The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases
Abstract
1. Introduction
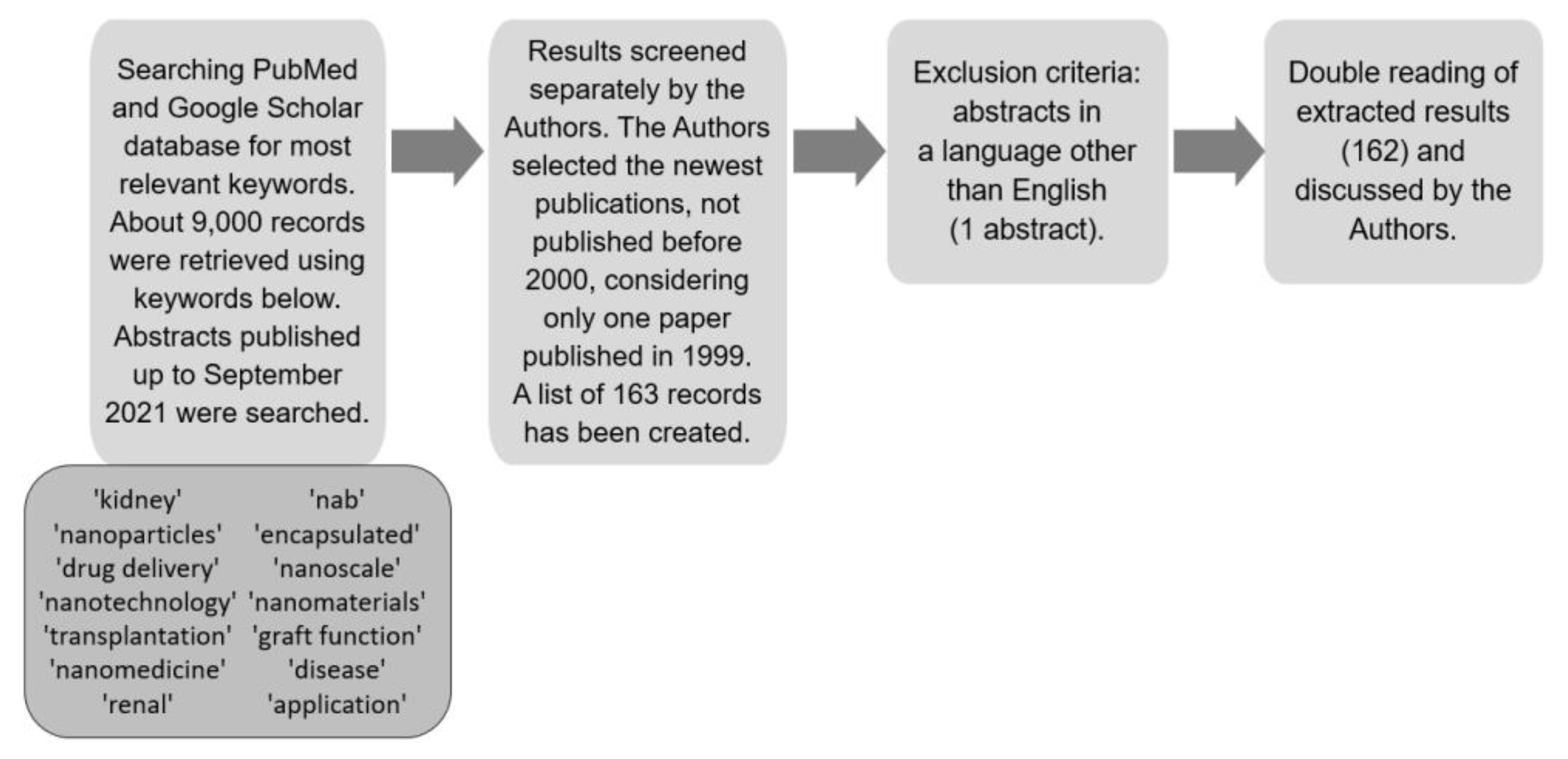
2. Materials and Methods
3. Properties of Nanoparticles
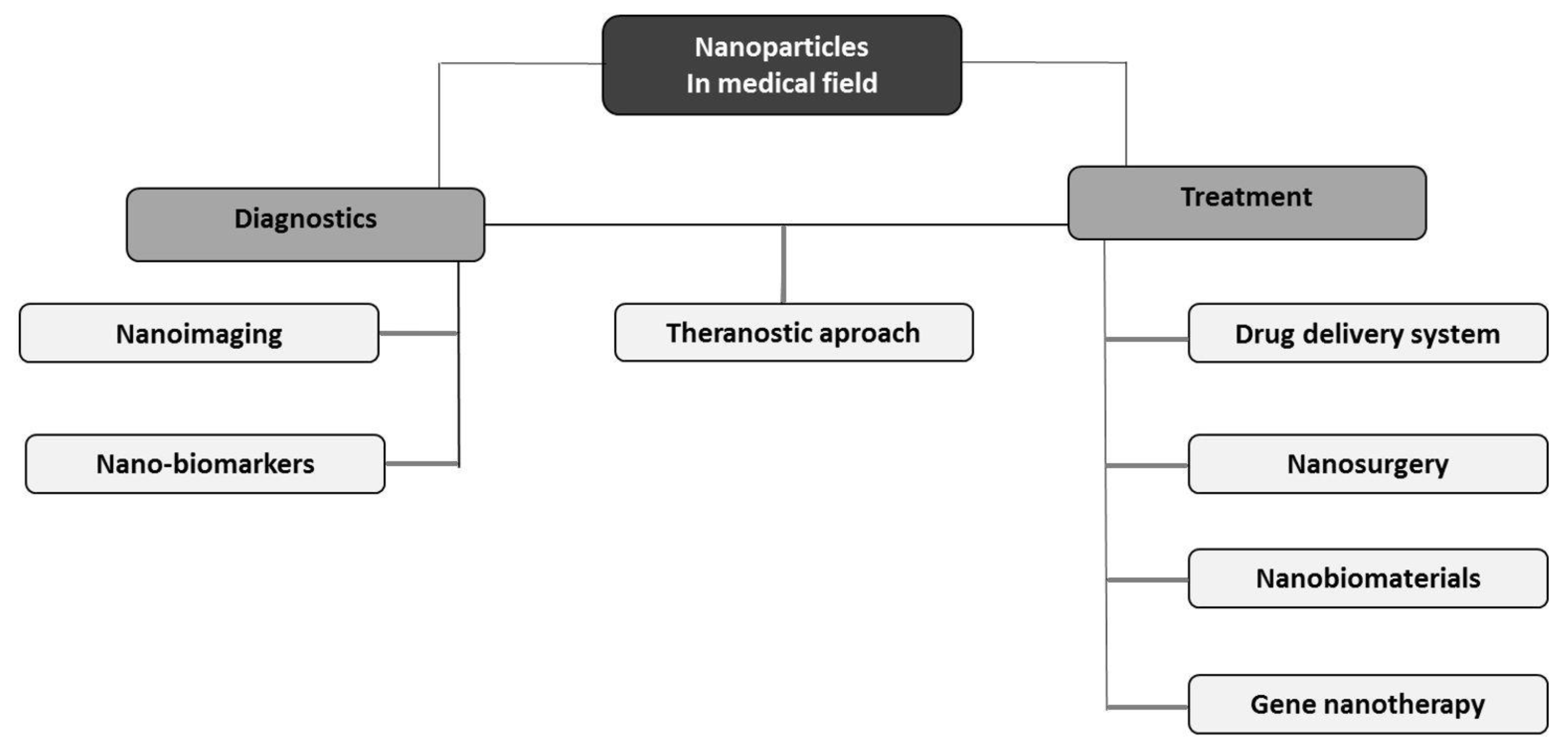
4. Medical Applications of Nanomaterials
4.1. Nanoimaging and Detection of Various Disorders
4.2. Drug Delivery System
4.3. Theranostic Approach
4.4. Nanosurgery and Nanobiomaterials
4.5. Gene Nanotherapy
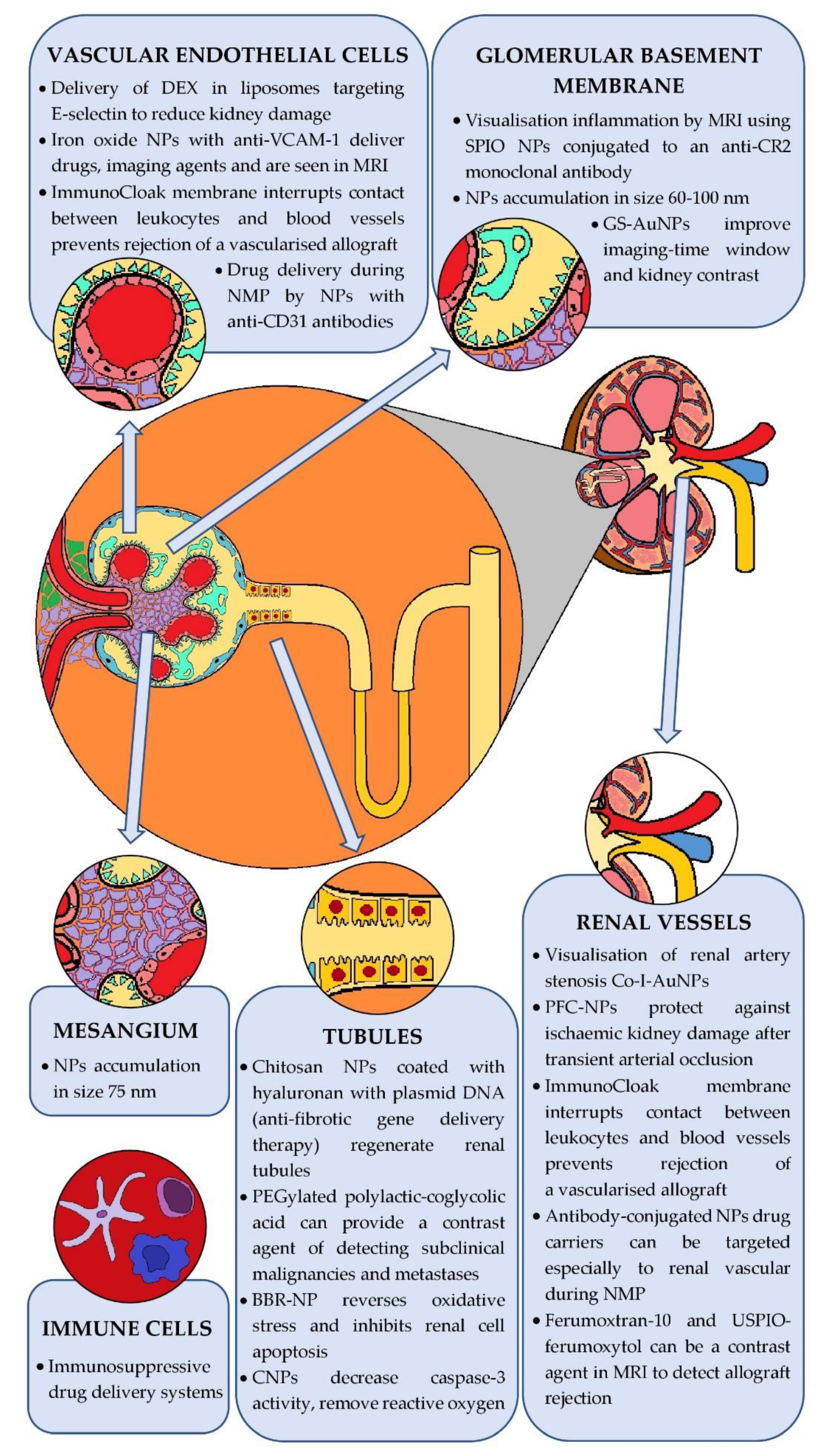
5. Nanoparticles in Diagnosis and Treatment of Kidney Diseases
5.1. Nanoparticles in Kidney Diseases
5.1.1. Monitoring of Kidney Function and Structure
5.1.2. Potential Therapeutic Application
5.2. Nanoparticles in Kidney Transplants
5.2.1. Prevention of Transplant Rejection
5.2.2. Inhibition of Premature Transplant Insufficiency Because of Nonimmunological Factors
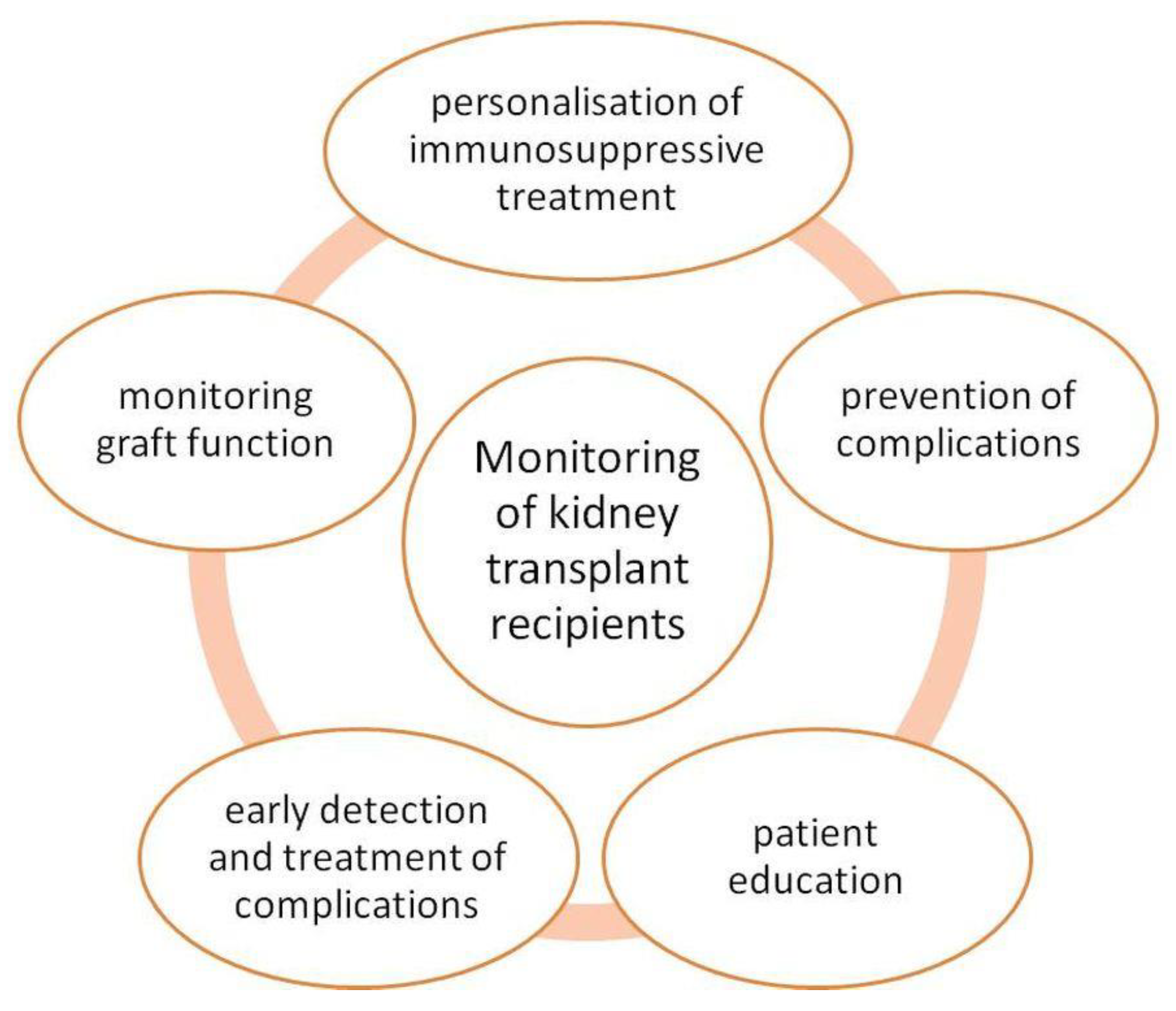
5.3. Monitoring Patients after KTx
5.4. Limitations of Nanoparticles in Nephrology
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Shatrohan Lal, R.K. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 11, 3. [Google Scholar] [CrossRef]
- Madkour, L.H. Introduction to Nanotechnology (NT) and Nanomaterials (NMs). In Nanoelectronic Materials; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2019; Volume 116, pp. 1–47. ISBN 978-3-030-21620-7. [Google Scholar]
- Kumar Teli, M.; Mutalik, S.; Rajanikant, G.K. Nanotechnology and Nanomedicine: Going Small Means Aiming Big. Curr. Pharm. Des. 2010, 16, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Bucheli, T.D. Occurrence, Behavior and Effects of Nanoparticles in the Environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Ma, Y.; Cai, F.; Li, Y.; Chen, J.; Han, F.; Lin, W. A Review of the Application of Nanoparticles in the Diagnosis and Treatment of Chronic Kidney Disease. Bioact. Mater. 2020, 5, 732–743. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-Hydroxyapatite Use in Dentistry: A Systematic Review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Jiang, K.; Chung, E.J. Improving Kidney Targeting: The Influence of Nanoparticle Physicochemical Properties on Kidney Interactions. J. Control. Release 2021, 334, 127–137. [Google Scholar] [CrossRef]
- Mac, Q.D.; Mathews, D.V.; Kahla, J.A.; Stoffers, C.M.; Delmas, O.M.; Holt, B.A.; Adams, A.B.; Kwong, G.A. Non-Invasive Early Detection of Acute Transplant Rejection via Nanosensors of Granzyme B Activity. Nat. Biomed. Eng. 2019, 3, 281–291. [Google Scholar] [CrossRef]
- Woud, W.W.; Merino, A.; Hoogduijn, M.J.; Boer, K.; van den Hoogen, M.W.F.; Baan, C.C.; Minnee, R.C. Nanoparticle Release by Extended Criteria Donor Kidneys During Normothermic Machine Perfusion. Transplantation 2019, 103, e110–e111. [Google Scholar] [CrossRef]
- Asha, A.B.; Narain, R. Nanomaterials Properties. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–359. ISBN 978-0-12-816806-6. [Google Scholar]
- Trotta, F.; Mele, A. Nanomaterials: Classification and Properties. In Nanosponges; Trotta, F., Mele, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–26. ISBN 978-3-527-34100-9. [Google Scholar]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, Properties, Preparation and Behaviour in Environmental Media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective Oxidation with Dioxygen by Gold Nanoparticle Catalysts Derived from 55-Atom Clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Pal, A.; Pal, T. Size Regime Dependent Catalysis by Gold Nanoparticles for the Reduction of Eosin. J. Phys. Chem. B 2001, 105, 9266–9272. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 63–70. ISBN 978-1-60327-197-4. [Google Scholar]
- Kumar, N.; Sinha Ray, S. Synthesis and Functionalization of Nanomaterials. In Processing of Polymer-based Nanocomposites; Sinha Ray, S., Ed.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2018; Volume 277, pp. 15–55. ISBN 978-3-319-97778-2. [Google Scholar]
- Sanvicens, N.; Marco, M.P. Multifunctional Nanoparticles—Properties and Prospects for Their Use in Human Medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in Developmental Organic and Inorganic Nanomaterial: A Review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and Multifunctional Applications in Biomedical Sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef]
- Kumar, N.; Sinha Ray, S.; Ngila, J.C. Ionic Liquid-Assisted Synthesis of Ag/Ag2 Te Nanocrystals via a Hydrothermal Route for Enhanced Photocatalytic Performance. New J. Chem. 2017, 41, 14618–14626. [Google Scholar] [CrossRef]
- Gusain, R.; Singhal, N.; Singh, R.; Kumar, U.; Khatri, O.P. Ionic-Liquid-Functionalized Copper Oxide Nanorods for Photocatalytic Splitting of Water. ChemPlusChem 2016, 81, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional Inorganic Nanoparticles: Recent Progress in Thermal Therapy and Imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in Magnetic Resonance Imaging: From Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727. [Google Scholar] [CrossRef]
