle Undifferentiated Spermatogonia1. Biol. Reprod. 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Nath, S.C.; Harper, L.; Rancourt, D.E. Rancourt. Cell-Based Therapy Manufacturing in Stirred Suspension Bioreactor: Thoughts for Cgmp Compliance. Front. Bioeng. Biotechnol. 2020, 8, 599674. [Google Scholar] [CrossRef]
- Burrell, K.; Dardari, R.; Goldsmith, T.; Toms, D.; Villagomez, D.A.F.; King, W.A.; Ungrin, M.D.; West, F.; Dobrinski, I. Stirred Suspension Bioreactor Culture of Porcine Induced Pluripotent Stem Cells. Stem Cells Dev. 2019, 28, 1264–1275. [Google Scholar] [CrossRef]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y. Oxygenation in Cell Culture: Critical Parameters for Reproducibility Are Routinely Not Reported. PLoS ONE 2018, 13, e0204269. [Google Scholar]
- Wenger, R.H.; Kurtcuoglu, V.; Scholz, C.C.; Marti, H.H.; Hoogewijs, D. Frequently Asked Questions in Hypoxia Research. Hypoxia 2015, 3, 35–43. [Google Scholar] [CrossRef]
- Badger, J.L.; Byrne, M.L.; Veraitch, F.S.; Mason, C.; Wall, I.B.; Caldwell, M.A. Hypoxic Culture of Human Pluripotent Stem Cell Lines Is Permissible Using Mouse Embryonic Fibroblasts. Regen. Med. 2012, 7, 675–683. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, C.T.; Wei, Y.H. Inhibitory Effects of Hypoxia on Metabolic Switch and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2013, 31, 2779–2788. [Google Scholar] [CrossRef]
- Renault, V.M.; Rafalski, V.A.; Morgan, A.A.; Salih, D.A.; Brett, J.O.; Webb, A.E.; Villeda, S.A.; Thekkat, P.U.; Guillerey, C.; Denko, N.C.; et al. Foxo3 Regulates Neural Stem Cell Homeostasis. Cell Stem Cell 2009, 5, 527–539. [Google Scholar] [CrossRef]
- Helsel, A.R.; Oatley, M.J.; Oatley, J.M. Glycolysis-Optimized Conditions Enhance Maintenance of Regenerative Integrity in Mouse Spermatogonial Stem Cells During Long-Term Culture. Stem Cell Rep. 2017, 8, 1430–1441. [Google Scholar] [CrossRef]
- Vodicka, P.; Smetana, K., Jr.; Dvorankova, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; Motlik, J. The Miniature Pig as an Animal Model in Biomedical Research. Ann. N.Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef]
- Bode, G.; Clausing, P.; Gervais, F.; Loegsted, J.; Luft, J.; Nogues, V.; Sims, J. The Utility of the Minipig as an Animal Model in Regulatory Toxicology. J. Pharmacol. Toxicol. Methods 2010, 62, 196–220. [Google Scholar] [CrossRef]
- França, L.R.; Silva, V.A., Jr.; Chiarini-Garcia, H.; Garcia, S.K.; Debeljuk, L. Cell Proliferation and Hormonal Changes During Postnatal Development of the Testis in the Pig. Biol. Reprod. 2000, 63, 1629–1636. [Google Scholar] [CrossRef]
- Foster, D.L.; Hileman, S.M. Chapter 31-Puberty in the Sheep. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1441–1485. [Google Scholar]
- Shafa, M.; Day, B.; Yamashita, A.; Meng, G.; Liu, S.; Krawetz, R.; Rancourt, D.E. Derivation of Ipscs in Stirred Suspension Bioreactors. Nat. Methods 2012, 9, 465–466. [Google Scholar] [CrossRef]
- Fluri, D.A.; Tonge, P.D.; Song, H.; Baptista, R.P.; Shakiba, N.; Shukla, S.; Clarke, G.; Nagy, A.; Zandstra, W.P. Derivation, Expansion and Differentiation of Induced Pluripotent Stem Cells in Continuous Suspension Cultures. Nat. Methods 2012, 9, 509–516. [Google Scholar] [CrossRef]
