Abstract
In pathological brain conditions, glial cells become reactive and show a variety of responses. We examined Ca2+ signals in pathological brains and found that reactive astrocytes share abnormal Ca2+ signals, even in different types of diseases. In a neuropathic pain model, astrocytes in the primary sensory cortex became reactive and showed frequent Ca2+ signals, resulting in the production of synaptogenic molecules, which led to misconnections of tactile and pain networks in the sensory cortex, thus causing neuropathic pain. In an epileptogenic model, hippocampal astrocytes also became reactive and showed frequent Ca2+ signals. In an Alexander disease (AxD) model, hGFAP-R239H knock-in mice showed accumulation of Rosenthal fibers, a typical pathological marker of AxD, and excessively large Ca2+ signals. Because the abnormal astrocytic Ca2+ signals observed in the above three disease models are dependent on type II inositol 1,4,5-trisphosphate receptors (IP3RII), we reanalyzed these pathological events using IP3RII-deficient mice and found that all abnormal Ca2+ signals and pathologies were markedly reduced. These findings indicate that abnormal Ca2+ signaling is not only a consequence but may also be greatly involved in the cause of these diseases. Abnormal Ca2+ signals in reactive astrocytes may represent an underlying pathology common to multiple diseases.
1. Introduction
Under pathological conditions, astrocytes become reactive and exhibit a wide variety of responses, which are closely associated with the onset or development of several brain diseases. Thus, reactive astrocytes have received much attention, and researchers are continuing intense investigations to understand the nature and roles of these cells. The term reactive astrocyte includes a variety of astrocyte states. Researchers are attempting to identify simpler ways to describe these astrocytes, such as neurotoxic A1 astrocytes and neuroprotective A2 astrocytes [1]. However, reactive astrocytes are not so simple, and it turns out that the pattern of molecules expressed by reactive astrocytes varies greatly depending on the type of disease, degree of progression, and other factors [2]. In addition to changes in the expression of molecules, various indicators such as functional changes, anatomical changes, or a combination of the two must also be taken into account. One such indicator is aberrant Ca2+ signaling in astrocytes.
Astrocytes are non-excitable cells in terms of electrophysiological properties. However, with regard to Ca2+ signals, astrocytes are highly excitable and active. Astrocytes release so-called gliotransmitters such as ATP, glutamate, and D-serine [3,4], which are dependent on intracellular Ca2+ signals [5]. Astrocytes dynamically regulate neuronal functions via these gliotransmitters by controlling synaptic transmission [6]. The types and amounts of gliotransmitters vary in different pathological conditions, suggesting that various reactive astrocytes influence synaptic transmission in markedly different ways [7]. In addition to their rapid and acute control of synaptic activities, astrocytes also play an important role in the control of long-term neuronal activities. Reactive astrocytes produce a wide variety of synaptogenic molecules such as thrombospondins [8], hevin [9], and glypicans [10], for which astrocytic Ca2+ has also an important role. These molecules lead to highly uncontrolled synapses and affect neuronal activities and network connectivity. Astrocytes also affect long-term neuronal activities by forming synapses [11,12]. In early developmental stages [13], pathological conditions in adults [14], and the process of learning [15], astrocytes eliminate synapses by phagocytosis, thereby producing long-lasting network activities. Therefore, phenotypical changes in astrocytes in various pathological conditions are closely associated with both acute and chronic neuronal activities. Interestingly, Ca2+ signals are greatly enhanced in these reactive astrocytes [16]. This suggests that aberrant Ca2+ signals in reactive astrocytes may be a common characteristic of astrocyte-mediated brain diseases. In this review, we will focus on aberrant Ca2+ signals as a phenotype of reactive astrocytes. We show some examples of brain dysfunctions such as neuropathic pains [11,12,17,18], epileptogenesis [19,20,21], and Alexander disease (AxD) [22], where aberrant Ca2+ signals in astrocytes are commonly observed and are highly involved in the cause of these pathogenesis.