- Das, S.; Kotcherlakota, R.; Patra, C.R. Noninvasive Imaging Techniques of Metal Nanoparticles and Their Future Diagnostic Applications. In Medical Imaging Methods; Shukla, A.K., Ed.; Springer: Singapore, 2019; pp. 119–141. ISBN 9789811391200. [Google Scholar]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jørnsgård, H.; Tegnelius, D.; Eriksson, S.; et al. The Nordic SentiMag Trial: A Comparison of Super Paramagnetic Iron Oxide (SPIO) Nanoparticles versus Tc99 and Patent Blue in the Detection of Sentinel Node (SN) in Patients with Breast Cancer and a Meta-Analysis of Earlier Studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakamura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter Clinical Trial on Sentinel Lymph Node Biopsy Using Superparamagnetic Iron Oxide Nanoparticles and a Novel Handheld Magnetic Probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.E.; Yoffe, S.; Meerasa, A. Nanotechnology and Diagnostic Imaging: New Advances in Contrast Agent Technology. J. Nanomedic. Nanotechnol. 2011, 2, 1–12. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Zeng, E.-Z. Application of Functional Quantum Dot Nanoparticles as Fluorescence Probes in Cell Labeling and Tumor Diagnostic Imaging. Nanoscale Res. Lett. 2015, 10, 171. [Google Scholar] [CrossRef]
- Tavernaro, I.; Cavelius, C.; Peuschel, H.; Kraegeloh, A. Bright Fluorescent Silica-Nanoparticle Probes for High-Resolution STED and Confocal Microscopy. Beilstein J. Nanotechnol. 2017, 8, 1283–1296. [Google Scholar] [CrossRef]
- Kosareva, A.; Abou-Elkacem, L.; Chowdhury, S.; Lindner, J.R.; Kaufmann, B.A. Seeing the Invisible—Ultrasound Molecular Imaging. Ultrasound Med. Biol. 2020, 46, 479–497. [Google Scholar] [CrossRef]
- Perera, R.; de Leon, A.; Wang, X.; Wang, Y.; Ramamurthy, G.; Peiris, P.; Abenojar, E.; Basilion, J.P.; Exner, A.A. Real Time Ultrasound Molecular Imaging of Prostate Cancer with PSMA-Targeted Nanobubbles. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102213. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hernandez, C.; Yuan, H.-X.; Lilly, J.; Kota, P.; Zhou, H.; Wu, H.; Exner, A.A. Ultrasound Molecular Imaging of Ovarian Cancer with CA-125 Targeted Nanobubble Contrast Agents. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2159–2168. [Google Scholar] [CrossRef]
- Ishigami, K.; Nishie, A.; Nakayama, T.; Asayama, Y.; Kakihara, D.; Fujita, N.; Ushijima, Y.; Okamoto, D.; Ohtsuka, T.; Mori, Y.; et al. Superparamagnetic Iron-Oxide-Enhanced Diffusion-Weighted Magnetic Resonance Imaging for the Diagnosis of Intrapancreatic Accessory Spleen. Abdom. Radiol. 2019, 44, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Kumano, S.; Murakami, T.; Kim, T.; Hori, M.; Okada, A.; Sugiura, T.; Noguchi, Y.; Kawata, S.; Tomoda, K.; Nakamura, H. Using Superparamagnetic Iron Oxide–Enhanced MRI to Differentiate Metastatic Hepatic Tumors and Nonsolid Benign Lesions. Am. J. Roentgenol. 2003, 181, 1335–1339. [Google Scholar] [CrossRef]
- Umetsu, M.; Goto, H.; Nakamura, Y.; Ota, H.; Shimizu, T.; Hashimoto, M.; Akamatsu, D.; Kamei, T. Detection of Macrophage Localization in the Abdominal Aortic Aneurysm Wall Using Ex Vivo Superparamagnetic Iron Oxide–Enhanced Magnetic Resonance Imaging. Ann. Vasc. Surg. 2020, 68, 344–350. [Google Scholar] [CrossRef]
- Sharfuddin, A. Renal Relevant Radiology: Imaging in Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2014, 9, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for Diagnosis and Therapy of Atherosclerosis and Myocardial Infarction: Evolution toward Prospective Theranostic Approaches. Theranostics 2018, 8, 4710–4732. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-W.; Wu, P.-T.; Liao, M.-Y.; Chung, I.-J.; Yang, K.-C.; Tseng, W.-Y.; Yu, J. Magnetic Nanoparticles Conjugated with Peptides Derived from Monocyte Chemoattractant Protein-1 as a Tool for Targeting Atherosclerosis. Pharmaceutics 2018, 10, 62. [Google Scholar] [CrossRef]
- Lagan, J.; Naish, J.H.; Simpson, K.; Zi, M.; Cartwright, E.J.; Foden, P.; Morris, J.; Clark, D.; Birchall, L.; Caldwell, J.; et al. Substrate for the Myocardial Inflammation–Heart Failure Hypothesis Identified Using Novel USPIO Methodology. JACC Cardiovasc. Imaging 2021, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tahir, A.; Wang, H.; Chang, J. Applications of Nanotechnology in Virus Detection, Tracking, and Infection Mechanisms. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1700. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum Dots: Synthesis, Bioapplications, and Toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Shafiee, H. Applications of Gold Nanoparticles in Virus Detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef] [PubMed]
- Aithal, S.; Mishriki, S.; Gupta, R.; Sahu, R.P.; Botos, G.; Tanvir, S.; Hanson, R.W.; Puri, I.K. SARS-CoV-2 Detection with Aptamer-Functionalized Gold Nanoparticles. Talanta 2022, 236, 122841. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Drelich, A.J.; Hopkins, C.M.; Mecozzi, S.; Li, L.; Kwon, G.; Hong, S. Gold Nanoparticles in Virus Detection: Recent Advances and Potential Considerations for SARS-CoV-2 Testing Development. WIREs Nanomed. Nanobiotechnol. 2021, e1754. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Chandra Ray, P. The Rapid Diagnosis and Effective Inhibition of Coronavirus Using Spike Antibody Attached Gold Nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Aung, K.M.M.; Ow, S.Y.; Amrun, S.N.; Sutarlie, L.; Ng, L.F.P.; Su, X. Epitope-Functionalized Gold Nanoparticles for Rapid and Selective Detection of SARS-CoV-2 IgG Antibodies. ACS Nano 2021, 15, 12286–12297. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A. (Ed.) Biotechnology Business-Concept to Delivery; EcoProduction, Environmental Issues in Logistics and Manufacturing; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-36130-3. [Google Scholar]