- Nath, S.C.; Day, B.; Harper, L.; Yee, J. Fluid Shear Stress Promotes Embryonic Stem Cell Pluripotency Via Interplay between Β-Catenin and Vinculin in Bioreactor Culture. Stem Cells 2021, 39, 1166–1177. [Google Scholar] [CrossRef]
- Tang, W.R.; Liu, Y.; Li, L.H.; Wu, Z.B.; He, Y. Fluid Shear Stress and Raloxifene Stimulates the Proliferation of Osteoblast through Regulating the Expresstion of Beta-Catenin and Estrogen Receptor Alpha. Sichuan Da Xue Xue Bao Yi Xue Ban 2014, 45, 913–918. [Google Scholar]
- Li, C.; Zeng, Y.; Hu, J.; Yu, H. Effects of Fluid Shear Stress on Expression of Proto-Oncogenes C-Fos and C-Myc in Cultured Human Umbilical Vein Endothelial Cells. Clin. Hemorheol. Microcirc. 2002, 26, 117–123. [Google Scholar]
- Norvell, S.M.; Alvarez, M.; Bidwell, J.P.; Pavalko, F.M. Fluid Shear Stress Induces Beta-Catenin Signaling in Osteoblasts. Calcif. Tissue Int. 2004, 75, 396–404. [Google Scholar] [CrossRef]
- Verma, D.; Ye, N.; Hua, S.Z. Role of Fluid Shear Stress on E-Cadherin Dynamics and Cytoskeletal Stresses. In Proceedings of the Paper Presented at the 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, NY, USA, 17–19 April 2015. [Google Scholar]
- Takase, H.M.; Nusse, R. Paracrine Wnt/Beta-Catenin Signaling Mediates Proliferation of Undifferentiated Spermatogonia in the Adult Mouse Testis. Proc. Natl. Acad. Sci. USA 2016, 113, E1489–E1497. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Rathi, R.; Dobrinski, I. Protein Gene Product 9.5 Is a Spermatogonia-Specific Marker in the Pig Testis: Application to Enrichment and Culture of Porcine Spermatogonia. Mol. Reprod. Dev. 2006, 73, 1531–1540. [Google Scholar] [CrossRef]
- Voigt, A.L.; Kondro, D.A.; Powell, D.; Valli-Pulaski, H.; Ungrin, M.; Stukenborg, J.B.; Klein, C.; Lewis, I.A.; Orwig, K.E.; Dobrinski, I. Unique Metabolic Phenotype and Its Transition During Maturation of Juvenile Male Germ Cells. FASEB J. 2021, 35, e21513. [Google Scholar] [CrossRef]
- Kehoe, D.E.; Jing, D.; Lock, L.T.; Tzanakakis, E.S. Scalable Stirred-Suspension Bioreactor Culture of Human Pluripotent Stem Cells. Tissue Eng. Part A 2010, 16, 405–421. [Google Scholar] [CrossRef]
- Gareau, T.; Lara, G.G.; Shepherd, R.D.; Krawetz, R.; Rancourt, D.E.; Rinker, K.D.; Kallos, M.S. Shear Stress Influences the Pluripotency of Murine Embryonic Stem Cells in Stirred Suspension Bioreactors. J. Tissue Eng. Regen. Med. 2014, 8, 268–278. [Google Scholar] [CrossRef]
- Sen, A.; Kallos, M.S.; Behie, L.A. Expansion of Mammalian Neural Stem Cells in Bioreactors: Effect of Power Input and Medium Viscosity. Dev. Brain Res. 2002, 134, 103–113. [Google Scholar] [CrossRef]
- Cormier, J.T.; Nieden, N.I.z.; Rancourt, D.E.; Kallos, M.S. Expansion of Undifferentiated Murine Embryonic Stem Cells as Aggregates in Suspension Culture Bioreactors. Tissue Eng. 2006, 12, 3233–3245. [Google Scholar] [CrossRef]
- Canadian Climate Normals 1981–2010 Station Data. Available online: https://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?searchType=stnProx&txtRadius=25&optProxType=city&selCity=51%7C2%7C114%7C4%7CCalgary&selPark=&txtCentralLatDeg=&txtCentralLatMin=0&txtCentralLatSec=0&txtCentralLongDeg=&txtCentralLongMin=0&txtCentralLongSec=0&txtLatDecDeg=&txtLongDecDeg=&stnID=2205&dispBack=0 (accessed on 3 November 2020).