2. Neuropathic Pain and Excess Ca2+ Signals in Astrocytes of the Primary Sensory (S1) Cortex
Neuropathic pain is an intractable chronic pain condition, and one of its main symptoms is mechanical allodynia. The causes of neuropathic pain have remained unknown for many years, but advances in glial research are beginning to shed light on some of its underlying issues. One study that has made a substantial contribution is that of Tsuda and colleagues, who found that abnormalities in spinal microglia expressing P2X4 receptors led to abnormal synaptic transmission, thereby resulting in mechanical allodynia [23]. Since then, research on spinal microglia and neuropathic pain has progressed considerably, and many important discoveries have been made. However, many patients still suffer from neuropathic pain, suggesting the need for other approaches to determine the molecular pathogenesis of neuropathic pain. In humans, it is reported that neuropathic pain is associated with infra-slow oscillatory activities in some brain regions such as somatosensory thalamus and primary somatosensory cortex, which interestingly, occur at frequencies similar to Ca2+ waves in reactive astrocytes [18]. This finding strongly suggests that brain astrocytes would be involved in the pathogenesis of neuropathic pain. Our research has focused on abnormalities in the neural network of the primary somatosensory cortex (S1) and has shown that during the development of mechanical allodynia, synaptic remodeling in the S1 cortex is enhanced. This leads to the reorganization of the S1 network and misconnection of the pain and touch circuits. Once these circuits are reorganized, they are maintained, resulting in chronic neuropathic pain [11,12]. Reactive astrocytes and their synaptogenic molecule thrombospondin-1 (TSP-1) play essential roles in this type of synaptic and network remodeling (Figure 1). Importantly, reactive astrocytes are highly associated with aberrant Ca2+ signals, which are dependent on Ca2+ release from type II inositol 1,4,5-trisphosphate receptors (IP3RII). To clarify whether such astrocytic Ca2+ signaling is a result or a cause of synaptic remodeling and neuropathic pain, we examined IP3RII-deficient (IP3RIIKO) mice and found that all of the above events, i.e., abnormal Ca2+ signals, TSP-1 production, synapse remodeling, and mechanical allodynia, were abolished in these mice. Thus, it is concluded that abnormal Ca2+ signals in reactive astrocytes may cause a series of pain signaling cascades leading to mechanical allodynia [11,12].
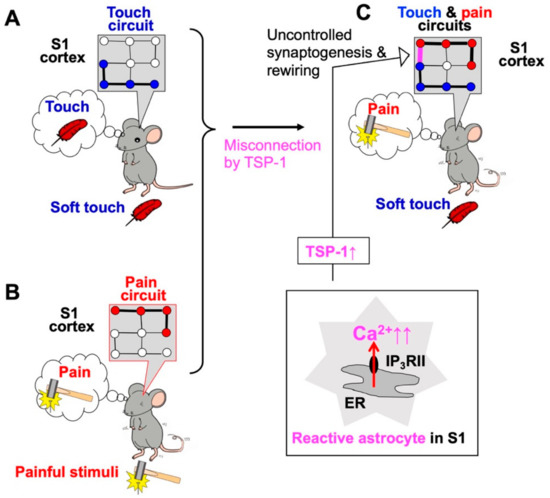
Figure 1.
Abnormal Ca2+ signals in primary somatosensory (S1) cortex astrocytes cause neuropathic pain and allodynia. Soft touch stimulation (A) and pain stimulation (B) activate touch circuits and pain circuits, respectively. In a model of neuropathic pain induced by sciatic nerve ligation, S1 astrocytes become reactive and exhibit excess Ca2+ signaling via type II inositol 1,4,5-trisphosphate receptors (IP3RII) (C). Reactive astrocytes in the S1 cortex produce synaptogenic molecules such as thrombospondin-1 (TSP-1) and cause excessive, uncontrolled synaptogenesis and network rewiring of S1 circuits (C, arrow), thereby leading to mechanical allodynia.
3. Other Brain Diseases and Aberrant Astrocytic Ca2+-mediated Synapse Remodeling
Similar astrocyte-mediated Ca2+ signals and synapse remodeling were also observed in the other brain regions, which are involved in different brain disorders. For example, activation of striatal medium spiny neurons (MSNs) results in GABA release, which stimulates GABAB receptors and Ca2+ elevation in adjacent astrocytes. Then, striatal astrocytes produce the synaptogenic molecule TSP-1 and boost excitatory synapse formation via an a2d-1-mediated mechanism. This in turn increases the firing of MSNs, which results in hyperactivity with disrupted attention phenotypes in mice. When the GABAB receptor-mediated Ca2+ responses were mimicked by stimulating Gi-designer receptors exclusively activated by designer drugs (Gi-DREADD) gated by clozapine-N-oxide (CNO), all subsequent responses, i.e., TSP-1 production, excessive synaptogenesis, and abnormal behaviors, were reproduced [24]. Therefore, aberrant Ca2+ signals in striatal astrocytes could also be a cause of the abnormal behavior, i.e., hyperactivity and disturbances of attention.