- Wang, H.; Cheng, G.; Du, Y.; Ye, L.; Chen, W.; Zhang, L.; Wang, T.; Tian, J.; Fu, F. Hypersensitivity Reaction Studies of a Polyethoxylated Castor Oil-Free, Liposome-Based Alternative Paclitaxel Formulation. Mol. Med. Rep. 2013, 7, 947–952. [Google Scholar] [CrossRef]
- Gardner, E.R.; Dahut, W.L.; Scripture, C.D.; Jones, J.; Aragon-Ching, J.B.; Desai, N.; Hawkins, M.J.; Sparreboom, A.; Figg, W.D. Randomized Crossover Pharmacokinetic Study of Solvent-Based Paclitaxel and Nab-Paclitaxel. Clin. Cancer Res. 2008, 14, 4200–4205. [Google Scholar] [CrossRef]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Schmatloch, S.; Aktas, B.; Denkert, C.; Schem, C.; Wiebringhaus, H.; Kümmel, S.; Warm, M.; et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69–GeparSepto. JCO 2019, 37, 2226–2234. [Google Scholar] [CrossRef]
- Mahtani, R.L.; Parisi, M.; Glück, S.; Ni, Q.; Park, S.; Pelletier, C.; Faria, C.; Braiteh, F. Comparative Effectiveness of Early-Line Nab-Paclitaxel vs. Paclitaxel in Patients with Metastatic Breast Cancer: A US Community-Based Real-World Analysis. CMAR 2018, 10, 249–256. [Google Scholar] [CrossRef]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. Nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival from a Phase III Trial. JNCI J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef]
- Weiss, J.; Gilbert, J.; Deal, A.M.; Weissler, M.; Hilliard, C.; Chera, B.; Murphy, B.; Hackman, T.; Liao, J.J.; Grilley Olson, J.; et al. Induction Chemotherapy with Carboplatin, Nab-Paclitaxel and Cetuximab for at Least N2b Nodal Status or Surgically Unresectable Squamous Cell Carcinoma of the Head and Neck. Oral Oncol. 2018, 84, 46–51. [Google Scholar] [CrossRef]
- Mudad, R.; Patel, M.B.; Margunato-Debay, S.; Garofalo, D.; Lal, L.S. Comparative Effectiveness and Safety of Nab-Paclitaxel plus Carboplatin vs Gemcitabine plus Carboplatin in First-Line Treatment of Advanced Squamous Cell Non-Small Cell Lung Cancer in a US Community Oncology Setting. LCTT 2017, 8, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Design Considerations for Nanotherapeutics in Oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced Cardiotoxicity and Preserved Antitumor Efficacy of Liposome-Encapsulated Doxorubicin and Cyclophosphamide Compared with Conventional Doxorubicin and Cyclophosphamide in a Randomized, Multicenter Trial of Metastatic Breast Cancer. JCO 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Valero, V.; Buzdar, A.U.; Theriault, R.L.; Azarnia, N.; Fonseca, G.A.; Willey, J.; Ewer, M.; Walters, R.S.; Mackay, B.; Podoloff, D.; et al. Phase II Trial of Liposome-Encapsulated Doxorubicin, Cyclophosphamide, and Fluorouracil as First-Line Therapy in Patients with Metastatic Breast Cancer. JCO 1999, 17, 1425. [Google Scholar] [CrossRef]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. IJMS 2018, 19, 3253. [Google Scholar] [CrossRef]
- Lei, J.; Wang, H.; Zhu, D.; Wan, Y.; Yin, L. Combined Effects of Avasimibe Immunotherapy, Doxorubicin Chemotherapy, and Metal–Organic Frameworks Nanoparticles on Breast Cancer. J. Cell Physiol. 2020, 235, 4814–4823. [Google Scholar] [CrossRef]
- Graziani, S.R.; Vital, C.G.; Morikawa, A.T.; Van Eyll, B.M.; Fernandes Junior, H.J.; Kalil Filho, R.; Maranhão, R.C. Phase II Study of Paclitaxel Associated with Lipid Core Nanoparticles (LDE) as Third-Line Treatment of Patients with Epithelial Ovarian Carcinoma. Med. Oncol. 2017, 34, 151. [Google Scholar] [CrossRef]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Fullerene as a Doxorubicin Nanotransporter for Targeted Breast Cancer Therapy: Capillary Electrophoresis Analysis. Electrophoresis 2018, 39, 2370–2379. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef]
- Wartlick, H.; Michaelis, K.; Balthasar, S.; Strebhardt, K.; Kreuter, J.; Langer, K. Highly Specific HER2-Mediated Cellular Uptake of Antibody-Modified Nanoparticles in Tumour Cells. J. Drug Target. 2004, 12, 461–471. [Google Scholar] [CrossRef]
