Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses
Abstract
1. Introduction
2. Results
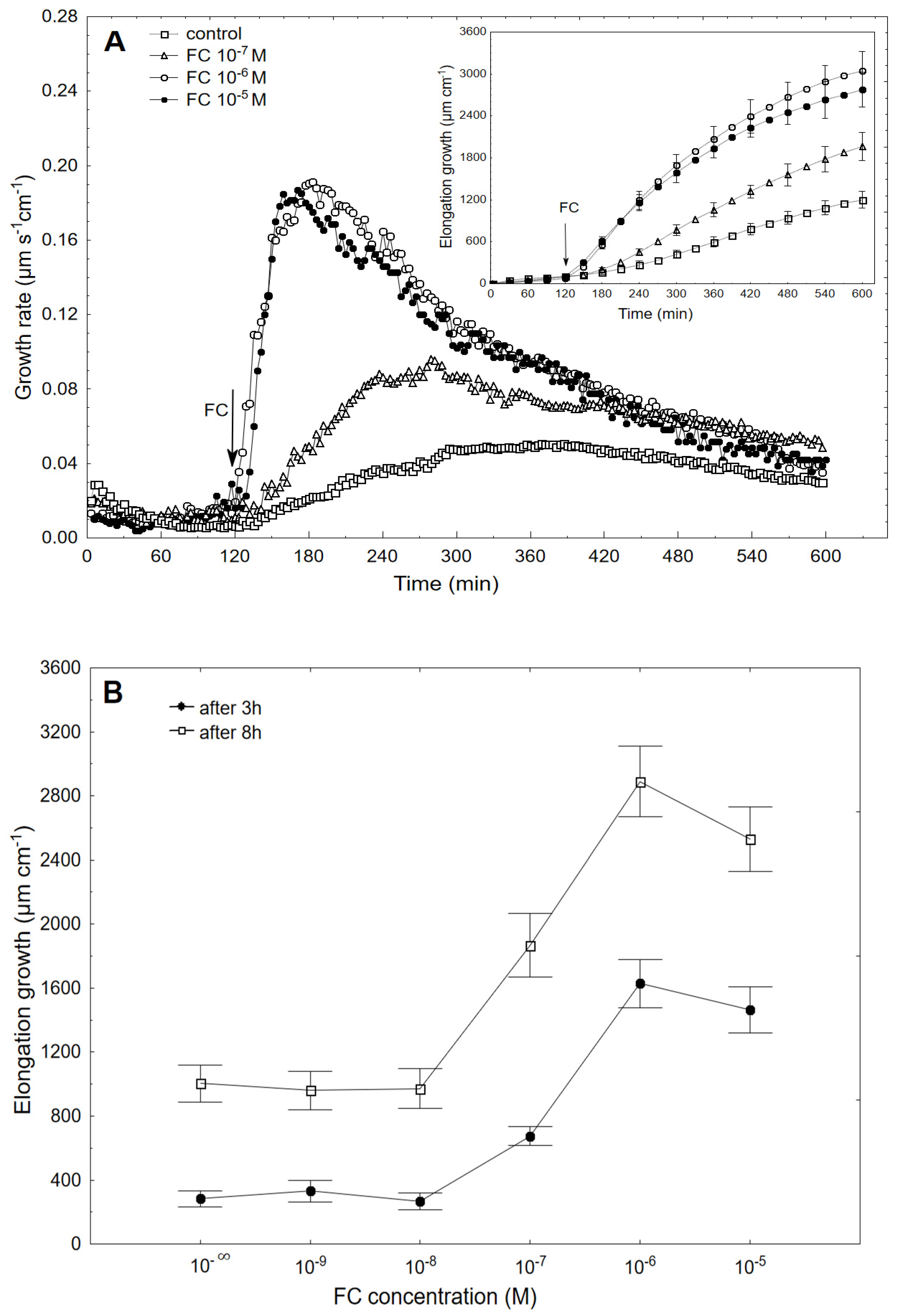
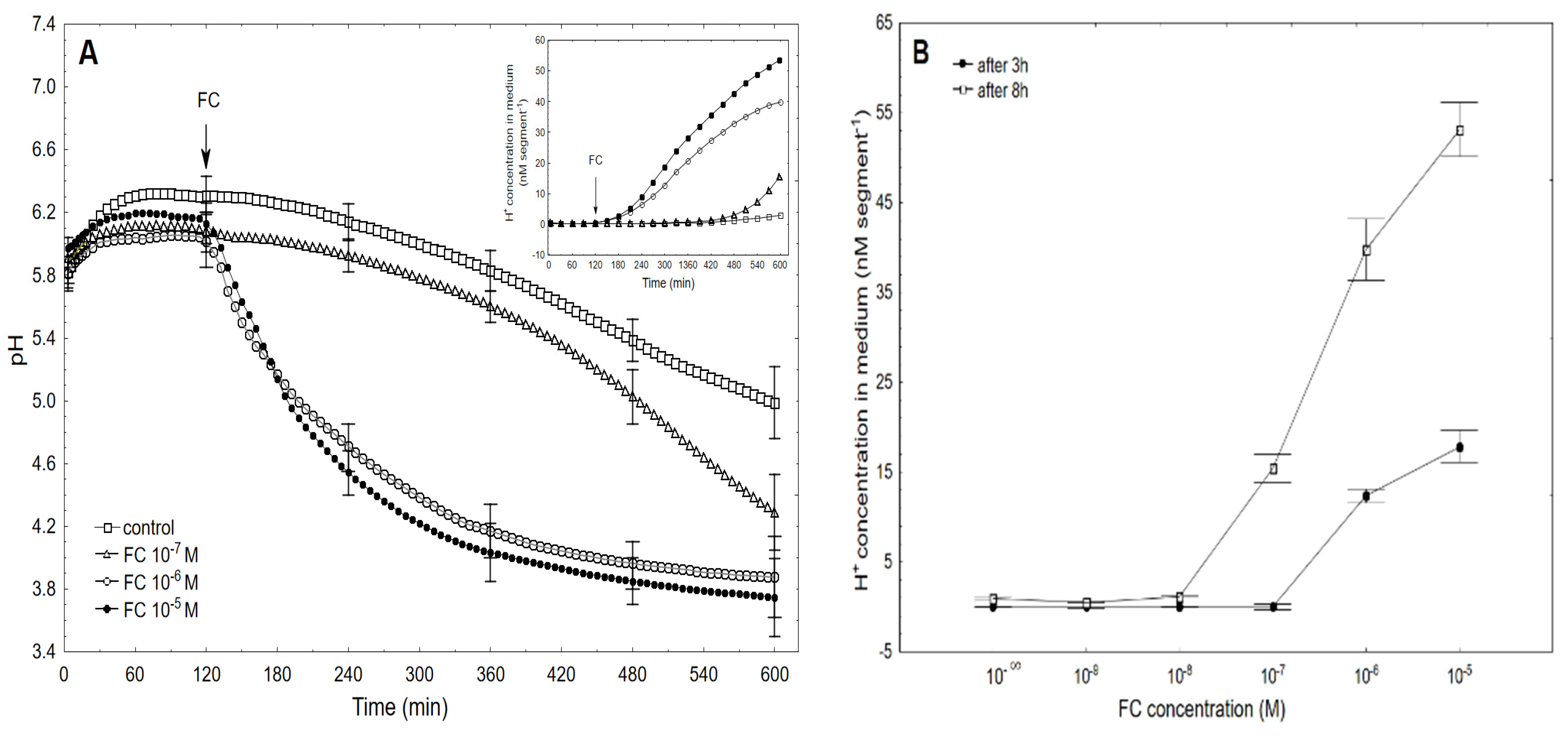
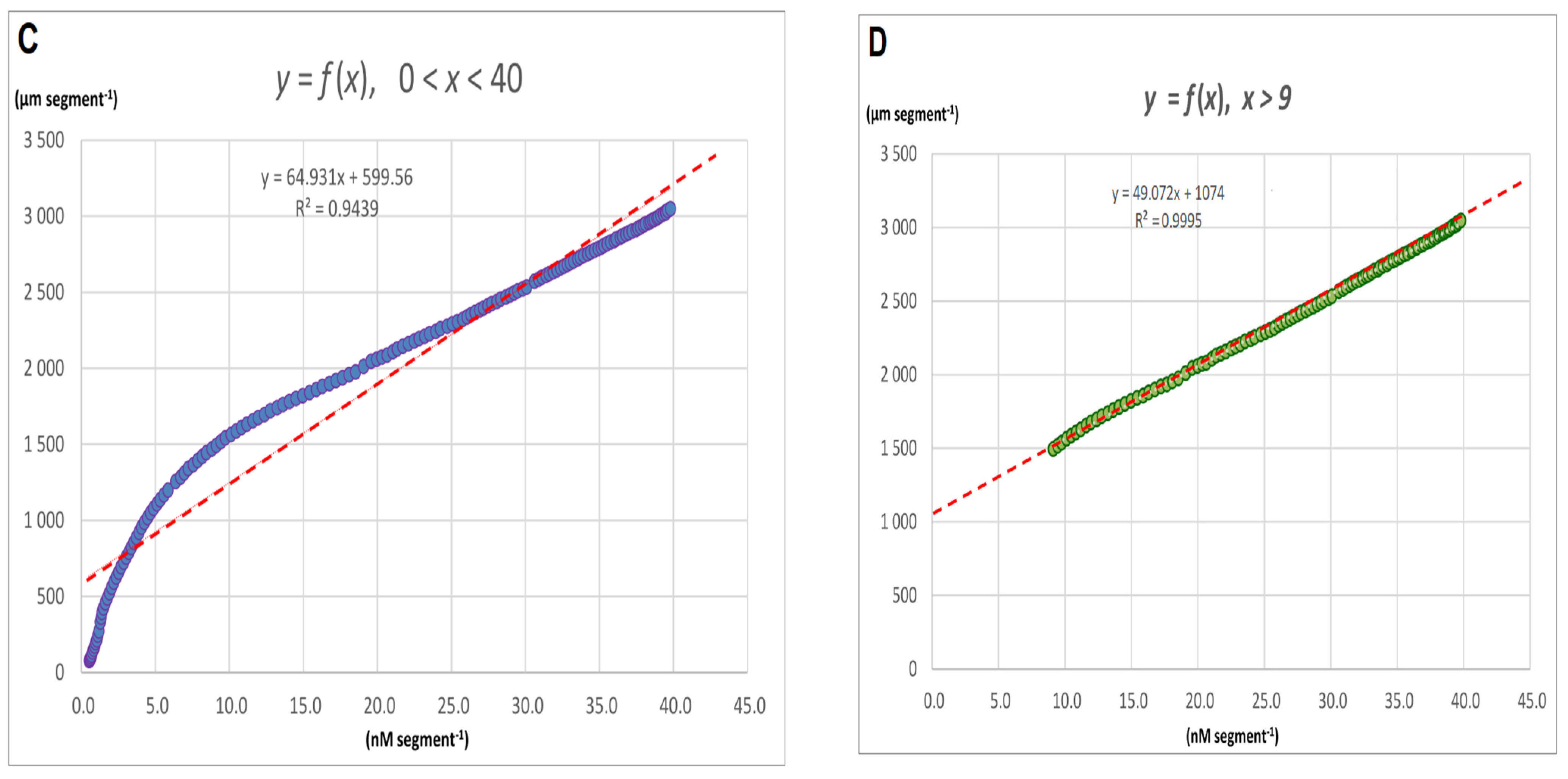
2.1. FC-Induced Elongation Growth of the Maize Coleoptile Segments and Medium pH Changes Measured Simultaneously with the Growth
2.2. The Kinetics of FC- and IAA-Induced Elongation Growth, Medium pH and Membrane Potential Measured Simultaneously at Their Optimal and Suboptimal Concentrations
2.3. High Temperatures Change the Biphasic Kinetics of IAA-Induced Growth to a Monophasic One
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Growth, Medium pH and Membrane Potential Measurements
4.4. Electrophysiological Experiments
4.5. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleland, R.E. Cell wall extension. Annu. Rev. Plant Physiol. 1971, 22, 197–222. [Google Scholar] [CrossRef]
- Hager, A.; Menzel, H.; Krauss, A. Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta 1971, 100, 1–15. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The acid-growth theory of auxin induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U. The current status of the acid-growth hypothesis. New Phytol. 1994, 126, 549–569. [Google Scholar] [CrossRef]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Dünser, K.; Kleine-Vehn, J. Differential growth regulation in plants- the acid growth balloon theory. Cur. Opin. Plant Biol. 2015, 28, 55–59. [Google Scholar] [CrossRef]