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of Oxygen Delivery to Cells in Culture: An Underappreciated Problem in Basic and Translational Research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth Factors Essential for Self-Renewal and Expansion of Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- He, Z.; Jiang, J.; Hofmann, M.C.; Dym, M. Gfra1 Silencing in Mouse Spermatogonial Stem Cells Results in Their Differentiation Via the Inactivation of Ret Tyrosine Kinase. Biol. Reprod. 2007, 77, 723–733. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Liao, H.F.; Yu, C.Y.; Mo, C.F.; Lin, S.P. Epigenetic Factors in the Regulation of Prospermatogonia and Spermatogonial Stem Cells. Reproduction 2015, 150, R77–R91. [Google Scholar] [CrossRef]
- Avvisato, C.L.; Yang, X.; Shah, S.; Hoxter, B.; Li, W.; Gaynor, R.; Pestell, R.; Tozeren, A.; Byers, S.W. Mechanical Force Modulates Global Gene Expression and Beta-Catenin Signaling in Colon Cancer Cells. J. Cell Sci. 2007, 120, 2672–2682. [Google Scholar] [CrossRef]
- Cha, B.; Geng, X.; Mahamud, M.R.; Fu, J.; Mukherjee, A.; Kim, Y.; Jho, E.H.; Kim, T.H.; Kahn, M.L.; Xia, L.; et al. Mechanotransduction Activates Canonical Wnt/Β-Catenin Signaling to Promote Lymphatic Vascular Patterning and the Development of Lymphatic and Lymphovenous Valves. Genes. Dev. 2016, 30, 1454–1469. [Google Scholar] [CrossRef]
- Fernández-Sánchez, M.E.; Barbier, S.; Whitehead, J.; Béalle, G.; Michel, A.; Latorre-Ossa, H.; Rey, C.; Fouassier, L.; Claperon, A.; Brullé, L.; et al. Mechanical Induction of the Tumorigenic Β-Catenin Pathway by Tumour Growth Pressure. Nature 2015, 523, 92–95. [Google Scholar] [CrossRef]