- Ranghar, S.; Sirohi, P.; Verma, P.; Agarwal, V. Nanoparticle-Based Drug Delivery Systems: Promising Approaches against Infections. Braz. Arch. Biol. Technol. 2013, 57, 209–222. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Skalickova, S.; Kepinska, M.; Cihalova, K.; Docekalova, M.; Stankova, M.; Uhlirova, D.; Fernandez, C.; Sochor, J.; Milnerowicz, H.; et al. Development of New Silver Nanoparticles Suitable for Materials with Antimicrobial Properties. J. Nanosci. Nanotechnol. 2019, 19, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 Influenza Virus-Induced Apoptosis by Functionalized Selenium Nanoparticles with Amantadine through ROS-Mediated AKT Signaling Pathways. IJN 2018, 13, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory Activity of Selenium Nanoparticles Functionalized with Oseltamivir on H1N1 Influenza Virus. IJN 2017, 12, 5733–5743. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Reynolds, J.L.; Law, W.-C.; Maponga, C.C.; Prasad, P.N.; Morse, G.D. Multimodal Nanoparticles That Provide Immunomodulation and Intracellular Drug Delivery for Infectious Diseases. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 831–838. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood–Brain Barrier to Treat Neurodegenerative Diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.-L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced Efficacy of Combined Temozolomide and Bromodomain Inhibitor Therapy for Gliomas Using Targeted Nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Rajala, R. Lipid Nanoparticles for Ocular Gene Delivery. JFB 2015, 6, 379–394. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Sarecka-Hujar, B. The Use of Nanotechnology in Modern Pharmacotherapy. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–158. ISBN 978-0-323-52725-5. [Google Scholar]
- Lin, Y.-L.; Tsai, N.-M.; Chen, C.-H.; Liu, Y.-K.; Lee, C.-J.; Chan, Y.-L.; Wang, Y.-S.; Chang, Y.-C.; Lin, C.-H.; Huang, T.-H.; et al. Specific Drug Delivery Efficiently Induced Human Breast Tumor Regression Using a Lipoplex by Non-Covalent Association with Anti-Tumor Antibodies. J. Nanobiotechnol. 2019, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer Assembled Nano-Drug Delivery Systems for Cancer Treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Rizzo, L.Y.; Ehling, J.; Kiessling, F.; Lammers, T. The Theranostic Path to Personalized Nanomedicine. Clin. Transl. Imaging 2014, 2, 67–76. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of Nanoparticles for Diagnosis and Therapy of Cancer. BJR 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent Advances in Metal Nanoparticles in Cancer Therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Li, H.; Zeng, Y.; Zhang, H.; Gu, Z.; Gong, Q.; Luo, K. Functional Gadolinium-Based Nanoscale Systems for Cancer Theranostics. J. Control. Release 2021, 329, 482–512. [Google Scholar] [CrossRef]
- Li, L.; Tong, R.; Li, M.; Kohane, D.S. Self-Assembled Gemcitabine–Gadolinium Nanoparticles for Magnetic Resonance Imaging and Cancer Therapy. Acta Biomater. 2016, 33, 34–39. [Google Scholar] [CrossRef]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.L.; Tran, V.-L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C.; et al. Gadolinium-Based Nanoparticles and Radiation Therapy for Multiple Brain Melanoma Metastases: Proof of Concept before Phase I Trial. Theranostics 2016, 6, 418–427. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting Brain Metastases with Ultrasmall Theranostic Nanoparticles, a First-in-Human Trial from an MRI Perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef]
- Lammers, T.; Koczera, P.; Fokong, S.; Gremse, F.; Ehling, J.; Vogt, M.; Pich, A.; Storm, G.; van Zandvoort, M.; Kiessling, F. Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood-Brain Barrier Permeation. Adv. Funct. Mater. 2015, 25, 36–43. [Google Scholar] [CrossRef]
- Deb, S.; Ghosh, K.; Shetty, S. Nanoimaging in Cardiovascular Diseases: Current State of the Art. Indian J. Med. Res. 2015, 141, 285. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial αν β3 Integrin–Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. ATVB 2006, 26, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Memic, A.; Akbari, M.; Brafman, D.A.; Nikkhah, M. Nano-Enabled Approaches for Stem Cell-Based Cardiac Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1533–1553. [Google Scholar] [CrossRef]
- Yin, R.-X.; Yang, D.-Z.; Wu, J.-Z. Nanoparticle Drug- and Gene-Eluting Stents for the Prevention and Treatment of Coronary Restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef]
- Tsukie, N.; Nakano, K.; Matoba, T.; Masuda, S.; Iwata, E.; Miyagawa, M.; Zhao, G.; Meng, W.; Kishimoto, J.; Sunagawa, K.; et al. Pitavastatin-Incorporated Nanoparticle-Eluting Stents Attenuate In-Stent Stenosis without Delayed Endothelial Healing Effects in a Porcine Coronary Artery Model. JAT 2013, 20, 32–45. [Google Scholar] [CrossRef]
- Leary, S.P.; Liu, C.Y.; Apuzzo, M.L.J. Toward the Emergence of Nanoneurosurgery: Part III—Nanomedicine: Targeted Nanotherapy, Nanosurgery, and Progress Toward the Realization of Nanoneurosurgery. Neurosurgery 2006, 58, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Sardesai, M.; Gaud, R.S. Micro to Nanoneedles: A Trend of Modernized Transepidermal Drug Delivery System. Artif. Cells Nanomed. Biotechnol. 2018, 46, 19–25. [Google Scholar] [CrossRef]
- Zhu, X.; Kwok, S.Y.; Yuen, M.F.; Yan, L.; Chen, W.; Yang, Y.; Wang, Z.; Yu, K.N.; Zhu, G.; Zhang, W.; et al. Dense Diamond Nanoneedle Arrays for Enhanced Intracellular Delivery of Drug Molecules to Cell Lines. J. Mater. Sci. 2015, 50, 7800–7807. [Google Scholar] [CrossRef]