- Arsuffi, G.; Braybrook, S. Acid growth: An ongoing trip. J. Exp. Bot. 2019, 69, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Karcz, W. Some new methodological and conceptual aspects of the “acid growth theory” for the auxin action in maize (Zea mays L.) coleoptile segments: Do acid- and auxin-induced rapid growth differ in their mechanisms? Int. J. Mol. Sci. 2021, 22, 2317. [Google Scholar] [CrossRef]
- Becker, D.; Hedrich, R. Channeling auxin action: Modulation of ion transport by indole-3-acetic acid. Plant Mol. Biol. 2002, 49, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Philippar, K.; Fuchs, I.; Lüthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Thiel, G.; Ljung, K.; Sandberg, G.; Böttger, M.; et al. Auxin-induced K+ channel expression an essential step in coleoptile growth and gravitropism. Proc. Nat. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef]
- Thiel, G.; Weise, R. Auxin augments conductance of K+ inward rectifier in maize protoplasts. Planta 1999, 208, 38–45. [Google Scholar] [CrossRef]
- Hager, A.; Debus, G.; Edel, H.G.; Stransky, H.; Serrano, R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma membrane H+-ATPase. Planta 1991, 185, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Claussen, M.; Lüthen, H.; Blatt, M.; Böttger, M. Auxin-induced growth and its linkage to potassium channels. Planta 1997, 201, 227–234. [Google Scholar] [CrossRef]
- Thiel, G.; Brüdern, A.; Gradmann, D. Small inward rectifying K+ channels in coleoptiles: Inhibition by external Ca2+ and function in cell elongation. J. Membr. Biol. 1996, 149, 9–20. [Google Scholar] [CrossRef]
