STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma
Abstract
:1. Introduction
2. Results
2.1. Changes of SOCE Levels in STIM1 Variants-Overexpressing U2OS Cells
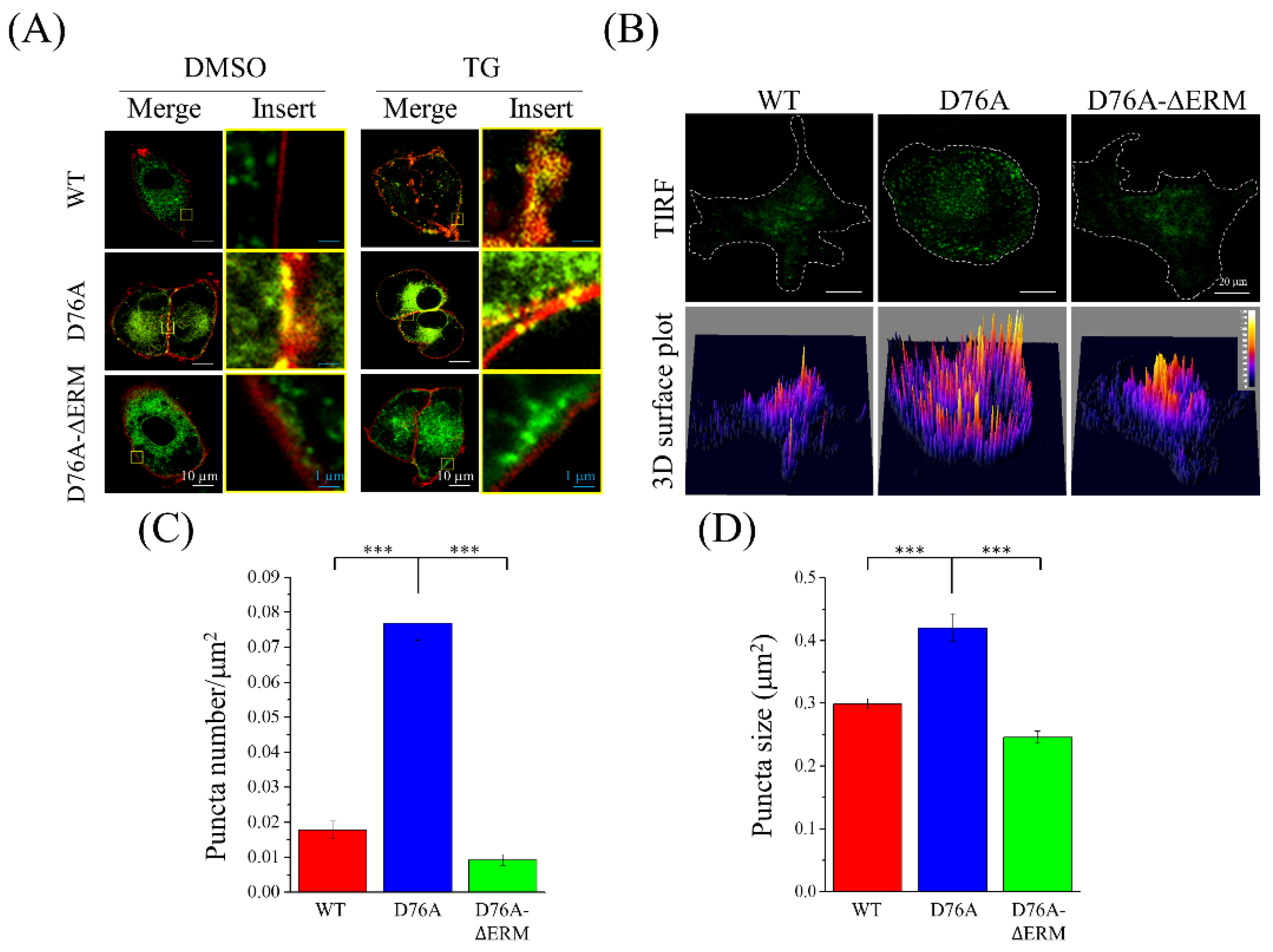
2.2. Localization of Different STIM1 Variants on the Cell Membrane
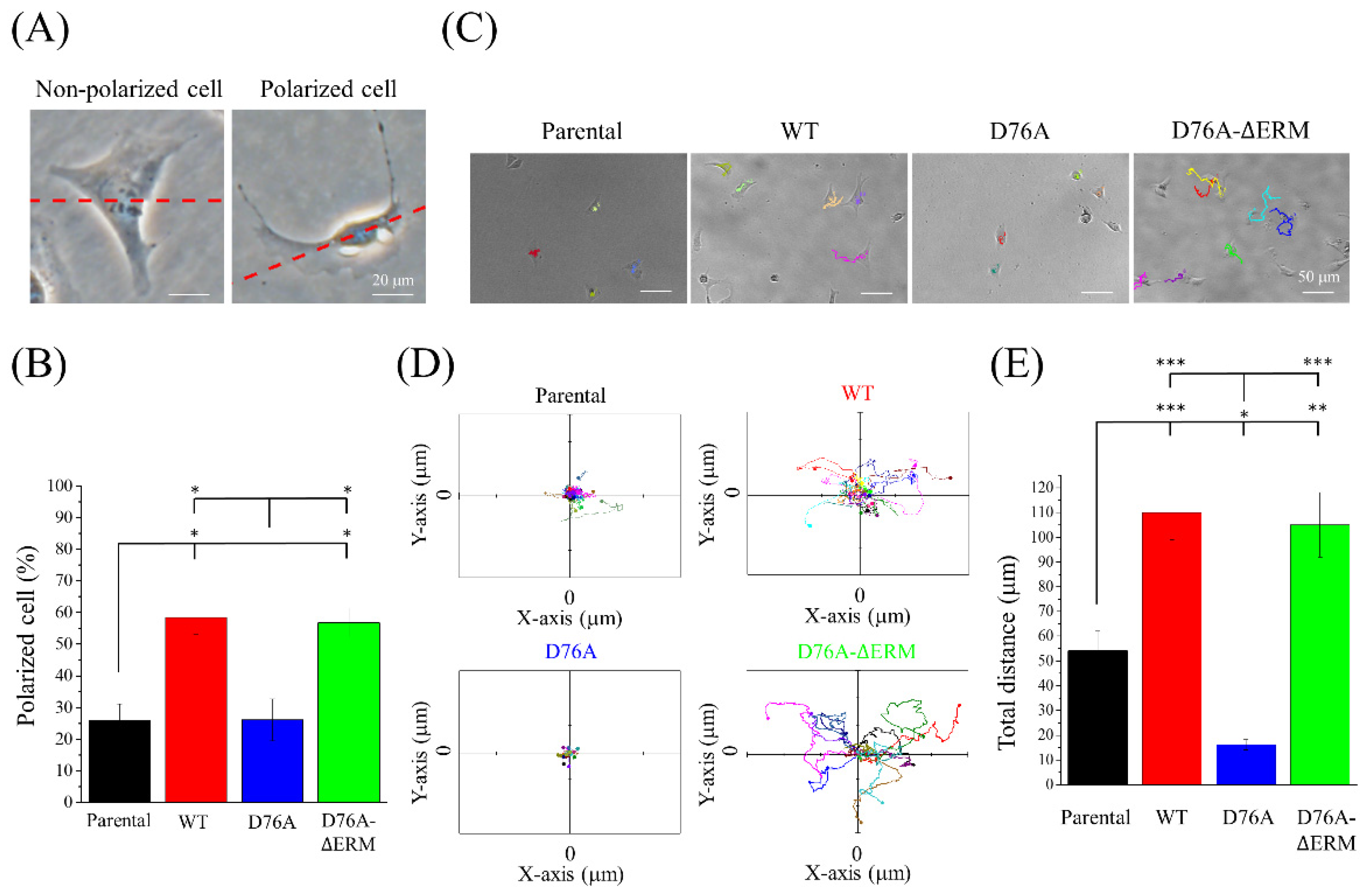
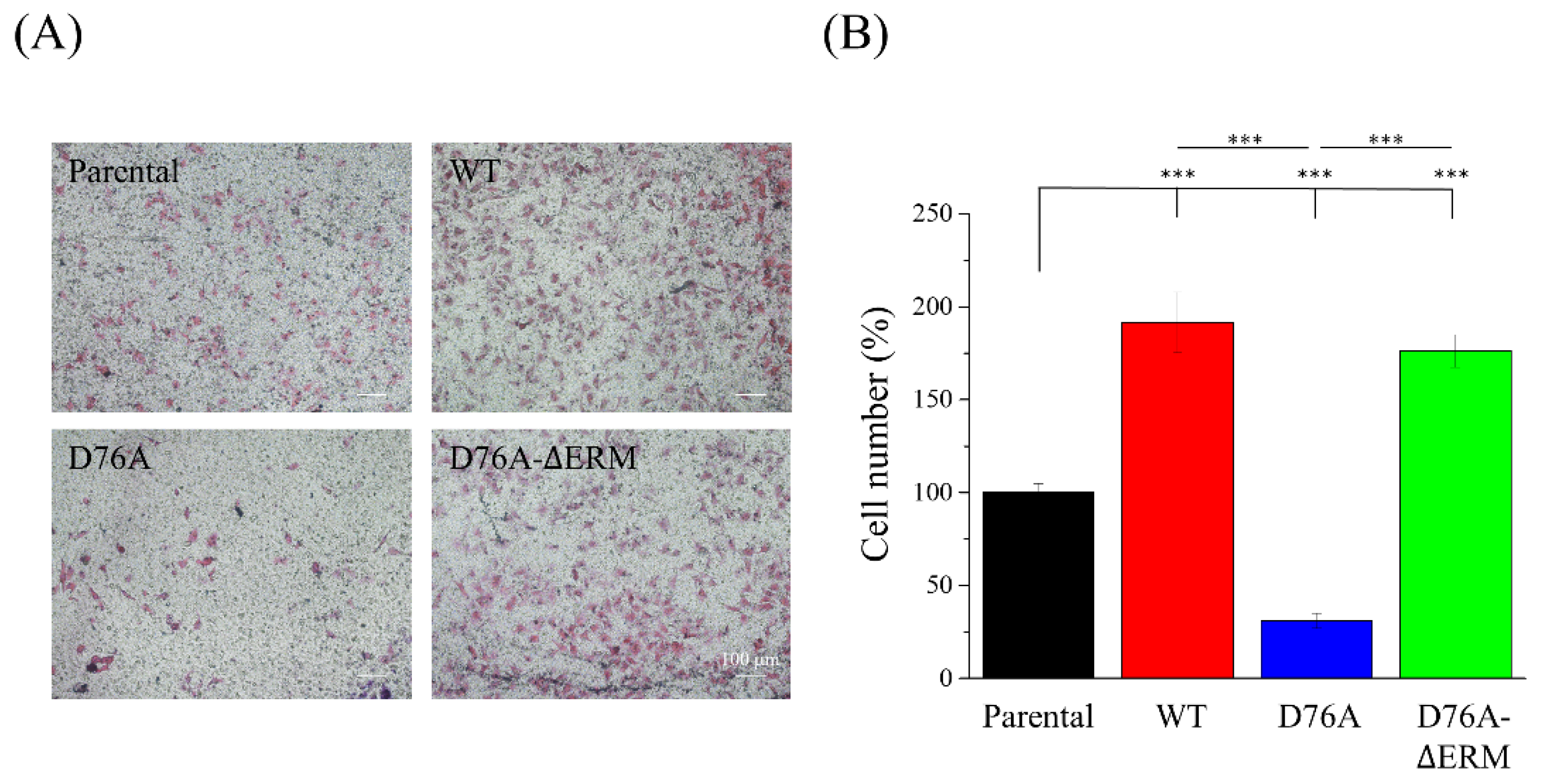
2.3. Cells Expressing Different STIM1 Variants Show Distinct Cell Migration Abilities
2.4. Focal Adhesion Dynamics Were Changed by STIM1 Overexpression
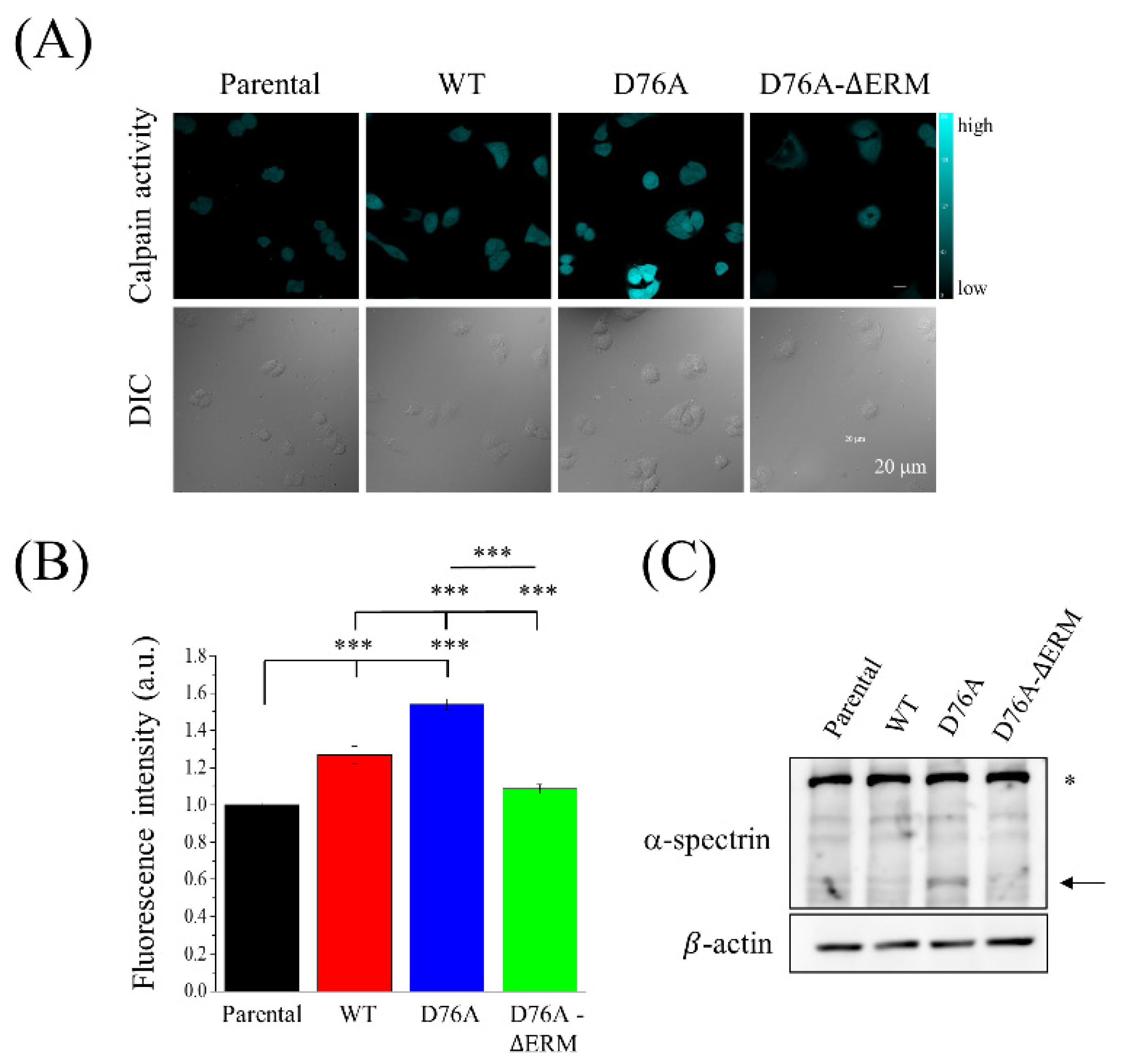
2.5. STIM1 Activation Meditated the Calpain Activity
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Bioinformatics Analysis
4.3. Single-Cell Ca2+ Measurement
4.4. Western Blotting
4.5. Confocal Microscopy
4.6. TIRF Microscopy and Image Analysis
4.7. Polarity Assay
4.8. Wound Healing Assay
4.9. 2D-Cell Migration Assay
4.10. Transwell Assay
4.11. Calpain Activity
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Prakriya, M. Store-operated Orai channels: Structure and function. Curr. Top. Membr. 2013, 71, 149–179. [Google Scholar] [CrossRef] [Green Version]
- Putney, J.W. The physiological function of store-operated calcium entry. Neurochem. Res. 2011, 36, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S.; et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salido, G.M.; Sage, S.O.; Rosado, J.A. TRPC channels and store-operated Ca2+ entry. Biochim. Biophys. Acta. 2009, 1793, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandman, O.; Liou, J.; Park, W.S.; Meyer, T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007, 131, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Bird, G.S.; Hwang, S.Y.; Smyth, J.T.; Fukushima, M.; Boyles, R.R.; Putney, J.W., Jr. STIM1 is a calcium sensor specialized for digital signaling. Curr. Biol. 2009, 19, 1724–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Chen, Y.F.; Chiu, W.T.; Wang, Y.K.; Chang, H.C.; Shen, M.R. The ER Ca2+; sensor STIM1 regulates actomyosin contractility of migratory cells. J. Cell Sci. 2013, 126, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Sun, J.; Huang, M.Y.; Wang, Y.S.; Hou, M.F.; Sun, Y.; He, H.; Krishna, N.; Chiu, S.J.; Lin, S.; et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene 2015, 34, 4358–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Miao, Y.; Zheng, X.; Gong, Y.; Zhang, J.; Zou, F.; Cai, C. STIM1 and STIM2 differently regulate endogenous Ca2+ entry and promote TGF-β-induced EMT in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 488, 74–80. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Pan, T.; Li, L.; Li, J.; Yang, H. STIM1 silencing inhibits the migration and invasion of A549 cells. Mol. Med. Rep. 2017, 16, 3283–3289. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, J.J.; Huang, X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009, 15, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Manji, S.S.; Parker, N.J.; Williams, R.T.; van Stekelenburg, L.; Pearson, R.B.; Dziadek, M.; Smith, P.J. STIM1: A novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta 2000, 1481, 47–155. [Google Scholar] [CrossRef]