In addition, cocaine-induced abnormal behaviors such as drug seeking and relapse after withdrawal are mediated by aberrant Ca2+ signals in astrocytes in the nucleus accumbens shell (NAcSh). The administration of cocaine increases aberrant Ca2+ signals in NAcSh astrocytes, which stimulates the production of the synaptogenic molecule TSP-2, leading to the activation of its receptor a2d-1 and the generation of AMPA receptor-silent glutamatergic synapses [25]. Importantly, the cocaine-evoked formation of glutamatergic silent synapses in NacSh functions as a memory trace of cocaine experience, and thus, the aberrant Ca2+ signals in astrocytes could be a cause also of cue-associated memory traces that promote cocaine relapse.
In the examples shown in Section 2 and Section 3, aberrant Ca2+ signals in astrocytes produce an excess of synaptogenic molecules such as TSP-1, leading to uncontrolled synapse remodeling and network remodeling. Therefore, it is suggested that aberrant astrocytic Ca2+ signals are decoded in the form of the production of a synaptogenic molecule and cause various different brain disorders. With regard to astrocytic synaptogenic molecules, many other molecules besides TSPs have been reported. Since Barres’s group first showed that astrocytes dramatically control synapse formation, maturation, and even elimination, as well as synaptic efficacy by releasing soluble proteins [26,27], a range of astrocyte-secreted molecules that can induce synapse remodeling during development has been identified: brain-derived neurotrophic factor (BDNF), cholesterol, glypicans, hevin, SPARC, transforming growth factor β (TGF-β), tumor necrosis factor α (TNF-α), TSPs [28,29] .(Briefly, BDNF released from astrocyte-like non-neuronal cells, called supporting cells, in the vestibular organ promotes synapse formation between hair cells and primary vestibular sensory neurons [30]. Cholesterol is the first identified astrocyte-derived molecule that induces excitatory synapse formation in retinal ganglion cell (RGC) cultures [31]. Glypicans 4 and 6 promote the formation of postsynaptically functional excitatory synapses by increasing the levels of AMPA receptors on the surface of the postsynaptic membrane [10]. Hevin induces the formation and/or maturation of retinocollicular and thalamocortical synapses, whereas SPARC antagonizes the synaptogenic effect of hevin [32,33]. Chordin-like 1 drives synapse maturation by increasing GluA2 AMPA receptors [34]. TGF-β also showed synaptogenic properties in cultured cortical neurons and in neuromuscular junction [35,36], and TNF-α increases the surface expression of AMPA receptors to maintain synaptic strength and contributes to the homeostatic activity-dependent refinement of neural circuits during development [37,38]. Finally, TSPs are suggested to have a primary role in astrocyte-mediated excitatory synapse formation [28,39]. Astrocytes express high levels of TSP-1 and -2 during development when neural circuits are massively forming, and double knockout mice have fewer excitatory synapses in the brain as compared to wild-type mice [8]. Adhesion molecules also have important roles in the formation of excitatory synapses by stimulating protein kinase C-mediated signals [40]. Astrocytes express many types of molecules that induce the formation and maturation of synapses. The identification of the mechanisms by which astrocytes use these molecules differentially will require further research [41].