- Siemieniuk, A.; Karcz, W. Effect of K+ and Ca2+ on the indole-3-acetic acid- and fusicoccin-induced growth and membrane potential in maize coleoptile cells. AoB PLANTS 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of transport processes in plants: Roles of the plasma membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Boutry, M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflügers Arch. 2009, 457, 645–655. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.S.; Song, C.P.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14−3−3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef]
- Ballio, A.; Chain, E.B.; De Leo, P.; Erlanger, B.F.; Mauri, M.; Rouola, A. Fusicoccin: A new wilting toxin produced by Fusicoccum amygdali Del. Nature. 1964, 203, 297. [Google Scholar] [CrossRef]
- Marrè, E. Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Physiol. 1979, 30, 273–288. [Google Scholar] [CrossRef]
- Jahn, T.; Fuglsang, A.T.; Olsson, A.; Brüntrup, I.M.; Collinge, D.B.; Volkmann, D.; Sommarin, M.; Palmgren, M.G.; Larsson, C. The 14−3−3 protein interacts directly with the C-terminal region of the plasma membrane H+-ATPase. Plant Cell 1997, 9, 1805–1814. [Google Scholar]
- Baunsgaard, L.; Fuglsang, A.T.; Jahn, T.; Korthout, H.A.; de Boer, A.H.; Palmgren, M.G. The 14−3−3 proteins associate with the plant plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant, J. 1998, 13, 661–671. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Visconti, S.; Drumm, K.; Jahn, T.; Stensballe, A.; Mattei, B.; Jensen, O.N.; Adducci, P.; Palmgren, M.G. Binding of 14−3−3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946 – Tyr – Val and requires phosphorylation of Thr947. J. Biol. Chem. 1999, 274, 36774–36780. [Google Scholar] [CrossRef]
