Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection
Abstract
1. Introduction
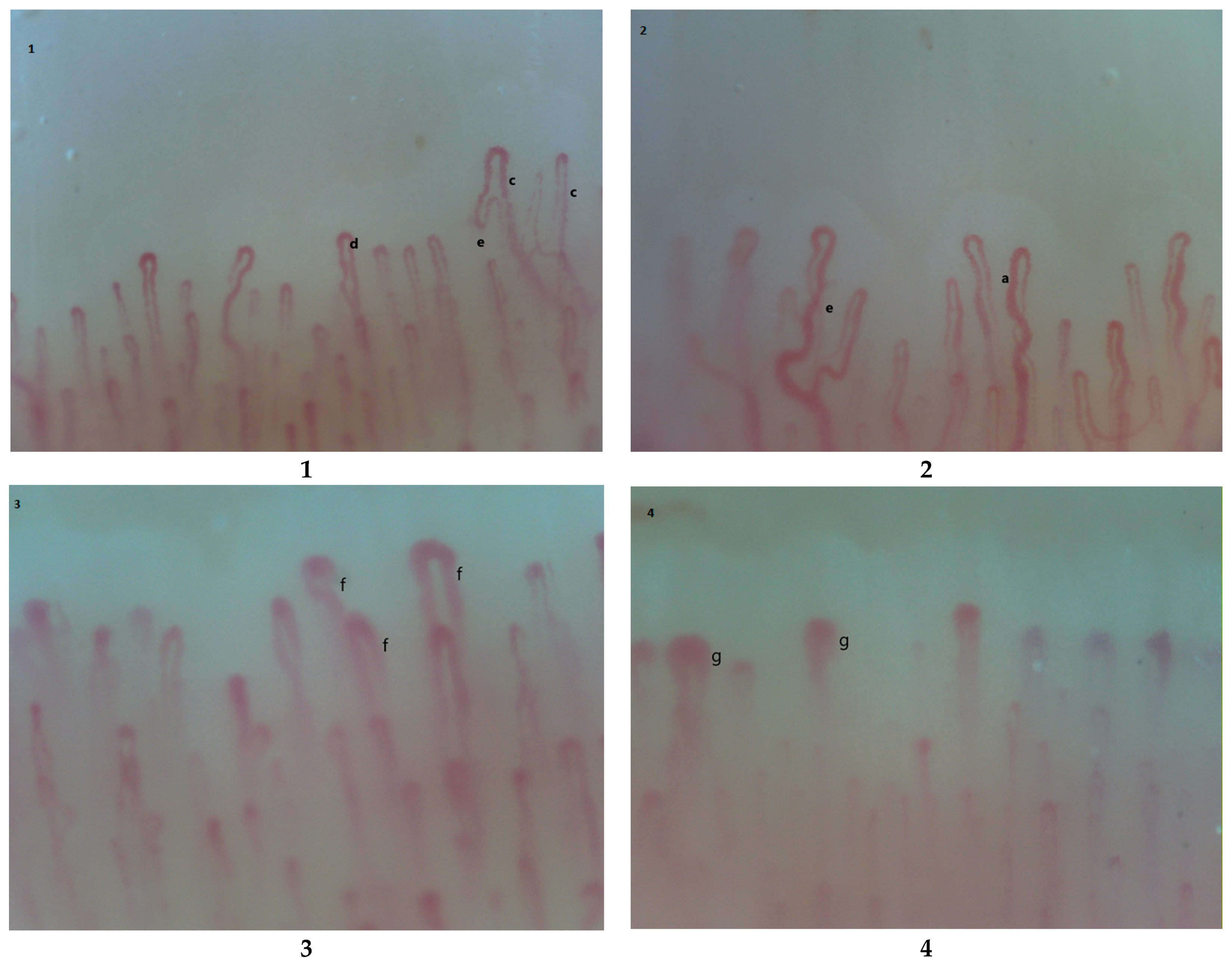
2. Pathogenesis of RP
3. RP, Metabolism, and Oxidative Status
4. AN, Inflammation, and Immunity
5. RP and Nutritional Status
6. AN and Autonomic Dysfunction
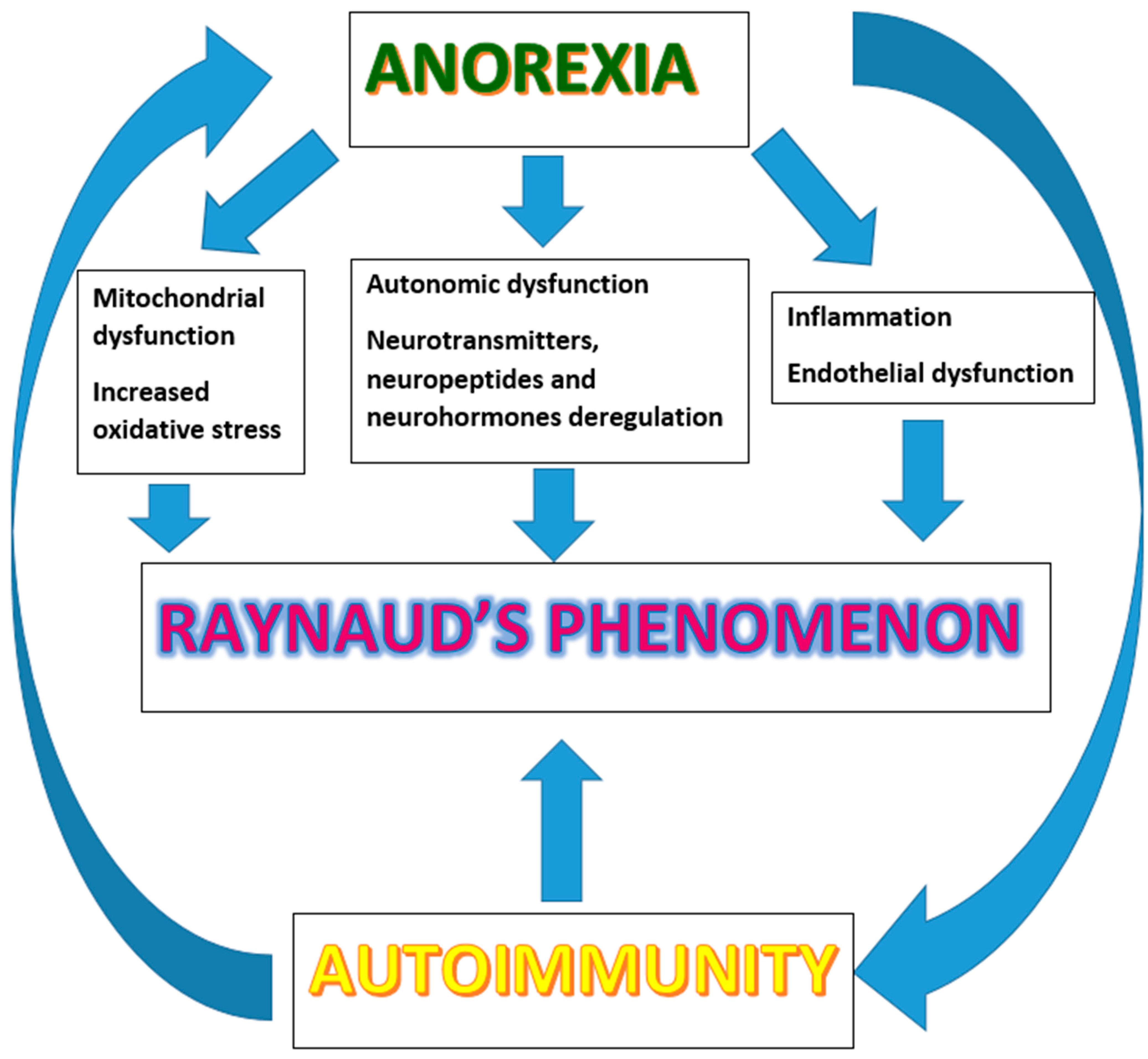
7. The Metabolic-Neuro-Immune Cross-Talk
8. Autoimmunity and Eating Disorders
9. Anorexia Nervosa and the Microbiota
10. Conclusions
Funding
Conflicts of Interest
References
- Mitchell, J.E.; Peterson, C.B. Anorexia Nervosa. N. Engl. J. Med. 2020, 382, 1343–1351. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Eating Disorders Working Group of the Psychiatric Genomics Consortium; Thornton, L.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Hübel, C.; Marzi, S.J.; Breen, G.; Bulik, C.M. Epigenetics in eating disorders: A systematic review. Mol. Psychiatry 2019, 24, 901–915. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Hardaway, J.A.; Bulik, C.M. Genetics and Epigenetics of Eating Disorders. Adv. Genom. Genet. 2015, 5, 131–150. [Google Scholar] [CrossRef]
- Booij, L.; Steiger, H. Applying epigenetic science to the understanding of eating disorders: A promising paradigm for research and practice. Curr. Opin. Psychiatry 2020, 33, 515–520. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Monteleone, P.; Serino, I.; Scognamiglio, P.; Di Genio, M.; Maj, M. Childhood trauma and cortisol awakening response in symptomatic patients with anorexia nervosa and bulimia nervosa. Int. J. Eat. Disord. 2015, 48, 615–621. [Google Scholar] [CrossRef]
- Luck, P.; Wakeling, A. Increased cutaneous vasoreactivity to cold in anorexia nervosa. Clin. Sci. 1981, 61, 559–567. [Google Scholar] [CrossRef]
- Karbalaie, A.; Emrani, Z.; Fatemi, A.; Etehadtavakol, M.; Erlandsson, B.E. Practical issues in assessing nailfold capillaroscopic images: A summary. Clin. Rheumatol. 2019, 38, 2343–2354. [Google Scholar] [CrossRef]
- Etehad Tavakol, M.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B.E. Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? Biomed. Res. Int. 2015, 2015, 974530. [Google Scholar] [CrossRef]
- Pain, C.E.; Constantin, T.; Toplak, N.; Moll, M.; Iking-Konert, C.; Piotto, D.P.; Aktay Ayaz, N.; Nemcova, D.; Hoeger, P.H.; Cutolo, M.; et al. Raynaud’s syndrome in children: Systematic review and development of recommendations for assessment and monitoring. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 100), 200–206. [Google Scholar]