4. Epileptogenesis and Aberrant Ca2+ Signals in Epileptogenic Astrocytes
Astrocytes are known to be reactive in the brain of epilepsy patients [42] and animal models of epilepsy [43], and a link between the molecular pathogenesis of epilepsy and reactive astrocytes has long been suggested. Epileptic seizures and ictogenesis result from abnormal and excessive excitability of neurons and networks and astrocytic dysregulation of neuronal excitability, including reduced uptake of extracellular glutamate by GLT-1 [44] and K+ by Kir4.1 [45]. Numerous reports have examined the association between epileptic seizures and dysfunction of these astrocytic molecules, including Ca2+ [46]. Epilepsy not only results from overexcitation of neurons but also is a condition in which excitation occurs repeatedly. Thus, in addition to the clarification of ictogenesis and neuronal overexcitation, the mechanism by which epileptic seizures are repeated needs to be elucidated, or in other words, the mechanism that makes epilepsy more likely to occur needs to be determined [19,20]. This situation, in which the brain becomes prone to recurrent epileptic seizures is called epileptogenesis. We examined epileptogenesis and reactive astrocytes and found that excess Ca2+ signals in reactive astrocytes cause epileptogenesis [21]. Pilocarpine (Pilo) was used to induce epileptogenesis. When Pilo is injected in mice, the animals soon show a strong epileptic seizure, known as status epilepticus (SE). After SE, the mice become progressively more sensitive to Pilo and more prone to epileptic seizure, and 4 weeks after SE, they become completely epileptogenic and show spontaneous ictal spikes (Figure 2). We analyzed the time course of glial activation in the hippocampus and found that after SE, microglia were immediately activated, but this was transient, and the microglia quickly returned to their original state. However, astrocytes gradually became reactive, which is highly consistent with the time course of epileptogenic acquisition. Thus, we hypothesized that reactive astrocytes are involved in epileptogenesis and tentatively named them “epileptogenic astrocytes”. We screened characteristic features of epileptogenic astrocytes and found that they showed very frequent, intense, and wide-spreading Ca2+ signals. These abnormal Ca2+ signals were mediated by IP3RII. We thus determined whether Pilo-evoked epileptogenic astrocytes, excess Ca2+ signals, and epileptogenesis were observed in IP3RIIKO mice and found that all epileptic events were abolished in these mice. Therefore, we concluded that the abnormal Ca2+ signals observed in reactive astrocytes cause epileptogenesis [21].
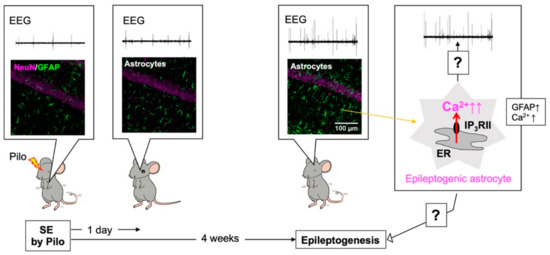
Figure 2.
Abnormal Ca2+ signals in hippocampal astrocytes cause epileptogenesis. Four weeks, but not 1 day, after pilocarpine (Pilo)-evoked status epilepticus (SE), hippocampal astrocytes became reactive and showed increased glial fibrillary acidic protein (GFAP) expression and frequent abnormal Ca2+ signaling (right cartoon). Reactive astrocytes were highly associated with the development of epileptogenesis as assessed by ictal spikes (electroencephalogram; EEG) or seizure susceptibility to Pilo and, thus, they were named “epileptogenic astrocytes”. Epileptogenesis appeared dependent on abnormal Ca2+ signaling in epileptogenic astrocytes. The mechanism by which epileptogenic astrocytes cause epileptogenesis is a subject for future research.
5. Alexander Disease (AxD) and Aberrant Ca2+ Signals in AxD Astrocytes
AxD is a very rare neurodegenerative disease that was first described by Steward Alexander in 1949 [47]. AxD is caused by mutation of the gene encoding glial fibrillary acidic protein (GFAP), an astrocyte-specific intermediate filament. In AxD patients and animal models, astrocytes become reactive and show intracellular aggregates called Rosenthal fibers including GFAP, ab-crystallin, vimentin, and heat shock protein27, which is a typical pathological feature [48]. Since AxD is a primary astrocytic disease, it is speculated that astrocytic dysfunction triggers it, which in turn causes severe neurological damage. Despite a great deal of vigorous research, little is known about how Rosenthal fibers-containing astrocytes become abnormal and the mechanisms involved in AxD development. In addition, the symptoms of AxD have multiple clinical forms, and its onset also varies from newborns to adults. It also remains unclear how mutations in GFAP cause such diverse and severe neurodegenerative diseases. We conducted a detailed analysis of AxD astrocytes using a mouse model of AxD overexpressing a mutated human GFAP gene (hGFAP-R239H knock-in mice) [49]. AxD astrocytes are reactive, and interestingly, also show abnormal Ca2+ signals, called AxCa (aberrant extra-large Ca2+) signals (Figure 3) [22]. AxCa signals are highly associated with AxD pathology and are caused by the activation of IP3RII. It was not known whether AxCa signals are a cause or a result of AxD. To determine this, we crossed the AxD mice with IP3RIIKO mice (AxD–IP3RIIKO). AxD-IP3RIIKO mice dramatically decreased AxCa signals as well as aggregated GFAP accumulation, a typical pathological indicator of AxD. Therefore, we concluded that abnormal Ca2+ signals in reactive astrocytes (AxD astrocytes) are a cause of AxD pathology [22].