- Würtele, M.; Jelich-Ottmann, C.; Wittinghofer, A.; Oecking, G. Structural view of a fungal toxin acting on a 14−3−3 regulatory complex. EMBO J. 2003, 22, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, A.; Porro, A.; Chaves-Sanjuan, A.; Nardini, M.; Rauh, O.; Thiel, G.; Moroni, A. Fusicoccin activates KAT1 channels by stabilizing their interaction with 14−3−3 proteins. Plant Cell 2017, 29, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Burdach, Z.; Siemieniuk, A.; Trela, Z.; Kurtyka, R.; Karcz, W. Role of auxin (IAA) in the regulation of slow vacuolar (SV) channels and the volume of red beet taproot vacuoles. BMC Plant Biol. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burdach, Z.; Siemieniuk, A.; Karcz, W. Effect of auxin (IAA) on the fast vacuolar (FV) channels in red beet (Beta vulgaris L.) taproot vacuoles. Inter. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Nissl, D.; Zenk, M.H. Evidence against induction of protein synthesis during auxin-induced initial elongation of Avena coleoptiles. Planta 1969, 89, 323–341. [Google Scholar] [CrossRef]
- Yamagata, Y.; Masuda, Y. Comparative studies on auxin and fusicoccin actions on plant growth. Plant Cell Physiol. 1975, 16, 41–52. [Google Scholar] [CrossRef]
- Cleland, R.E. Fusicoccin-induced growth and hydrogen ion excretion of Avena coleoptiles: Relation to auxin responses. Planta 1976, 128, 201–206. [Google Scholar] [CrossRef]
- Rubinstein, B.; Cleland, R.E. Responses of Avena coleoptiles to suboptimal Fusicoccin: Kinetics and comparison with indoleacetic acid. Plant Physiol. 1981, 68, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Rücke, A.; Palme, K.; Venis, M.A.; Napier, R.M.; Felle, H.H. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant, J. 1993, 4, 41–46. [Google Scholar] [CrossRef]