- Sabbioni, S.; Barbanti-Brodano, G.; Croce, C.M.; Negrini, M. GOK: A gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997, 57, 4493–4497. [Google Scholar] [PubMed]
- Hammad, A.S.; Machaca, K. Store operated calcium entry in cell migration and cancer metastasis. Cells 2021, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Niu, H.; Ye, Y.; Hu, F.; Chen, S.; Li, X.; Luo, X.; Jiang, S.; Liu, Y.; et al. STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to-mesenchymal transition in prostate cancer. Sci. Rep. 2015, 5, 11754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [Green Version]
- Skalski, M.; Yi, Q.; Kean, M.J.; Myers, D.W.; Williams, K.C.; Burtnik, A.; Coppolino, M.G. Lamellipodium extension and membrane ruffling require different SNARE-mediated trafficking pathways. BMC Cell Biol. 2010, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Broussard, J.A.; Webb, D.J.; Kaverina, I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 2008, 20, 85–90. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21 (Suppl. 7), vii320–vii325. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone microenvironment and osteosarcoma metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chiu, W.T.; Chen, Y.T.; Lin, P.Y.; Huang, H.J.; Chou, C.Y.; Chang, H.C.; Tang, M.J.; Shen, M.R.; Tang, M.J.; et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15225–15230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karacicek, B.; Erac, Y.; Tosun, M. Functional consequences of enhanced expression of STIM1 and Orai1 in