- Ingegnoli, F.; Ughi, N.; Crotti, C.; Mosca, M.; Tani, C. Outcomes, rates and predictors of transition of isolated Raynaud’s phenomenon: A systematic review and meta-analysis. Swiss Med. Wkly. 2017, 147, w14506. [Google Scholar] [CrossRef][Green Version]
- García-González, M.; Rodríguez-Lozano, B.; Bustabad, S.; Ferraz-Amaro, I. Undifferentiated connective tissue disease: Predictors of evolution into definite disease. Clin. Exp. Rheumatol. 2017, 35, 739–745. [Google Scholar]
- Herrick, A.L.; Wigley, F.M. Raynaud’s phenomenon. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101474. [Google Scholar] [CrossRef]
- Gorski, S.; Bartnicka, M.; Citko, A.; Żelazowska-Rutkowska, B.; Jablonski, K.; Gorska, A. Microangiopathy in Naifold Videocapillaroscopy and Its Relations to sE- Selectin, Endothelin-1, and hsCRP as Putative Endothelium Dysfunction Markers among Adolescents with Raynaud’s Phenomenon. J. Clin. Med. 2019, 8, 567. [Google Scholar] [CrossRef]
- Bañuls, C.; de Marañon, A.M.; Veses, S.; Castro-Vega, I.; López-Domènech, S.; Salom-Vendrell, C.; Orden, S.; Álvarez, Á.; Rocha, M.; Víctor, V.M.; et al. Malnutrition impairs mitochondrial function and leukocyte activation. Nutr. J. 2019, 18, 89. [Google Scholar] [CrossRef]
- Víctor, V.M.; Rovira-Llopis, S.; Saiz-Alarcón, V.; Sangüesa, M.C.; Rojo-Bofill, L.; Bañuls, C.; de Pablo, C.; Álvarez, Á.; Rojo, L.; Rocha, M.; et al. Involvement of leucocyte/endothelial cell interactions in anorexia nervosa. Eur. J. Clin. Investig. 2015, 45, 670–678. [Google Scholar] [CrossRef]
- Abdulle, A.E.; Arends, S.; van Goor, H.; Brouwer, E.; van Roon, A.M.; Westra, J.; Herrick, A.L.; de Leeuw, K.; Mulder, D.J. Low body weight and involuntary weight loss are associated with Raynaud’s phenomenon in both men and women. Scand. J. Rheumatol. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, A.G.; Bottaccioli, F.; Minelli, A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: A concise review. Ann. N. Y. Acad. Sci. 2019, 1437, 31–42. [Google Scholar] [CrossRef]
- Houben, A.J.; Eringa, E.C.; Jonk, A.M.; Serne, E.H.; Smulders, Y.M.; Stehouwer, C.D. Perivascular fat and the microcirculation: Relevance to insulin resistance, diabetes, and cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2012, 6, 80–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Raynaud’s phenomenon and nailfold capillaroscopic findings in anorexia nervosa. Curr. Med. Res. Opin. 2018, 34, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Taher, R.; Sara, J.D.; Toya, T.; Shepherd, R.; Moder, K.; Lerman, L.O.; Lerman, A. Secondary Raynaud’s phenomenon is associated with microvascular peripheral endothelial dysfunction. Microvasc. Res. 2020, 132, 104040. [Google Scholar] [CrossRef] [PubMed]