- Kurtyka, R.; Kita, A.; Karcz, W. Fusicocin conteracts the toxic effect of cadmium on the growth of maize coleoptile segments. Arch. Environ. Contam. Toxicol. 2011, 61, 568–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedrich, R.; Bregante, M.; Dreyer, I.; Gambale, F. The voltage-dependent potassium-uptake channel of corn coleoptiles has permeation properties different from other K+ channels. Planta 1995, 197, 193–199. [Google Scholar] [CrossRef]
- Paponov, I.A.; Dindas, J.; Król, E.; Friz, T.; Budryk, V.; Teale, W.; Paponov, M.; Hedrich, R.; Palme, K. Auxin-induced plasma membrane depolarization is regulated by auxin transport and not by AUXIN BINDING PROTEIN1. Front. Plant. Sci. 2019. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the centre of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Karcz, W.; Burdach, Z. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regul. 2007, 52, 141–150. [Google Scholar] [CrossRef]
- Ilan, N.; Moran, N.; Schwartz, A. The role of potassium channels in temperature control of stomata aperture. Plant Physiol. 1995, 108, 1161–1170. [Google Scholar] [CrossRef]
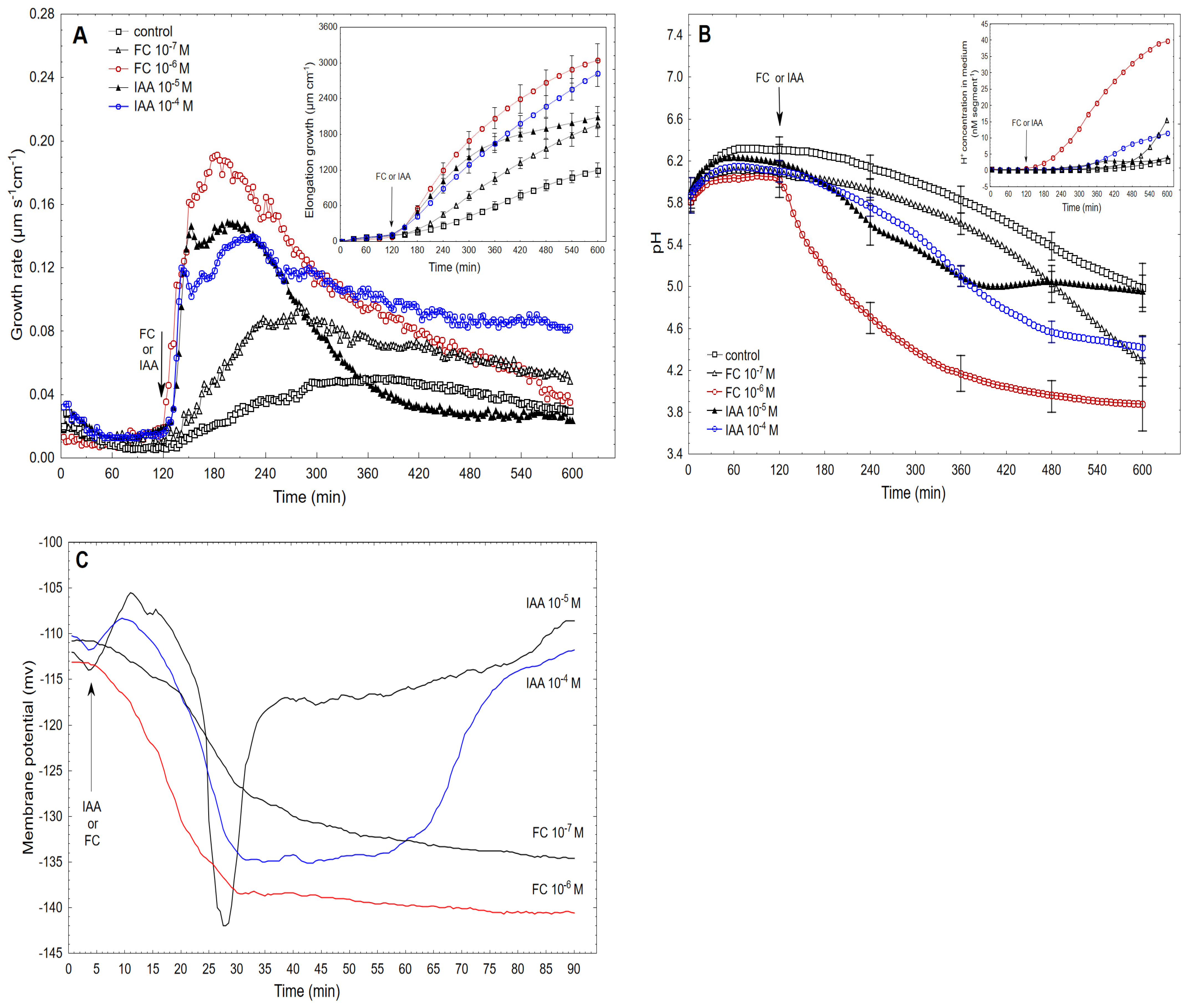

| Rapid growth, medium pH and membrane potential in the maize coleoptile cells that had been treated with the optimal concentrations of FC and IAA for growth | |||||
|---|---|---|---|---|---|
| Optimal Concentration of FC or IAA | Parameters under Consideration | Initial Value of Response | Lag Time of Response (min) | Maximal Value of Response | Rate of Response Approximated by its Steady State (from … to …min) |