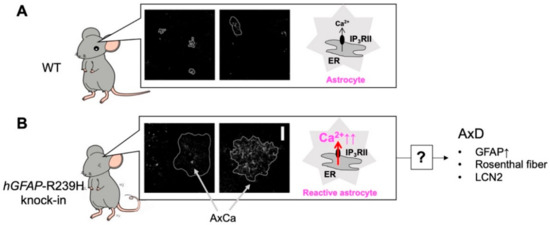
Figure 3.
Abnormal Ca2+ (AxCa) signals in reactive astrocytes cause Alexander disease (AxD). Unlike in WT mice (A), in hGFAP-R239H knock-in mice, hippocampal astrocytes are reactive and show abnormal extremely large Ca2+ signals called “AxCa” signals (B). AxCa signals in AxD astrocytes are mediated by type II inositol 1,4,5-trisphosphate receptors (IP3RII) and are abolished in AxD-IP3RII knockout (KO) mice. Importantly, typical indicators of AxD such as an increase in glial fibrillary acidic protein (GFAP), the formation of Rosenthal fibers, and the induction of lipocalin 2 (LCN2) are dramatically decreased in AxD-IP3RIIKO mice. Although the mechanisms underlying AxCa signal-mediated AxD pathology remain largely unknown, AxCa signals in AxD astrocytes are a cause of AxD.
Although aberrant Ca2+ signals mediated by IP3RII in AxD astrocytes were found to be associated with the molecular pathogenesis of AxD, it remains unclear whether these are cell-autonomous actions of astrocytes or whether they involve the communication with other cells, such as neurons, microglia, and blood vessels. Recently, in iPS cell-derived astrocytes from AxD human patients, aberrant Ca2+ signals were also observed [50]. AxD iPS cell-derived astrocytes showed decreased Ca2+ propagation, which was due to impaired extracellular release of ATP. In this experimental system, only iPS cell-derived astrocytes are present, and there is no communication with other types of cells. Therefore, aberrant Ca2+ signaling in AxD astrocytes occurs as a cell-autonomous action of astrocytes, suggesting that GFAP mutations would be translated into abnormal Ca2+ signals in astrocytes, leading to the development of AxD. However, the molecular mechanisms and cascades leading from aberrant Ca2+ signals to AxD remain unclear. By focusing on Ca2+ abnormalities in astrocytes, future research on AxD is expected to make significant progress.
6. Complexity of Ca2+ Signals in Astrocytes
In this manuscript, we have simply described astrocyte dysfunctions in terms of “aberrant astrocyte Ca2+ signals”. However, Ca2+ signal shows more complex spatiotemporal variations than we thought, and the mechanisms by which these occur are different. For example, Ca2+ signals have three different patterns in striatal astrocytes, defined as global Ca2+ signals, local Ca2+ signals, and Ca2+ microdomains [51]. Global Ca2+ signals are well observed in the somata of astrocytes and are mainly due to Ca2+ release from IP3RII, followed by Ca2+ entry via store-operated Ca2+ entry mechanisms. In addition, Ca2+ shut-off mechanisms mediated by the endoplasmic reticulum Ca2+ pump, the plasma membrane Ca2+ pump, and the Na+/Ca2+ exchanger also affect Ca2+ signals there. On the contrary, Ca2+ microdomains are mainly observed in the fine processes of astrocytes and are rather produced by Ca2+ entry via Ca2+ entry channels such as TRPA1 [52]. Of course, these classifications are not exhaustive, but it should be noted that even healthy astrocytes in the same brain region show such functional heterogeneity in Ca2+ signals. In addition, the word “aberrant Ca2+ signal” means not only an abnormal increase but also an abnormal decrease in Ca2+ signals. However, other than the fact that differences in the spatiotemporal pattern of Ca2+ signals in astrocytes have a significant effect on astrocyte function, research in this field has not progressed, and the actual situation is not well understood. Therefore, in future studies, we should investigate and categorize the aberrant Ca2+ signals in astrocytes from the point of view of temporal and spatial differences as well as of qualitative differences, so to classify them more precisely.