- Yamahata, C.; Collard, D.; Legrand, B.; Takekawa, T.; Kumemura, M.; Hashiguchi, G.; Fujita, H. Silicon Nanotweezers With Subnanometer Resolution for the Micromanipulation of Biomolecules. J. Microelectromech. Syst. 2008, 17, 623–631. [Google Scholar] [CrossRef]
- Chang, W.C.; Hawkes, E.A.; Kliot, M.; Sretavan, D.W. In Vivo Use of a Nanoknife for Axon Microsurgery. Neurosurgery 2007, 61, 683–692. [Google Scholar] [CrossRef]
- Jourabchi, N.; Beroukhim, K.; Tafti, B.A.; Kee, S.T.; Lee, E.W. Irreversible Electroporation (NanoKnife) in Cancer Treatment. Gastrointest. Interv. 2014, 3, 8–18. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A. Current Status of Nanomedicine and Nanosurgery. Anesth. Essays Res. 2013, 7, 237. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Sun, H.; Lv, L.; Bai, Y.; Yang, H.; Zhou, H.; Li, C.; Yang, L. Nanotechnology-Enabled Materials for Hemostatic and Anti-Infection Treatments in Orthopedic Surgery. IJN 2018, 13, 8325–8338. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery Materials for SiRNA Therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Solanki, A.; Memoli, K.A.; Kamei, K.; Kim, H.; Drahl, M.A.; Williams, L.J.; Tseng, H.-R.; Lee, K. Selective Inhibition of Human Brain Tumor Cells through Multifunctional Quantum-Dot-Based SiRNA Delivery. Angew. Chem. Int. Ed. 2010, 49, 103–107. [Google Scholar] [CrossRef]
- Boca, S.; Gulei, D.; Zimta, A.-A.; Onaciu, A.; Magdo, L.; Tigu, A.B.; Ionescu, C.; Irimie, A.; Buiga, R.; Berindan-Neagoe, I. Nanoscale Delivery Systems for MicroRNAs in Cancer Therapy. Cell. Mol. Life Sci. 2020, 77, 1059–1086. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.M. Getting MiRNA Therapeutics into the Target Cells for Neurodegenerative Diseases: A Mini-Review. Front. Mol. Neurosci. 2016, 9, 129. [Google Scholar] [CrossRef]
- Joga, S.; Koyyala, V.P.B. Nanotechnology in Oncology. Indian J. Med. Paediatr. Oncol. 2021, 42, 093–095. [Google Scholar] [CrossRef]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein SiRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an Optimal Drug/SiRNA Combination Using Mesoporous Silica Nanoparticles to Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef]
- Brede, C.; Labhasetwar, V. Applications of Nanoparticles in the Detection and Treatment of Kidney Diseases. Adv. Chronic Kidney Dis. 2013, 20, 454–465. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Zou, X.; Mukherjee, R.; Yu, Z.; Ferguson, C.M.; Zhou, W.; McCollough, C.H.; Lerman, L.O. Targeted Imaging of Renal Fibrosis Using Antibody-Conjugated Gold Nanoparticles in Renal Artery Stenosis. Investig. Radiol. 2018, 53, 623–628. [Google Scholar] [CrossRef]
- Wang, J.; Masehi-Lano, J.J.; Chung, E.J. Peptide and Antibody Ligands for Renal Targeting: Nanomedicine Strategies for Kidney Disease. Biomater. Sci. 2017, 5, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Liu, J. Bioapplications of Renal-Clearable Luminescent Metal Nanoparticles. Biomater. Sci. 2017, 5, 1393–1406. [Google Scholar] [CrossRef]
- Ordikhani, F.; Kasinath, V.; Uehara, M.; Akbarzadeh, A.; A Yilmam, O.; Dai, L.; Aksu, H.; Jung, S.; Jiang, L.; Li, X.; et al. Selective Trafficking of Light Chain-Conjugated Nanoparticles to the Kidney and Renal Cell Carcinoma. Nano Today 2020, 35, 100990. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yu, G.; Shan, Z.; Li, Z. Phyto-Mediated Synthesized Multifunctional Zn/CuO NPs Hybrid Nanoparticles for Enhanced Activity for Kidney Cancer Therapy: A Complete Physical and Biological Analysis. J. Photochem. Photobiol. B Biol. 2018, 186, 131–136. [Google Scholar] [CrossRef]
- Minardi, S.; Shah, S.; Luo, X. Biomimetic Nanoparticles for Transplantation Tolerance. Curr. Opin. Organ Transplant. 2018, 23, 15–21. [Google Scholar] [CrossRef]
- Vemuri, C.; Upadhya, G.A.; Arif, B.; Jia, J.; Lin, Y.; Gaut, J.P.; Fazal, J.; Pan, H.; Wickline, S.A.; Chapman, W.C. Antithrombin Perfluorocarbon Nanoparticles Improve Renal Allograft Function in a Murine Deceased Criteria Donor Model. Transplant. Direct. 2018, 4, e384. [Google Scholar] [CrossRef]
- Uehara, M.; Bahmani, B.; Jiang, L.; Jung, S.; Banouni, N.; Kasinath, V.; Solhjou, Z.; Zhao, J.; Ordikhani, F.; Bae, M.; et al. Nanodelivery of Mycophenolate Mofetil to the Organ Improves Transplant Vasculopathy. ACS Nano 2019, 13, 12393–12407. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, J.; Li, H.; Yu, D.; Wu, T.; Wang, L.; Wang, Y.; Zhou, L.; Zheng, S. Albumin Based Nanomedicine for Enhancing Tacrolimus Safety and Lymphatic Targeting Efficiency. J. Biomed. Nanotechnol. 2019, 15, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.O.; Kireta, S.; McInnes, S.J.P.; Kette, F.D.; Sivanathan, K.N.; Kim, J.; Cueto-Diaz, E.J.; Cunin, F.; Durand, J.-O.; Drogemuller, C.J.; et al. Murine and Non-Human Primate Dendritic Cell Targeting Nanoparticles for in Vivo Generation of Regulatory T-Cells. ACS Nano 2018, 12, 6637–6647. [Google Scholar] [CrossRef]
- Brasile, L.; Henry, N.; Stubenitsky, B. Underlying Mechanisms of Protection Involved in Immunocloak. Transplantation 2017, 101, e49–e56. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Xu, Y.; Jing, W.; Juxiang, Z.; Hailun, L.; Yu, H.; Zheng, D.-H.; Lin, Y.-T. Berberine Nanoparticles Protects Tubular Epithelial Cells from Renal Ischemia-Reperfusion Injury. Oncotarget 2017, 8, 24154–24162. [Google Scholar] [CrossRef]
