Gadolinium Protects Arabidopsis thaliana against Botrytis cinerea through the Activation of JA/ET-Induced Defense Responses
Abstract
1. Introduction
2. Results
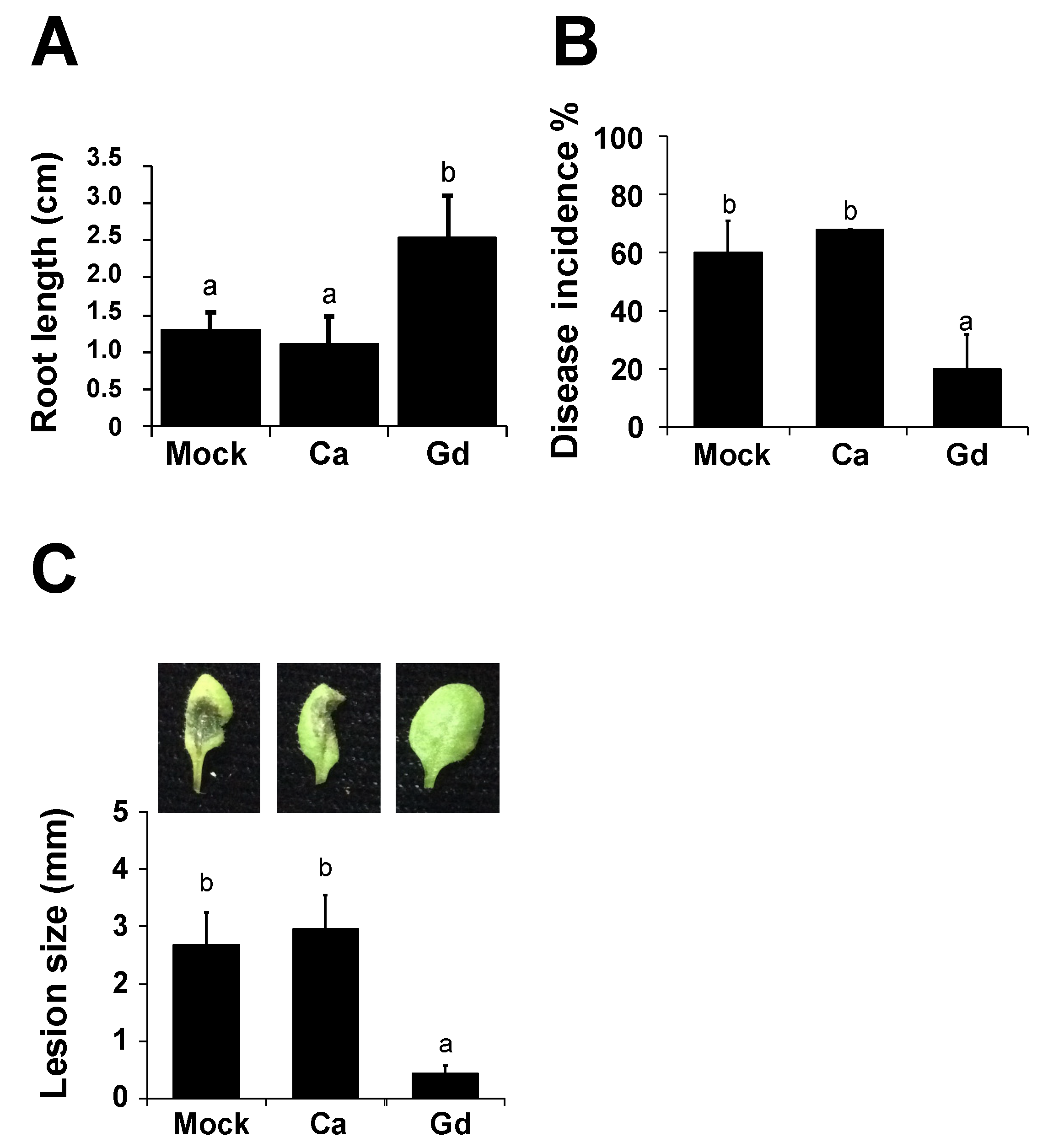
2.1. Gadolinium Improves Root Development of A. thaliana
2.2. Gadolinium Protects Arabidopsis thaliana Plants against the