- Rollando, D.; Bezante, G.P.; Sulli, A.; Balbi, M.; Panico, N.; Pizzorni, C.; Negrini, S.; Brunelli, C.; Barsotti, A.; Cutolo, M.; et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J. Rheumatol. 2010, 37, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Palova, S.; Charvat, J.; Chlumsky, J. Flow-mediated vasodilatation in the patients with anorexia nervosa. Bratisl. Lek. Listy. 2013, 114, 634–636. [Google Scholar] [CrossRef]
- Le, J.H.; Cho, K.I. Association between endothelial function and microvascular changes in patients with secondary Raynaud’s phenomenon. Clin. Rheumatol. 2014, 33, 1627–1633. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Predicting cardiovascular involvement in systemic sclerosis for a timely and better treatment approach. Eur. J. Prev. Cardiol. 2020, 15. [Google Scholar] [CrossRef]
- Sekaninova, N.; Bona Olexova, L.; Visnovcova, Z.; Ondrejka, I.; Tonhajzerova, I. Role of Neuroendocrine, Immune, and Autonomic Nervous System in Anorexia Nervosa-Linked Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7302. [Google Scholar] [CrossRef] [PubMed]
- Peyser, D.; Scolnick, B.; Hildebrandt, T.; Taylor, J.A. Heart rate variability as a biomarker for anorexia nervosa: A review. Eur. Eat. Disord. Rev. 2021, 29, 20–31. [Google Scholar] [CrossRef]
- Nakai, Y.; Fujita, M.; Nin, K.; Noma, S.; Teramukai, S. Relationship between duration of illness and cardiac autonomic nervous activity in anorexia nervosa. Biopsychosoc. Med. 2015, 9, 12. [Google Scholar] [CrossRef]
- Bruno, R.M.; Ghiadoni, L.; Seravalle, G.; Dell’oro, R.; Taddei, S.; Grassi, G. Sympathetic regulation of vascular function in health and disease. Front. Physiol. 2012, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Lorton, D. Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int. J. Mol. Sci. 2018, 19, 1188. [Google Scholar] [CrossRef] [PubMed]
- Tonhajzerova, I.; Mestanikova, A.; Jurko, A., Jr.; Grendar, M.; Langer, P.; Ondrejka, I.; Jurko, T.; Hrtanek, I.; Cesnekova, D.; Mestanik, M. Arterial stiffness and haemodynamic regulation in adolescent anorexia nervosa versus obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 81–90. [Google Scholar] [CrossRef]