- le Duc, Q.; Shi, Q.; Blonk, I.; Sonnenberg, A.; Wang, N.; Leckband, D.; de Rooij, J. Vinculin Potentiates E-Cadherin Mechanosensing and Is Recruited to Actin-Anchored Sites within Adherens Junctions in a Myosin Ii-Dependent Manner. J. Cell Biol. 2010, 189, 1107–1115. [Google Scholar] [CrossRef]
- Kemler, R. From Cadherins to Catenins: Cytoplasmic Protein Interactions and Regulation of Cell Adhesion. Trends Genet. 1993, 9, 317–321. [Google Scholar] [CrossRef]
- Tolkunova, E.N.; Malashicheva, A.B.; Chikhirzhina, E.V.; Kostyleva, E.I.; Zeng, W.; Luo, J.; Dobrinskiĭ, I.; Hierholzer, A.; Kemler, R.; Tomilin, A.N. E-Cadherin as a Novel Surface Marker of Spermatogonial Stem Cells. Tsitologiia 2009, 51, 212–218. [Google Scholar] [CrossRef][Green Version]
- Thompson, C.J.; Su, Z. Cadherin Clusters Stabilized by a Combination of Specific and Nonspecific Cis-Interactions. eLife 2020, 9, e59035. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Brabletz, T.; Fearon, E.; Willis, A.L.; Hu, C.Y.; Li, X.Y.; Weiss, S.J. Canonical Wnt Suppressor, Axin2, Promotes Colon Carcinoma Oncogenic Activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11312. [Google Scholar] [CrossRef]
- Lam, A.Q.; Freedman, B.S.; Morizane, R.; Lerou, P.H.; Valerius, M.T.; Bonventre, J.V. Rapid and Efficient Differentiation of Human Pluripotent Stem Cells into Intermediate Mesoderm That Forms Tubules Expressing Kidney Proximal Tubular Markers. J. Am. Soc. Nephrol. 2014, 25, 1211–1225. [Google Scholar] [CrossRef]