7. Conclusions and Future Perspectives
As shown in the above examples, astrocytes show common characteristics in three very different diseases, i.e., they appeared reactive and exhibited abnormal Ca2+ signals, and very importantly, aberrant Ca2+ signaling was found to be a cause of these diseases. As shown in Figure 4A, the mechanisms by which abnormal Ca2+ signals are induced in various diseases differ (Figure 4A, X1, Y1, and Z1), and the mechanisms by which abnormal Ca2+ signals affect the development and progression of various diseases also markedly differ (Figure 4A, X2, Y2, and Z2). However, evidence strongly suggests that each disease may develop and progress via abnormal astrocytic Ca2+ signaling or that abnormal astrocytic Ca2+ signals may be a common underlying dysfunction in neurodegenerative diseases, because inhibition of these abnormal Ca2+ signals by deletion of IP3RII dramatically reduced all three disease states (Figure 4B). Thus, the elucidation of the mechanisms of astrocytic Ca2+ signaling, especially abnormal Ca2+ signaling, is a fascinating subject that may reveal common principles of neurodegenerative diseases and directly lead to the development of high-impact therapeutic strategies common to many brain diseases. In the future, it will be necessary to clarify the actual Ca2+ abnormalities at the molecular level by comprehensive analyses, such as single-cell RNA sequencing and proteome analysis, and to analyze the mechanisms responsible for Ca2+ signaling abnormalities (Figure 4, X1, Y1, and Z1) and the diseases resulting from Ca2+ signaling abnormalities (Figure 4, X2, Y2, and Z2). In addition, although this review is focused on abnormal Ca2+ signaling in astrocytes, the nature of the “abnormality” in each disease is different. The degree to which Ca2+ signals are abnormal in astrocytes also needs to be carefully studied.
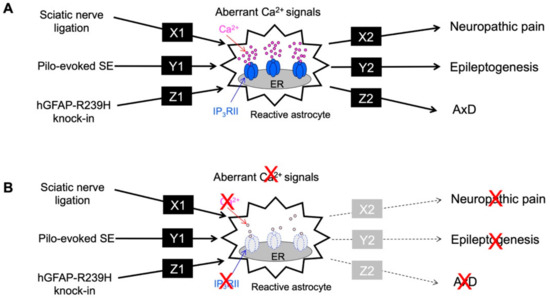
Figure 4.
Summary of aberrant Ca2+ signals and neurodegenerative disorders. (A). Sciatic nerve ligation (neuropathic pain model), Pilo-evoked SE (epileptogenesis model), and hGFAP-R239H knock-in (AxD model) caused a common phenomenon of aberrant Ca2+ signals in reactive astrocytes, although each pathway (X1, Y1 and Z1) is completely different. In addition, the aberrant astrocytic Ca2+ signal observed in each pathological model causes neuropathic pain, epileptogenesis, and AxD via different pathways (X2, Y2 and Z2). Molecules or signals of X1-Z1, X2-Z2 are unknown. (B). The aberrant astrocytic Ca2+ signal observed in each pathological model was mediated by IP3RII. Therefore, when IP3RIIKO mice were used, the aberrant Ca2+ signal induced by sciatic nerve ligation, SE, and hGFAP-R239H knock-in, respectively, was abolished. Importantly, the symptoms of neuropathic pain, epileptogenesis, and AxD also disappeared. Therefore, aberrant Ca2+ signals seen in astrocytes may be one of the common causes of these brain disorders.