Huh-7 hepatocellular carcinoma tumor-initiating cells. BMC Cancer 2019, 19, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, J.; Zuo, D.; Shogren, K.L.; Gustafson, C.T.; Zhou, Z.; Thompson, M.A.; Guo, R.; Prakash, Y.S.; Lu, L.; Guo, W.; et al. STIM1 expression is associated with osteosarcoma cell survival. Chin. J. Cancer Res. 2019, 31, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, Q.; Cheng, J.; Bian, Y.; Tian, C.; Hu, Y.; Li, H. Enhanced Stim1 expression is associated with acquired chemo-resistance of cisplatin in osteosarcoma cells. Hum. Cell 2017, 30, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Rosado, J.A.; Diez, R.; Smani, T.; Jardín, I. STIM and Orai1 variants in store-operated calcium entry. Front. Pharmacol. 2015, 6, 325. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.P.; Zeng, W.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 2007, 9, 636–645. [Google Scholar] [CrossRef]
- Rainusso, N.; Man, T.K.; Lau, C.C.; Hicks, J.; Shen, J.J.; Yu, A.; Wang, L.L.; Rosen, J.M. Identification and gene expression profiling of tumor-initiating cells isolated from human osteosarcoma cell lines in an orthotopic mouse model. Cancer Biol. Ther. 2011, 12, 278–287. [Google Scholar] [CrossRef]
- Shen, Y.; Dana, H.; Abdelfattah, A.S.; Patel, R.; Shea, J.; Molina, R.S.; Rawal, B.; Rancic, V.; Chang, Y.F.; Wu, L.; et al. A genetically encoded Ca2+ indicator based on circularly permutated sea anemone red fluorescent protein eqFP578. BMC Biol. 2018, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Nagano, M.; Hoshino, D.; Koshikawa, N.; Akizawa, T.; Seiki, M. Turnover of focal adhesions and cancer cell migration. Int. J. Cell Biol. 2012, 2012, 310616. [Google Scholar] [CrossRef] [Green Version]