- Borys, B.S.; Roberts, E.L.; Le, A.; Kallos, M.S. Scale-up of Embryonic Stem Cell Aggregate Stirred Suspension Bioreactor Culture Enabled by Computational Fluid Dynamics Modeling. Biochem. Eng. J. 2018, 133, 157–167. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Bai, Y.; Shi, R.; Wei, H.; Zhang, S. Mouse Undifferentiated Spermatogonial Stem Cells Cultured as Aggregates under Simulated Microgravity. Andrologia 2014, 46, 1013–1021. [Google Scholar] [CrossRef]
- King, J.A.; Miller, W.M. Bioreactor Development for Stem Cell Expansion and Controlled Differentiation. Curr. Opin. Chem. Biol. 2007, 11, 394–398. [Google Scholar] [CrossRef]
- Hayashi, Y.; Otsuka, K.; Ebina, M.; Igarashi, K.; Takehara, A.; Matsumoto, M.; Kanai, A. Distinct Requirements for Energy Metabolism in Mouse Primordial Germ Cells and Their Reprogramming to Embryonic Germ Cells. Proc. Natl. Acad. Sci. USA 2017, 114, 8289–8294. [Google Scholar] [CrossRef]
- Brinster, R.L.; Harstad, H. Energy Metabolism in Primordial Germ Cells of the Mouse. Exp. Cell Res. 1977, 109, 111–117. [Google Scholar] [CrossRef]
- Tischler, J.; Gruhn, W.H.; Reid, J.; Allgeyer, E. Metabolic Regulation of Pluripotency and Germ Cell Fate through A-Ketoglutarate. EMBO J. 2019, 38, e99518. [Google Scholar] [CrossRef] [PubMed]
- Verdikt, R.; Allard, P. Metabolo-Epigenetics: The Interplay of Metabolism and Epigenetics During Early Germ Cells Development. Biol. Reprod. 2021, 105, 616–624. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Tanaka, T.; Ogonuki, N.; Ogura, A.; Morimoto, H.; Cheng, P.F.; Eisenman, R.N.; Trumpp, A.; Shinohara, T. Myc/Mycn-Mediated Glycolysis Enhances Mouse Spermatogonial Stem Cell Self-Renewal. Genes. Dev. 2016, 30, 2637–2648. [Google Scholar] [CrossRef]