- Stephen Inbaraj, B.; Chen, B.-H. An Overview on Recent in Vivo Biological Application of Cerium Oxide Nanoparticles. Asian J. Pharm. Sci. 2020, 15, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.Y.; Song, E.J.; Sohn, J.Y.; Kim, S.-T.; Woo, C.W.; Gong, G.; Kang, H.J.; Lee, J.S. Allogeneic Renal Graft Rejection in a Rat Model: In Vivo MR Imaging of the Homing Trait of Macrophages. Radiology 2010, 256, 847–854. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal Clearable Catalytic Gold Nanoclusters for in Vivo Disease Monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
- Midgley, A.C.; Wei, Y.; Zhu, D.; Gao, F.; Yan, H.; Khalique, A.; Luo, W.; Jiang, H.; Liu, X.; Guo, J.; et al. Multifunctional Natural Polymer Nanoparticles as Antifibrotic Gene Carriers for CKD Therapy. JASN 2020, 31, 2292–2311. [Google Scholar] [CrossRef]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for Renal Disease: Current Status and Future Applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef]
- Akhtar, A.M.; Schneider, J.E.; Chapman, S.J.; Jefferson, A.; Digby, J.E.; Mankia, K.; Chen, Y.; McAteer, M.A.; Wood, K.J.; Choudhury, R.P. In Vivo Quantification of Vcam-1 Expression in Renal Ischemia Reperfusion Injury Using Non-Invasive Magnetic Resonance Molecular Imaging. PLoS ONE 2010, 5, e12800. [Google Scholar] [CrossRef]
- Liu, G.W.; Pippin, J.W.; Eng, D.G.; Lv, S.; Shankland, S.J.; Pun, S.H. Nanoparticles Exhibit Greater Accumulation in Kidney Glomeruli during Experimental Glomerular Kidney Disease. Physiol. Rep. 2020, 8, e14545. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.L.; Rodríguez-Benot, A.; Valcárcel, M. Bases nanotecnológicas de una «nueva» Nefrología. Nefrología 2018, 38, 368–378. [Google Scholar] [CrossRef]
- Martuszewski, A.; Paluszkiewicz, P.; Król, M.; Banasik, M.; Kepinska, M. Donor-Derived Cell-Free DNA in Kidney Transplantation as a Potential Rejection Biomarker: A Systematic Literature Review. JCM 2021, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Stanimirova, I.; Banasik, M.; Ząbek, A.; Dawiskiba, T.; Kościelska-Kasprzak, K.; Wojtowicz, W.; Krajewska, M.; Janczak, D.; Młynarz, P. Serum Metabolomics Approach to Monitor the Changes in Metabolite Profiles Following Renal Transplantation. Sci. Rep. 2020, 10, 17223. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral Theory of Transplantation: Humoral Theory of Transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Klinger, M.; Banasik, M. Immunological Characteristics of the Elderly Allograft Recipient. Transplant. Rev. 2015, 29, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Kamińska, D.; Bartoszek, D.; Żabińska, M.; Myszka, M.; Zmonarski, S.; Protasiewicz, M.; Nowakowska, B.; et al. The Influence of Non-HLA Antibodies Directed against Angiotensin II Type 1 Receptor (AT1R) on Early Renal Transplant Outcomes. Transpl. Int. 2014, 27, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.T.; Hosgood, S.A.; DiRito, J.; Cui, J.; Deep, D.; Song, E.; Kraehling, J.R.; Piotrowski-Daspit, A.S.; Kirkiles-Smith, N.C.; Al-Lamki, R.; et al. Nanoparticle Targeting to the Endothelium during Normothermic Machine Perfusion of Human Kidneys. Sci. Transl. Med. 2017, 9, eaam6764. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Hoff, M.; Nicholson, M.L. Treatment of Transplant Kidneys during Machine Perfusion. Transpl. Int. 2021, 34, 224–232. [Google Scholar] [CrossRef] [PubMed]
- DiRito, J.R.; Hosgood, S.A.; Tietjen, G.T.; Nicholson, M.L. The Future of Marginal Kidney Repair in the Context of Normothermic Machine Perfusion. Am. J. Transpl. 2018, 18, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- DiRito, J.R.; Hosgood, S.A.; Reschke, M.; Albert, C.; Bracaglia, L.G.; Ferdinand, J.R.; Stewart, B.J.; Edwards, C.M.; Vaish, A.G.; Thiru, S.; et al. Lysis of Cold-storage-induced Microvascular Obstructions for Ex Vivo Revitalization of Marginal Human Kidneys. Am. J. Transpl. 2021, 21, 161–173. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, L.; Lin, D.; Zhao, J.; Guan, Q.; Zheng, B.; Wang, K.; Li, H.; Chen, R.; Zeng, H.; et al. Assessment of Treatment Efficacy Using Surface-Enhanced Raman Spectroscopy Analysis of Urine in Rats with Kidney Transplantation or Kidney Disease. Clin. Exp. Nephrol. 2019, 23, 880–889. [Google Scholar] [CrossRef]
- Chi, J.; Ma, Y.; Weng, F.L.; Thiessen-Philbrook, H.; Parikh, C.R.; Du, H. Surface-Enhanced Raman Scattering Analysis of Urine from Deceased Donors as a Prognostic Tool for Kidney Transplant Outcome. J. Biophotonics 2017, 10, 1743–1755. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Sun, Y.; He, X.; Xue, D. A Circulating Exosomal MicroRNA Panel as a Novel Biomarker for Monitoring Post-transplant Renal Graft Function. J. Cell. Mol. Med. 2020, 24, 12154–12163. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Zeier, M.G.; Chapman, J.R.; Craig, J.C.; Ekberg, H.; Garvey, C.A.; Green, M.D.; Jha, V.; Josephson, M.A.; Kiberd, B.A.; et al. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients: A Summary. Kidney Int. 2010, 77, 299–311. [Google Scholar] [CrossRef]
- Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [CrossRef]
- Josephson, M.A. Monitoring and Managing Graft Health in the Kidney Transplant Recipient. CJASN 2011, 6, 1774–1780. [Google Scholar] [CrossRef]
- Benoît, G. Surgical view of a series of 3000 kidney transplantations. Bull. Acad. Natl. Med. 2011, 195, 351–362; discussion 362–363. [Google Scholar] [PubMed]
- Carvalho, J.A.; Nunes, P.; Antunes, H.; Parada, B.; Tavares da Silva, E.; Rodrigues, L.; Roseiro, A.; Bastos, C.; Macário, F.; Figueiredo, A. Surgical Complications in Kidney Transplantation: An Overview of a Portuguese Reference Center. Transpl. Proc. 2019, 51, 1590–1596. [Google Scholar] [CrossRef]
- Hakim, D.N.; Nader, M.A.; Sood, A.; Kandilis, A.; Hakim, N.S. Rescue of Transplanted Kidney Thanks to an Implantable Doppler Probe: Is This the Future? Exp. Clin. Transplant. 2016, 14, 454–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodgers, S.K.; Sereni, C.P.; Horrow, M.M. Ultrasonographic Evaluation of the Renal Transplant. Radiol. Clin. N. Am. 2014, 52, 1307–1324. [Google Scholar] [CrossRef]
- Malakasioti, G.; Marks, S.D.; Watson, T.; Williams, F.; Taylor-Allkins, M.; Mamode, N.; Morgan, J.; Hayes, W.N. Continuous Monitoring of Kidney Transplant Perfusion with Near-Infrared Spectroscopy. Nephrol. Dial. Transplant. 2018, 33, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. JCM 2020, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Wu, H.; Hughes, J. Macrophages and Kidney Transplantation. Semin. Nephrol. 2010, 30, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Wolf, G. The Pharmacokinetics of the Lymphotropic Nanoparticle MRI Contrast Agent Ferumoxtran-10. CBM 2009, 5, 69–73. [Google Scholar] [CrossRef]
- Aghighi, M.; Pisani, L.; Theruvath, A.J.; Muehe, A.M.; Donig, J.; Khan, R.; Holdsworth, S.J.; Kambham, N.; Concepcion, W.; Grimm, P.C.; et al. Ferumoxytol Is Not Retained in Kidney Allografts in Patients Undergoing Acute Rejection. Mol. Imaging Biol. 2018, 20, 139–149. [Google Scholar] [CrossRef]
- Yang, D.; Ye, Q.; Williams, M.; Sun, Y.; Hu, T.C.-C.; Williams, D.S.; Moura, J.M.F.; Ho, C. USPIO-Enhanced Dynamic MRI: Evaluation of Normal and Transplanted Rat Kidneys. Magn. Reson. Med. 2001, 46, 1152–1163. [Google Scholar] [CrossRef]
- Hauger, O.; Grenier, N.; Deminère, C.; Lasseur, C.; Delmas, Y.; Merville, P.; Combe, C. USPIO-Enhanced MR Imaging of Macrophage Infiltration in Native and Transplanted Kidneys: Initial Results in Humans. Eur. Radiol. 2007, 17, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Urmson, J.; Ramassar, V.; Melk, A.; Zhu, L.-F.; Halloran, B.P.; Bleackley, R.C. Lesions of T-Cell-Mediated Kidney Allograft Rejection in Mice Do Not Require Perforin or Granzymes A and B. Am. J. Transplant. 2004, 4, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A. Toxicity of Nanoparticles_ Challenges and Opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of Nanoparticles in Bone Tissue Engineering; a Review on the Molecular Mechanisms Driving Osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef] [PubMed]

| NCT Number | Title | Condition | Actual Enrolment 1 | Recruitment Status | Location | Age of Participants 2 |
|---|---|---|---|---|---|---|
| NCT05045872 | The Accuracy and Safety of Renal Artery Contrast-enhanced Magnetic Resonance Imaging with Polysaccharide Superparamagnetic Iron Oxide Nanoparticle | Chronic Kidney Diseases | 40 3 | Not yet recruiting | China | Adult, Older Adult |
| NCT04277377 | Nanoparticle for DSA Removal | Kidney Failure, Presence of Donor Specific Antibodies | 100 3 | Not yet recruiting | Switzerland | Adult, Older Adult |
| NCT02646319 | Nanoparticle Albumin-Bound Rapamycin in Treating Patients with Advanced Cancer with mTOR Mutations | Advanced Cancers 4 | 2 | Completed | United States | Adult, Older Adult |
| NCT04260360 | Trial of NanoDoce Intratumoral Injection in Renal Cell Carcinoma | Renal Cell Carcinoma, Kidney Cancer, Adenocarcinoma of Kidney, Adenocarcinoma, Renal, Renal Cell Cancer | 0 | Withdrawn (Not initiated) | No data | Adult, Older Adult |
| NCT02006108 | Imaging Kidney Transplant Rejection Using Ferumoxytol-Enhanced Magnetic Resonance | Renal Transplant Rejection | 21 | Completed | United States | Child, Adult |
| NCT03961698 | Evaluation of IPI-549 Combined with Front-line Treatments in Pts. With Triple-Negative Breast Cancer or Renal Cell Carcinoma (MARIO-3) (MARIO-3) | Breast Cancer, Renal Cell Carcinoma | 90 3 | Recruiting | United States | Adult, Older Adult |
| NCT00499291 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients with Advanced or Refractory Solid Tumors | Cancer | 0 | Withdrawn | No data | Adult, Older Adult |
| NCT02626663 | The Role of Microparticles as a Biomarker | Atypical Haemolytic Uremic Syndrome, Thrombotic Thrombocytopenic Purpura, Microparticles, Microangiopathic Haemolytic Anaemia | 0 | Withdrawn (PI left the University, sponsor pulled funding) | United States | Adult, Older Adult |
| NCT03678883 | 9-ING-41 in Patients with Advanced Cancers | Human malignancies 5 | 350 3 | Recruiting | United States, Netherlands, Spain | Adult, Older Adult |