Author Contributions
S.K. designed the manuscript outline, wrote the text, and constructed the figures. E.S., F.S., K.S., and S.K.K. commented on the Ca2+ imaging sections, the epileptogenesis section, the AxD section, and the neuropathic pain section, respectively. J.N. commented on the in vivo imaging section and the neuropathic pain section. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Numbers JP16H04669, JP19H04746, JP20H05060, JP20H05902, JP21H04786, and JP21K19309 and partially supported by JST Grant CREST(JPMJCR14G2) and AMED-CREST (JP21gm1310008). This study was also supported by grants from Takeda, Mitsubishi, and Yamanashi Brain-Immune Research.
Institutional Review Board Statement
All animals used in this study were obtained, housed, cared for, and used in accordance with the “Guiding Principles in the Care and Use of Animals in the Field of Physiological Sciences” published by The Physiological Society of Japan and with the approval of the Animal Care Committee of the University of Yamanashi (Approval number: A29-7).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Lisa Kreiner, from Edanz (https://www.jp.edanz.com/ac (accessed on 23 August 2021)) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Koizumi, S.; Fujishita, K.; Tsuda, M.; Shigemoto-Mogami, Y.; Inoue, K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. USA 2003, 100, 11023–11028. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Wang, H.K.; Ye, C.Q.; Ge, W.; Chen, Y.; Jiang, Z.L.; Wu, C.P.; Poo, M.M.; Duan, S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 2003, 40, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef]
- Haydon, P.G. GLIA: Listening and talking to the synapse. Nat. Rev. Neurosci. 2001, 2, 185–193. [Google Scholar] [CrossRef]
- Santello, M.; Bezzi, P.; Volterra, A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 2011, 69, 988–1001. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J. Cell Commun. Signal. 2009, 3, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.J.; Bennett, M.L.; Foo, L.C.; Wang, G.X.; Chakraborty, C.; Smith, S.J.; Barres, B.A. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012, 486, 410–414. [Google Scholar] [CrossRef]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef]
- Kim, S.K.; Nabekura, J.; Koizumi, S. Astrocyte-mediated synapse remodeling in the pathological brain. Glia 2017, 65, 1719–1727. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Author Correction: Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017, 8, 1598. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, J.Y.; Noh, S.; Lee, H.; Lee, S.Y.; Mun, J.Y.; Park, H.; Chung, W.S. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 2021, 590, 612–617. [Google Scholar] [CrossRef]
- Shigetomi, E.; Saito, K.; Sano, F.; Koizumi, S. Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int. J. Mol. Sci. 2019, 20, 996. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Eto, K.; Kim, S.K.; Wake, H.; Takeda, I.; Horiuchi, H.; Moorhouse, A.J.; Ishibashi, H.; Nabekura, J. Cortical astrocytes prime the induction of spine plasticity and mirror image pain. Pain 2018, 159, 1592–1606. [Google Scholar] [CrossRef]
- Alshelh, Z.; Di Pietro, F.; Youssef, A.M.; Reeves, J.M.; Macey, P.M.; Vickers, E.R.; Peck, C.C.; Murray, G.M.; Henderson, L.A. Chronic Neuropathic Pain: It’s about the Rhythm. J. Neurosci. 2016, 36, 1008–1018. [Google Scholar] [CrossRef] [Green Version]
- Heuser, K.; Nome, C.G.; Pettersen, K.H.; Abjorsbraten, K.S.; Jensen, V.; Tang, W.; Sprengel, R.; Tauboll, E.; Nagelhus, E.A.; Enger, R. Ca2+ Signals in Astrocytes Facilitate Spread of Epileptiform Activity. Cereb. Cortex 2018, 28, 4036–4048. [Google Scholar] [CrossRef] [Green Version]
- Heuser, K.; Enger, R. Astrocytic Ca2+ Signaling in Epilepsy. Front. Cell. Neurosci. 2021, 15, 695380. [Google Scholar] [CrossRef]