- Lord, T.; Nixon, B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev. Cell 2020, 52, 399–411. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.L.; Thiageswaran, S.; de Lima e Martins Lara, N.; Dobrinski, I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance in vivo and in vitro. Int. J. Mol. Sci. 2021, 22, 1998. [Google Scholar] [CrossRef]
- Taiani, J.T.; Krawetz, R.J.; Nieden, N.I.Z.; Wu, Y.E.; Kallos, M.S.; Matyas, J.R.; Rancourt, D.E. Reduced Differentiation Efficiency of Murine Embryonic Stem Cells in Stirred Suspension Bioreactors. Stem Cells Dev. 2010, 19, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.E.; Young, J.C.; Horvay, K.; Abud, H.E.; Loveland, K.L. Regulated Wnt/Beta-Catenin Signaling Sustains Adult Spermatogenesis in Mice. Biol. Reprod. 2014, 90, 1–12. [Google Scholar] [CrossRef]
- Chassot, A.A.; Le Rolle, M.; Jourden, M.; Taketo, M.M.; Ghyselinck, N.B.; Chaboissier, M.C. Constitutive Wnt/Ctnnb1 Activation Triggers Spermatogonial Stem Cell Proliferation and Germ Cell Depletion. Dev. Biol. 2017, 426, 17–27. [Google Scholar] [CrossRef]
- O’Flaherty, L.; Shnyder, S.D.; Cooper, P.A.; Cross, S.J.; Wakefield, J.G.; Pardo, O.E.; Seckl, M.J.; Tavaré, J.M. Tumor Growth Suppression Using a Combination of Taxol-Based Therapy and Gsk3 Inhibition in Non-Small Cell Lung Cancer. PLoS ONE 2019, 14, e0214610. [Google Scholar] [CrossRef] [PubMed]