- Carragher, N.O.; Frame, M.C. Calpain: A role in cell transformation and migration. Int. J. Biochem. Cell Biol. 2002, 34, 1539–1543. [Google Scholar] [CrossRef]
- Franco, S.J.; Huttenlocher, A. Regulating cell migration: Calpains make the cut. J. Cell Sci. 2005, 118, 3829–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorchid, A.; Ikura, M. How calpain is activated by calcium. Nat. Struct. Biol. 2002, 9, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.T.; Bennin, D.A.; Huttenlocher, A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). J. Biol. Chem. 2010, 285, 11418–11426. [Google Scholar] [CrossRef] [Green Version]
- Serrano, K.; Devine, D.V. Vinculin is proteolyzed by calpain during platelet aggregation: 95 kDa cleavage fragment associates with the platelet cytoskeleton. Cell Motil. Cytoskeleton 2004, 58, 242–252. [Google Scholar] [CrossRef]
- Cortesio, C.L.; Boateng, L.R.; Piazza, T.M.; Bennin, D.A.; Huttenlocher, A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J. Biol. Chem. 2011, 286, 9998–10006. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013, 27, 1351–1361. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Tsai, F.C.; Kuo, G.H.; Chang, S.W.; Tsai, P.J. Ca2+ signaling in cytoskeletal reorganization, cell migration, and cancer metastasis. BioMed Res. Int. 2015, 2015, 409245. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.Y.; Lin, Y.H.; Chang, H.A.; Yeh, T.Y.; Chang, Y.H.; Chen, Y.F.; Chen, Y.; Li, C.; Chiu, W.T. STIM1 knockout enhances PDGF-mediated Ca2+ signaling through upregulation of the PDGFR-PLCγ-STIM2 cascade. Int. J. Mol. Sci. 2018, 19, 1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascalis, C.; Etienne-Manneville, S. Single and collective cell migration: The mechanics of adhesions. Mol. Biol. Cell 2017, 28, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Nelson, W.J. Running with neighbors: Coordinating cell migration and cell-cell adhesion. Curr. Opin. Cell Biol. 2015, 36, 62–70. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-S.; Lin, Y.-H.; Nguyen Thi, M.; Hsiao, S.-C.; Chiu, W.-T. STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma. Int. J. Mol. Sci. 2022, 23, 162. https://doi.org/10.3390/ijms23010162
Lin Y-S, Lin Y-H, Nguyen Thi M, Hsiao S-C, Chiu W-T. STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma. International Journal of Molecular Sciences. 2022; 23(1):162. https://doi.org/10.3390/ijms23010162
Chicago/Turabian StyleLin, Yu-Shan, Yi-Hsin Lin, MyHang Nguyen Thi, Shih-Chuan Hsiao, and Wen-Tai Chiu. 2022. "STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma" International Journal of Molecular Sciences 23, no. 1: 162. https://doi.org/10.3390/ijms23010162
APA StyleLin, Y.-S., Lin, Y.-H., Nguyen Thi, M., Hsiao, S.-C., & Chiu, W.-T. (2022). STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma. International Journal of Molecular Sciences, 23(1), 162. https://doi.org/10.3390/ijms23010162