| NCT00689065 | Safety Study of CALAA-01 to Treat Solid Tumor Cancers | Cancer, Solid Tumour | 24 | Terminated | United States | Adult, Older Adult |
| Nanoparticles | Type | Role | Mechanism of Action | Type of Condition/ Pathology | References |
|---|---|---|---|---|---|
| Gold NPs with anti-collage-I antibody | Nanocarriers with molecules on the surface that interact with target cells | Diagnostics | CT-imaging contrast, increased retention of gold NPs, good safety profile. | Renal fibrosis | [113] |
| SPIO NPs conjugated to an anti-CR2 monoclonal antibody | SPIO/Nanocarriers with drugs | Therapeutic and diagnostic | Reduced inflammation -targeted drug delivery (e.g., dexamethasone) to glomerular endothelial cells, visualization of inflammation by MRI | Glomerulonephritis | [114] |
| Iron oxide NPs conjugated to anti-VCAM-1 antibody | SPIO/Nanocarriers with drugs | Therapeutic and diagnostic | MRI imaging of the kidney and targeted drug delivery to ischemic tissue (with increased VCAM-1 expression) | AKI (ischemia-reperfusion injury) | [114] |
| Mesoscale NPs | Nanocarriers with drugs | Therapeutic | Drug delivery with 5–7- fold higher efficacy in the kidney (tubular epithelium) than in other organs, safety profile. | Unspecified (various kidney pathologies) | [115] |
| Glutathione-coated luminescent gold NPs | Renal-clearable luminescent metal NPs | Diagnostic | Improved imaging-time window and kidney contrast compared to conventional dyes— superior assessment of renal function | Unspecified (various kidney pathologies) | [116] |
| PEGylated polylactic-coglycolic acid NPs with contrast/drug | Nanocarriers with molecules on the surface that interact with target cells | Therapeutic and diagnostic | Selective interaction with proximal tubule epithelial cells and renal clear cell carcinoma cells—new possibility of imaging a kidney tumour | Renal clear cell carcinoma | [117] |
| Zn/CuO NPs | Nanocarriers with cytotoxic agents | Therapeutic | Excellent inhibition of renal cancer cells- cytotoxic effect of zinc ions without affecting healthy cells | Renal tumours | [118] |
| Liposomes with immunosuppressive drugs | Nanocarriers with immunosuppressive drugs | Therapeutic | Targeted drug delivery with lower toxicity than immunosuppressive drugs alone | Graft rejection | [119] |
| Thrombin-targeted PFC-NP | Nanocarriers with anticoagulants | Therapeutic | Antithrombin activity- protection of the transplanted kidney after a period of ischaemia | Transient arterial occlusion after KTx | [120] |
| NP carrier of mycophenolate mofetil | Nanocarriers with immunosuppressive drugs | Therapeutic | Direct delivery of immunosuppressive drugs to the organ at the time of transplantation reduces intra- graft inflammation and alloimmune activation at a critical time | Graft rejection | [121] |
| NP carrier of tacrolimus | Nanocarriers with immunosuppressive drugs | Therapeutic | Inhibition of interleukin-2 production, suppression of T-cell activation with good safety profile | Graft rejection | [122] |
| Rapamycin and ovalbumin peptide loaded porous silicon NPs | Nanocarriers with immunosuppressive drugs | Therapeutics | Up-regulation of regulatory T-cells | Graft rejection | [123] |
| Immunocloaking membrane covering luminal surfaces of allograft vessels | Nanomaterial | Therapeutics | Interrupted antigen presentation, disrupted diapedesis and inhibited early T-cell activation | Graft rejection | [124] |
| Berberine NPs | Nanocarriers with antioxidative agents | Therapeutic | Reduced expression of oxidative and mitochondrial stress pathway proteins | AKI (ischemia-reperfusion injury) | [125] |
| CNPs | Nanocarriers with antioxidative agents | Therapeutic | Decreased caspase-3 activity, reduced level of reactive oxygen species | AKI (ischemia-reperfusion injury) | [126] |
| SPIO-labelled macrophages | SPIO | Diagnostic | Increased signal in MRI—new possibility of monitoring of macrophages accumulation in kidney and potential kidney allograft rejection | Renal ischemia (reperfusion injury after KTx) | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paluszkiewicz, P.; Martuszewski, A.; Zaręba, N.; Wala, K.; Banasik, M.; Kepinska, M. The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases. Int. J. Mol. Sci. 2022, 23, 131. https://doi.org/10.3390/ijms23010131
Paluszkiewicz P, Martuszewski A, Zaręba N, Wala K, Banasik M, Kepinska M. The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases. International Journal of Molecular Sciences. 2022; 23(1):131. https://doi.org/10.3390/ijms23010131
Chicago/Turabian StylePaluszkiewicz, Patrycja, Adrian Martuszewski, Natalia Zaręba, Kamila Wala, Mirosław Banasik, and Marta Kepinska. 2022. "The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases" International Journal of Molecular Sciences 23, no. 1: 131. https://doi.org/10.3390/ijms23010131
APA StylePaluszkiewicz, P., Martuszewski, A., Zaręba, N., Wala, K., Banasik, M., & Kepinska, M. (2022). The Application of Nanoparticles in Diagnosis and Treatment of Kidney Diseases. International Journal of Molecular Sciences, 23(1), 131. https://doi.org/10.3390/ijms23010131