| FC at 10−6 M | Growth rate µm s−1 cm−1 | <0.04 | 3 | 0.19–0.20 | 0.166 ± 0.014 from 150 to 270 0.101 ± 0.007 from 300 to 420 |
| Medium pH [H+] cm−1 nM cm−1 | 0.3 ± 0.071 (n = 7, ±SE) | 3 | 40 ± 3.7 (n = 7, ± SE) over 600 min | 0.88 pM s−1 cm−1 from 150 to 270 2.51 pM s−1 cm−1 from 300 to 420 | |
| Membrane potential mv | −114.1 ± 4.6 (n = 5, ± SE) | 1–2 | −139 ± 5.6 (n = 5, ± SE) | 1.13 mV min−1 from 15 to 30 | |
| IAA at 10−4 M | Growth rate µm s−1 cm−1 | <0.04 | 12–15 | 0.12 | 0.111 ± 0.012 from 150 to 270 0.097 ± 0.007 from 300 to 420 |
| Medium pH [H+] cm−1 nM cm−1 | 0.28 ± 0.084 (n = 9, ± SE) | 20–30 | 11 ± 0.9 (n = 9, ± SE) over 600 min | 0.096 pM s−1 cm−1 from 150 to 270 0.49 pM s−1 cm−1 from 300 to 420 | |
| Membrane potential mv | −113.3 ± 4.1 (n = 8, ± SE) | 3–4 | −135±5.1 (n = 5, ± SE) | 1.5 mV min−1 from 15 to 30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, M.; Karcz, W. Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses. Int. J. Mol. Sci. 2021, 22, 5017. https://doi.org/10.3390/ijms22095017
Polak M, Karcz W. Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses. International Journal of Molecular Sciences. 2021; 22(9):5017. https://doi.org/10.3390/ijms22095017
Chicago/Turabian StylePolak, Małgorzata, and Waldemar Karcz. 2021. "Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses" International Journal of Molecular Sciences 22, no. 9: 5017. https://doi.org/10.3390/ijms22095017
APA StylePolak, M., & Karcz, W. (2021). Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses. International Journal of Molecular Sciences, 22(9), 5017. https://doi.org/10.3390/ijms22095017