- Hasan, T.F.; Hasan, H. Anorexia nervosa: A unified neurological perspective. Int. J. Med. Sci. 2011, 8, 679–703. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Krama, T.; Krams, I. Eating Disorders: An Evolutionary Psychoneuroimmunological Approach. Front. Psychol. 2019, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fang, F.; Tomasson, G.; Arnberg, F.K.; Mataix-Cols, D.; Fernández de la Cruz, L.; Almqvist, C.; Fall, K.; Valdimarsdóttir, U.A. Association of Stress-Related Disorders with Subsequent Autoimmune Disease. JAMA 2018, 319, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Lukens, J.R.; Gurung, P.; Vogel, P.; Johnson, G.R.; Carter, R.A.; McGoldrick, D.J.; Bandi, S.R.; Calabrese, C.R.; Vande Walle, L.; Lamkanfi, M.; et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014, 516, 246–249. [Google Scholar] [CrossRef]
- Hedman, A.; Breithaupt, L.; Hübel, C.; Thornton, L.M.; Tillander, A.; Norring, C.; Birgegård, A.; Larsson, H.; Ludvigsson, J.F.; Sävendahl, L.; et al. Bidirectional relationship between eating disorders and autoimmune diseases. J. Child Psychol. Psychiatry 2019, 60, 803–812. [Google Scholar] [CrossRef]
- Zerwas, S.; Larsen, J.T.; Petersen, L.; Thornton, L.M.; Quaranta, M.; Koch, S.V.; Pisetsky, D.; Mortensen, P.B.; Bulik, C.M. Eating Disorders, Autoimmune, and Autoinflammatory Disease. Pediatrics 2017, 140, e20162089. [Google Scholar] [CrossRef] [PubMed]
- Hommer, R.E.; Swedo, S.E. Anorexia and Autoimmunity: Challenging the Etiologic Constructs of Disordered Eating. Pediatrics 2017, 140, e20173060. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Gautron, L.; Layé, S. Neurobiology of inflammation-associated anorexia. Front. Neurosci. 2010, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Uezono, Y.; Ueta, Y. Anorexia in human and experimental animal models: Physiological aspects related to neuropeptides. J. Physiol. Sci. 2015, 65, 385–395. [Google Scholar] [CrossRef]
- Caroleo, M.; Carbone, E.A.; Greco, M.; Corigliano, D.M.; Arcidiacono, B.; Fazia, G.; Rania, M.; Aloi, M.; Gallelli, L.; Segura-Garcia, C.; et al. Brain-Behavior-Immune Interaction: Serum Cytokines and Growth Factors in Patients with Eating Disorders atExtremes of the Body Mass Index (BMI) Spectrum. Nutrients 2019, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Plata-Salamán, C.R. Cytokine-induced anorexia. Behavioral, cellular, and molecular mechanisms. Ann. N. Y. Acad. Sci. 1998, 856, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, M.; Müller-Fielitz, H.; Sundaram, S.M.; Gallet, S.; Neve, V.; Shionoya, K.; Zager, A.; Quan, N.; Liu, X.; Schmidt-Ullrich, R.; et al. NF-κB signaling in tanycytes mediates inflammation-induced anorexia. Mol. Metab. 2020, 39, 101022. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef]