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus. JCI Insight 2021, 6, e135391. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Shigetomi, E.; Yasuda, R.; Sato, R.; Nakano, M.; Tashiro, K.; Tanaka, K.F.; Ikenaka, K.; Mikoshiba, K.; Mizuta, I.; et al. Aberrant astrocyte Ca2+ signals “AxCa signals” exacerbate pathological alterations in an Alexander disease model. Glia 2018, 66, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- Nagai, J.; Rajbhandari, A.K.; Gangwani, M.R.; Hachisuka, A.; Coppola, G.; Masmanidis, S.C.; Fanselow, M.S.; Khakh, B.S. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019, 177, 1280–1292. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.L.; Shukla, A.; Beroun, A.; Ishikawa, M.; Huang, X.; Wang, Y.; Wang, Y.Q.; Yang, Y.; Bastola, N.D.; et al. Cocaine Triggers Astrocyte-Mediated Synaptogenesis. Biol. Psychiatry 2021, 89, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Barres, B.A. Synaptic efficacy enhanced by glial cells in vitro. Science 1997, 277, 1684–1687. [Google Scholar] [CrossRef] [Green Version]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of synapse number by glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Stogsdill, J.A.; Eroglu, C. The interplay between neurons and glia in synapse development and plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Casati, M.E.; Murtie, J.C.; Rio, C.; Stankovic, K.; Liberman, M.C.; Corfas, G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17005–17010. [Google Scholar] [CrossRef] [Green Version]
- Mauch, D.H.; Nagler, K.; Schumacher, S.; Goritz, C.; Muller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef] [Green Version]
- Risher, W.C.; Patel, S.; Kim, I.H.; Uezu, A.; Bhagat, S.; Wilton, D.K.; Pilaz, L.J.; Singh Alvarado, J.; Calhan, O.Y.; Silver, D.L.; et al. Astrocytes refine cortical connectivity at dendritic spines. Elife 2014, 3, e04047. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Suarez, E.; Liu, T.F.; Kopelevich, A.; Allen, N.J. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 2018, 100, 1116–1132. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigao, P.; Stipursky, J.; Kahn, S.A.; Romao, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]
- Fuentes-Medel, Y.; Ashley, J.; Barria, R.; Maloney, R.; Freeman, M.; Budnik, V. Integration of a retrograde signal during synapse formation by glia-secreted TGF-beta ligand. Curr. Biol. 2012, 22, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFalpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Welsh, C.A.; Barres, B.A.; Stevens, B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015, 18, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Hama, H.; Hara, C.; Yamaguchi, K.; Miyawaki, A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron 2004, 41, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Kitaura, H.; Hiraishi, T.; Itoh, Y.; Oishi, M.; Fujii, Y.; Fukuda, M.; Kakita, A. Reactive astrocytes contribute to epileptogenesis in patients with cavernous angioma. Epilepsy Res. 2021, 176, 106732. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Tanaka, K.; Watase, K.; Manabe, T.; Yamada, K.; Watanabe, M.; Takahashi, K.; Iwama, H.; Nishikawa, T.; Ichihara, N.; Kikuchi, T.; et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 1997, 276, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Kinboshi, M.; Ikeda, A.; Ohno, Y. Role of Astrocytic Inwardly Rectifying Potassium (Kir) 4.1 Channels in Epileptogenesis. Front. Neurol. 2020, 11, 626658. [Google Scholar] [CrossRef]
- Tian, G.F.; Azmi, H.; Takano, T.; Xu, Q.; Peng, W.; Lin, J.; Oberheim, N.; Lou, N.; Wang, X.; Zielke, H.R.; et al. An astrocytic basis of epilepsy. Nat. Med. 2005, 11, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Alexander, W.S. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain 1949, 72, 373–381. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakagawa, M. Clinical aspects and pathology of Alexander disease, and morphological and functional alteration of astrocytes induced by GFAP mutation. Neuropathology 2012, 32, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.F.; Takebayashi, H.; Yamazaki, Y.; Ono, K.; Naruse, M.; Iwasato, T.; Itohara, S.; Kato, H.; Ikenaka, K. Murine model of Alexander disease: Analysis of GFAP aggregate formation and its pathological significance. Glia 2007, 55, 617–631. [Google Scholar] [CrossRef]
- Jones, J.R.; Kong, L.; Hanna, M.G.T.; Hoffman, B.; Krencik, R.; Bradley, R.; Hagemann, T.; Choi, J.; Doers, M.; Dubovis, M.; et al. Mutations in GFAP Disrupt the Distribution and Function of Organelles in Human Astrocytes. Cell Rep. 2018, 25, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Diaz-Castro, B.; Looger, L.L.; Khakh, B.S. Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J. Neurosci. 2016, 36, 3453–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).