- Honaramooz, A.; Snedaker, A.; Boiani, M.; Scholer, H.; Dobrinski, I.; Schlatt, S. Sperm from Neonatal Mammalian Testes Grafted in Mice. Nature 2002, 418, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Yu, Y.; Voigt, A.; Ungrin, M.; Dobrinski, I. Generation of Porcine Testicular Organoids with Testis Specific Architecture Using Microwell Culture. J. Vis. Exp. 2019, 152, e60387. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.R.; Zhang, X.; Nagano, M.C. Establishment of a Short-Term in Vitro Assay for Mouse Spermatogonial Stem Cells. Biol. Reprod. 2007, 77, 897–904. [Google Scholar] [CrossRef] [PubMed][Green Version]

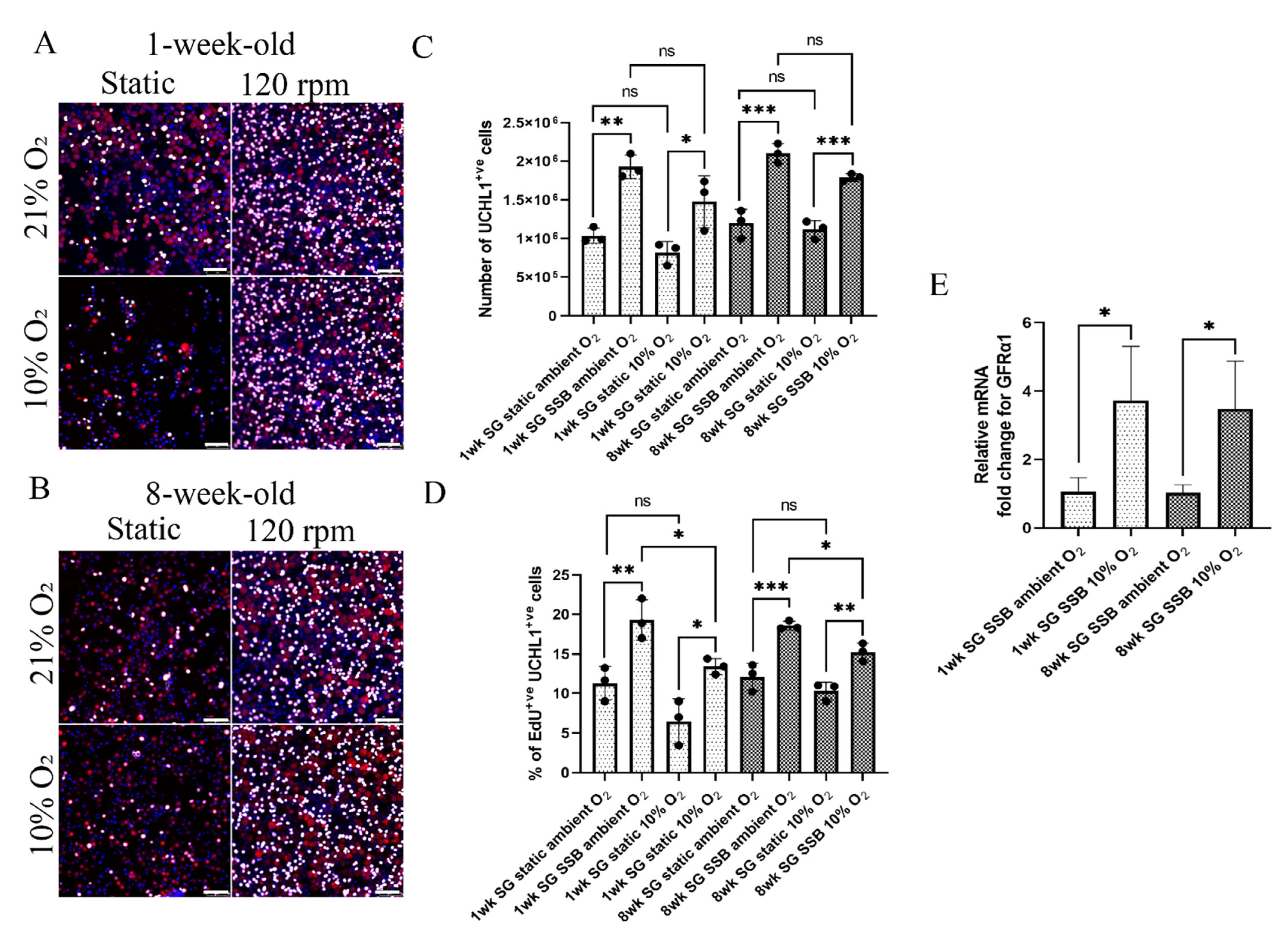
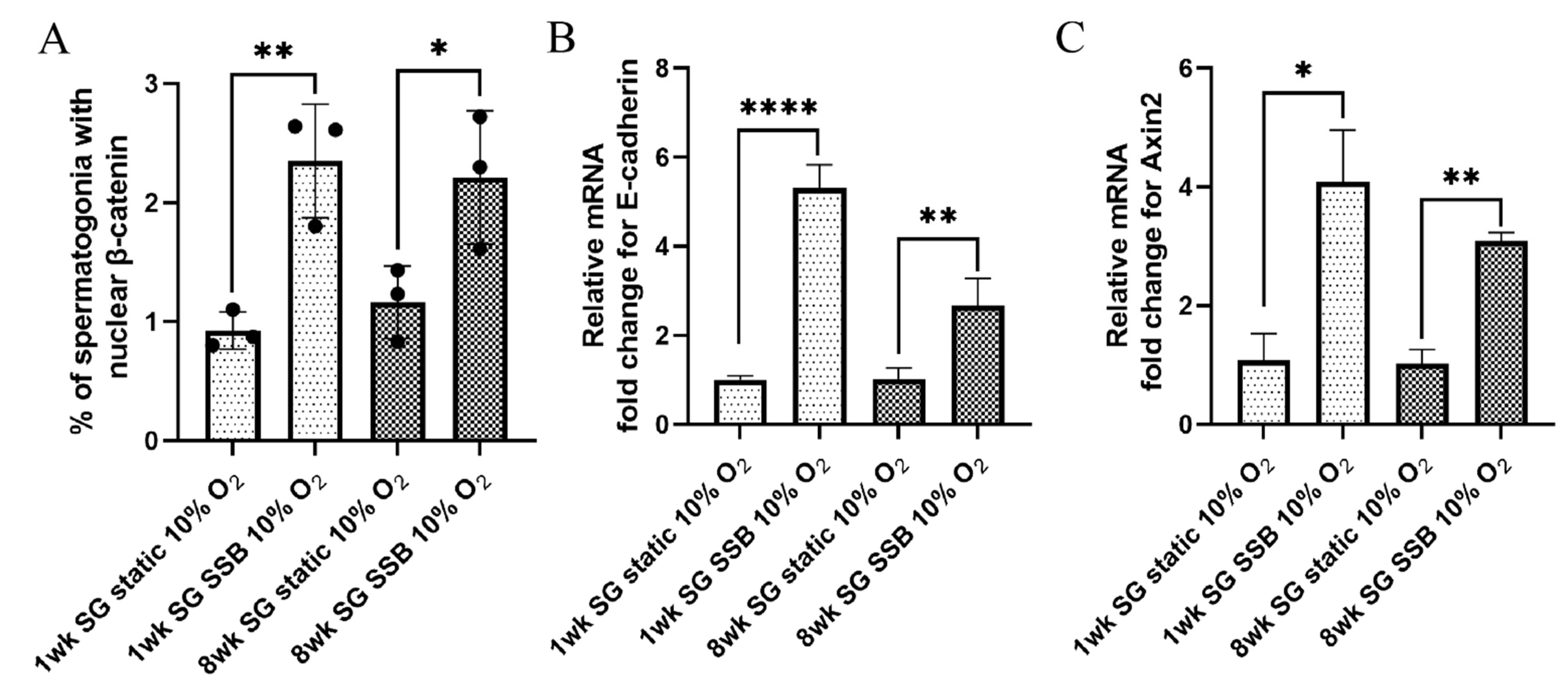
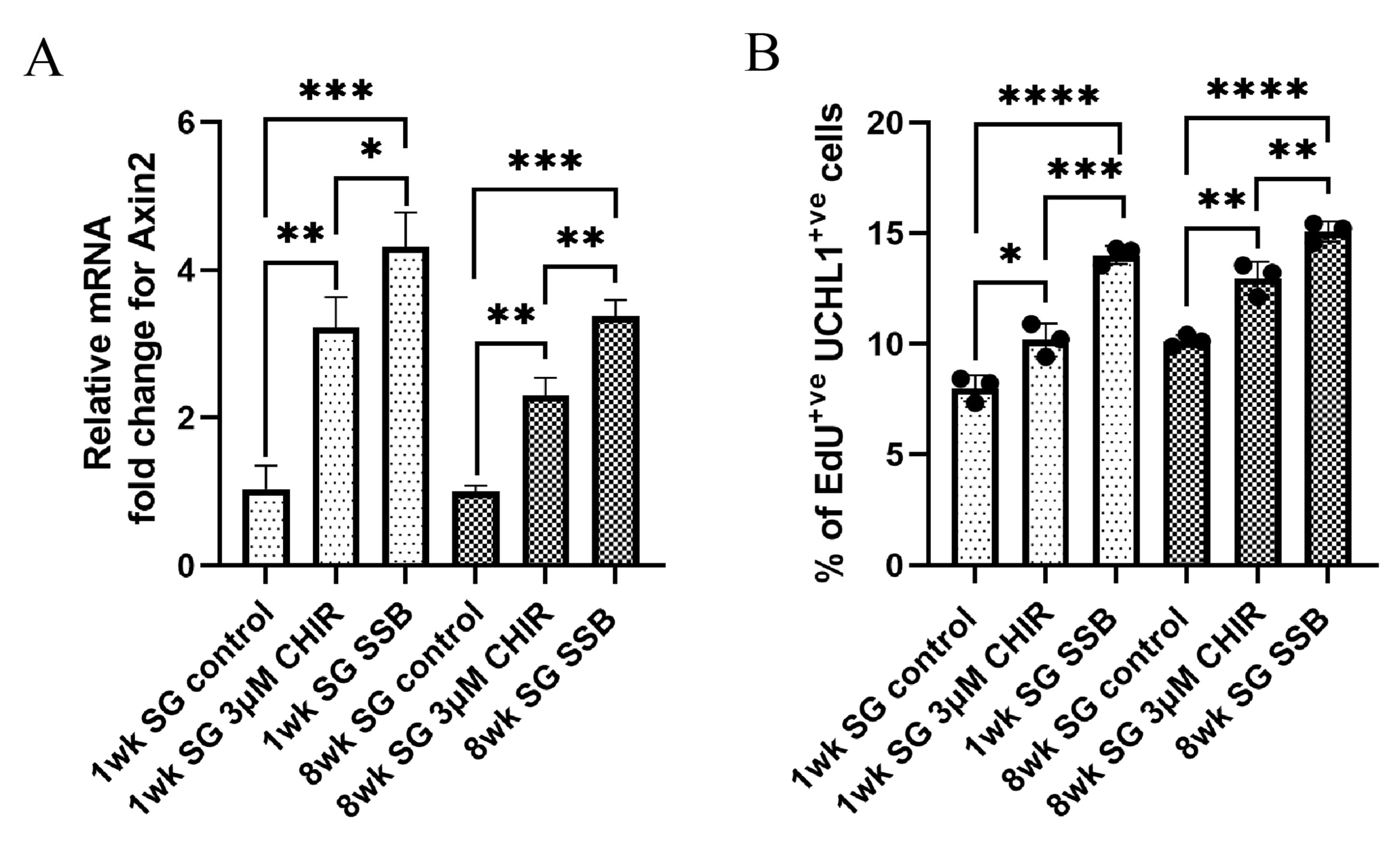
| Conditions | O2 Tension Pre-Set for the Incubator | Partial Pressure of O2 | True O2 Tension (at 1084 m Altitude) | Concentration in Gas Phase |
|---|---|---|---|---|
| Ambient | 20.9% | 16.34 kPa | 18.4% | 6.34 mM |
| 10% O2 | 10% | 8.89 kPa | 10% | 3.45 mM |
| Conditions | O2 Tension | Concentration |
|---|---|---|
| Static ambient O2 | 14.9 ± 0.04% | 5.2 ± 0.01 mM |
| SSB ambient O2 | 18.2 ± 0.17% | 6.3 ± 0.06 mM |
| Static 10% O2 | 3.28 ± 0.22% | 1.1 ± 0.07 mM |
| SSB 10% O2 | 3.74 ± 0.01% | 1.3 ± 0.003 mM |
| Gene Name | Direction | Sequence |
|---|---|---|
| GFRα1 | Forward | CATCTGCAGATCTCGCCTGGC |
| Reverse | GCCAAAGGCTTGAATTGCATTTTTGAGAC | |
| PLZF | Forward | GGG TGC ATA CAG GTG AGA AGC C |
| Reverse | CAC ACA TAG CAC AGG TAG AGG TAC GTC | |