- Tonhajzerova, I.; Sekaninova, N.; Bona Olexova, L.; Visnovcova, Z. Novel Insight into Neuroimmune Regulatory Mechanisms and Biomarkers Linking Major Depression and Vascular Diseases: The Dilemma Continues. Int. J. Mol. Sci. 2020, 21, 2317. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Favaro, A.; Santonastaso, P.; Manzato, E.; Sergi, G.; Correll, C.U. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology 2015, 51, 237–252. [Google Scholar] [CrossRef]
- Raevuori, A.; Haukka, J.; Vaarala, O.; Suvisaari, J.M.; Gissler, M.; Grainger, M.; Linna, M.S.; Suokas, J.T. The increased risk for autoimmune diseases in patients with eating disorders. PLoS ONE 2014, 9, e104845. [Google Scholar] [CrossRef] [PubMed]
- Ascherman, D.P.; Zang, Y.; Fernandez, I.; Clark, E.S.; Khan, W.N.; Martinez, L.; Greidinger, E.L. An Autoimmune Basis for Raynaud’s Phenomenon: Murine Model and Human Disease. Arthritis Rheumatol. 2018, 70, 1489–1499. [Google Scholar] [CrossRef]
- Shah, A.A.; Montagne, J.; Oh, S.Y.; Wigley, F.M.; Casciola-Rosen, L. Pilot study to determine whether transient receptor potential melastatin type 8 (TRPM8) antibodies are detected in scleroderma. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 91), S123–S126. [Google Scholar]
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J. Cell Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef]
- Bruni, C.; Frech, T.; Manetti, M.; Rossi, F.W.; Furst, D.E.; De Paulis, A.; Rivellese, F.; Guiducci, S.; Matucci-Cerinic, M.; Bellando-Randone, S. Vascular Leaking, a Pivotal and Early Pathogenetic Event in Systemic Sclerosis: Should the Door Be Closed? Front. Immunol. 2018, 9, 2045. [Google Scholar] [CrossRef] [PubMed]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Sato, S. Vasculopathy in scleroderma. Semin. Immunopathol. 2015, 37, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Freff, J.; Schwarte, K.; Bröker, L.; Bühlmeier, J.; Kraft, I.; Öztürk, D.; Hinney, A.; Arolt, V.; Dannlowski, U.; Romer, G.; et al. Alterations in B cell subsets correlate with body composition parameters in female adolescents with anorexia nervosa. Sci. Rep. 2021, 11, 1125. [Google Scholar] [CrossRef]
- Nilsson, I.A.; Millischer, V.; Göteson, A.; Hübel, C.; Thornton, L.M.; Bulik, C.M.; Schalling, M.; Landén, M. Aberrant inflammatory profile in acute but not recovered anorexia nervosa. Brain Behav. Immun. 2020, 88, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.A.; D’Amato, P.; Vicchio, G.; De Fazio, P.; Segura-Garcia, C. A systematic review on the role of microbiota in the pathogenesis and treatment of eating disorders. Eur. Psychiatry 2020, 64, e2. [Google Scholar] [CrossRef]