| E-cadherin | Forward | GAGAAGAGGACCAGGACTTTGACTTGAG |
| Reverse | TCACTATCAGCTGCCTTCAGGTTTTCATC | |
| Axin2 | Forward | GAGGGAGAAATGCGTGGATA |
| Reverse | GGTTTCAGCTGCTTGGAGAC | |
| DAZL | Forward | GTT ATT CCT CCG GCT TAT ACA GCT G |
| Reverse | GAT ACC ACT GTC TGT ATG CTT CGG TC | |
| HPRT1 | Forward | GAAGAGCTACTGTAATGACCAGTCAACGG |
| Reverse | TCATTGTAGTCAAGGGCATAGCCTACC |
| Parameters | Dimensions |
|---|---|
| Media volume | 0.00001 m3 |
| Impeller width | 0.0054 m |
| Impeller diameter | 0.0254 m |
| Bioreactor diameter | 0.032 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakib, S.; Voigt, A.; de Lima e Martins Lara, N.; Su, L.; Ungrin, M.; Rancourt, D.; Dobrinski, I. The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2021, 22, 13549. https://doi.org/10.3390/ijms222413549
Sakib S, Voigt A, de Lima e Martins Lara N, Su L, Ungrin M, Rancourt D, Dobrinski I. The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences. 2021; 22(24):13549. https://doi.org/10.3390/ijms222413549
Chicago/Turabian StyleSakib, Sadman, Anna Voigt, Nathalia de Lima e Martins Lara, Lin Su, Mark Ungrin, Derrick Rancourt, and Ina Dobrinski. 2021. "The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway" International Journal of Molecular Sciences 22, no. 24: 13549. https://doi.org/10.3390/ijms222413549
APA StyleSakib, S., Voigt, A., de Lima e Martins Lara, N., Su, L., Ungrin, M., Rancourt, D., & Dobrinski, I. (2021). The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences, 22(24), 13549. https://doi.org/10.3390/ijms222413549