- Sirufo, M.; Ginaldi, L.; De Martinis, M. Non–coding RNAs, osteoarthritis and the microbiome: New therapeutic targets? Arthritis Rheumatol. 2021, in press. [Google Scholar]
- Seitz, J.; Dahmen, B.; Keller, L.; Herpertz-Dahlmann, B. Gut Feelings: How Microbiota Might Impact the Development and Course of Anorexia Nervosa. Nutrients 2020, 12, 3295. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Allegra, A.; Sirufo, M.M.; Pioggia, G.; Tonacci, A.; Gangemi, S. The Osteoporosis/Microbiota Linkage: The Role of miRNA. Int. J. Mol. Sci. 2020, 21, 8887. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. MicroRNAs, bone and microbiota. Bone 2021, 144, 115824. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Microbiota-miRNA interactions: Opportunities in ankylosing spondylitis. Autoimmun. Rev. 2021, in press. [Google Scholar]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Fava, F.; Tuohy, K.M. Gut microbiota: Inulin regulates endothelial function: A prebiotic smoking gun? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Nailfold capillaroscopic findings in a semi-professional volleyball player. Clin. Hemorheol. Microcirc. 2020, 74, 281–285. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM 2019, 112, 531–533. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Nailfold Capillaroscopic Findings in an Orthopedic Surgeon: Reversible Abnormalities after the Cessation of Radiation Exposure. Radiat. Res. 2020, 193, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Microvascular Damage in a Young Female Archer Assessed by Nailfold Videocapillaroscopy: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 4218. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Nailfold capillaroscopy: Clinical practice in non-rheumatic conditions. Microvasc. Res. 2021, 134, 104122. [Google Scholar] [CrossRef]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection. Int. J. Mol. Sci. 2021, 22, 5043. https://doi.org/10.3390/ijms22095043
Sirufo MM, Ginaldi L, De Martinis M. Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection. International Journal of Molecular Sciences. 2021; 22(9):5043. https://doi.org/10.3390/ijms22095043
Chicago/Turabian StyleSirufo, Maria Maddalena, Lia Ginaldi, and Massimo De Martinis. 2021. "Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection" International Journal of Molecular Sciences 22, no. 9: 5043. https://doi.org/10.3390/ijms22095043
APA StyleSirufo, M. M., Ginaldi, L., & De Martinis, M. (2021). Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection. International Journal of Molecular Sciences, 22(9), 5043. https://doi.org/10.3390/ijms22095043