Necrotrophic Pathogen B. cinerea
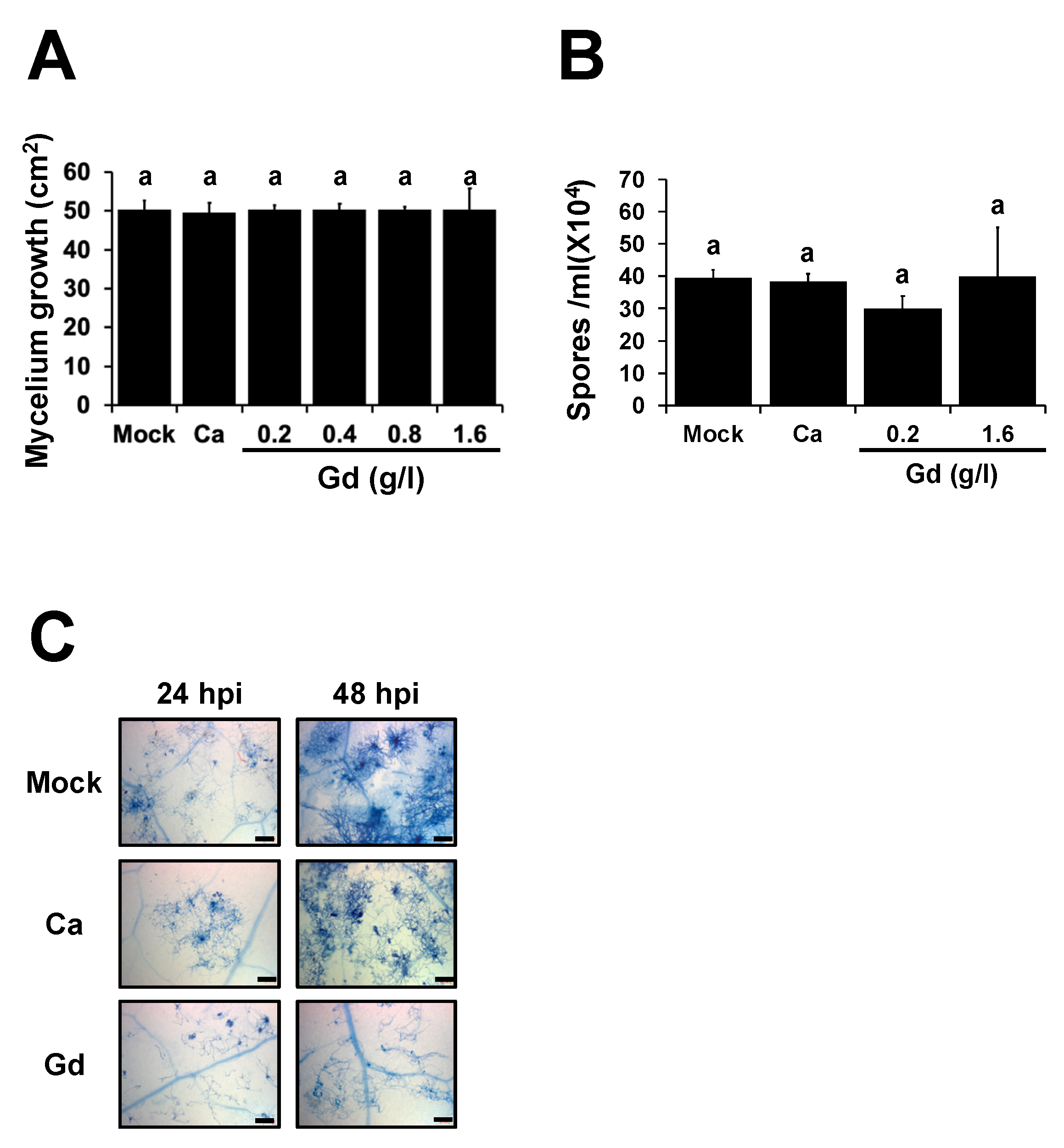
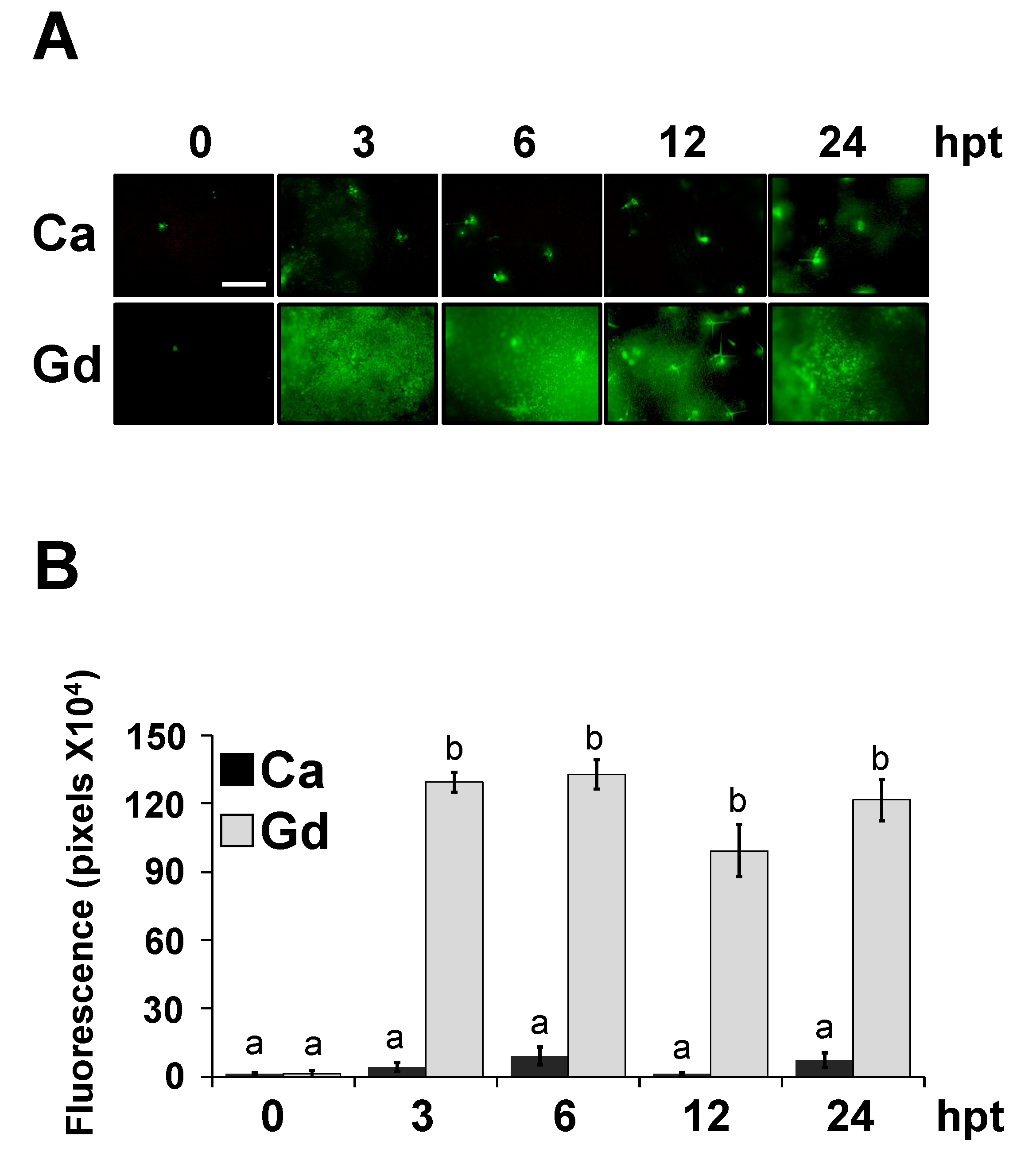
2.3. Gd Does Not Affect the Development of B. cinerea
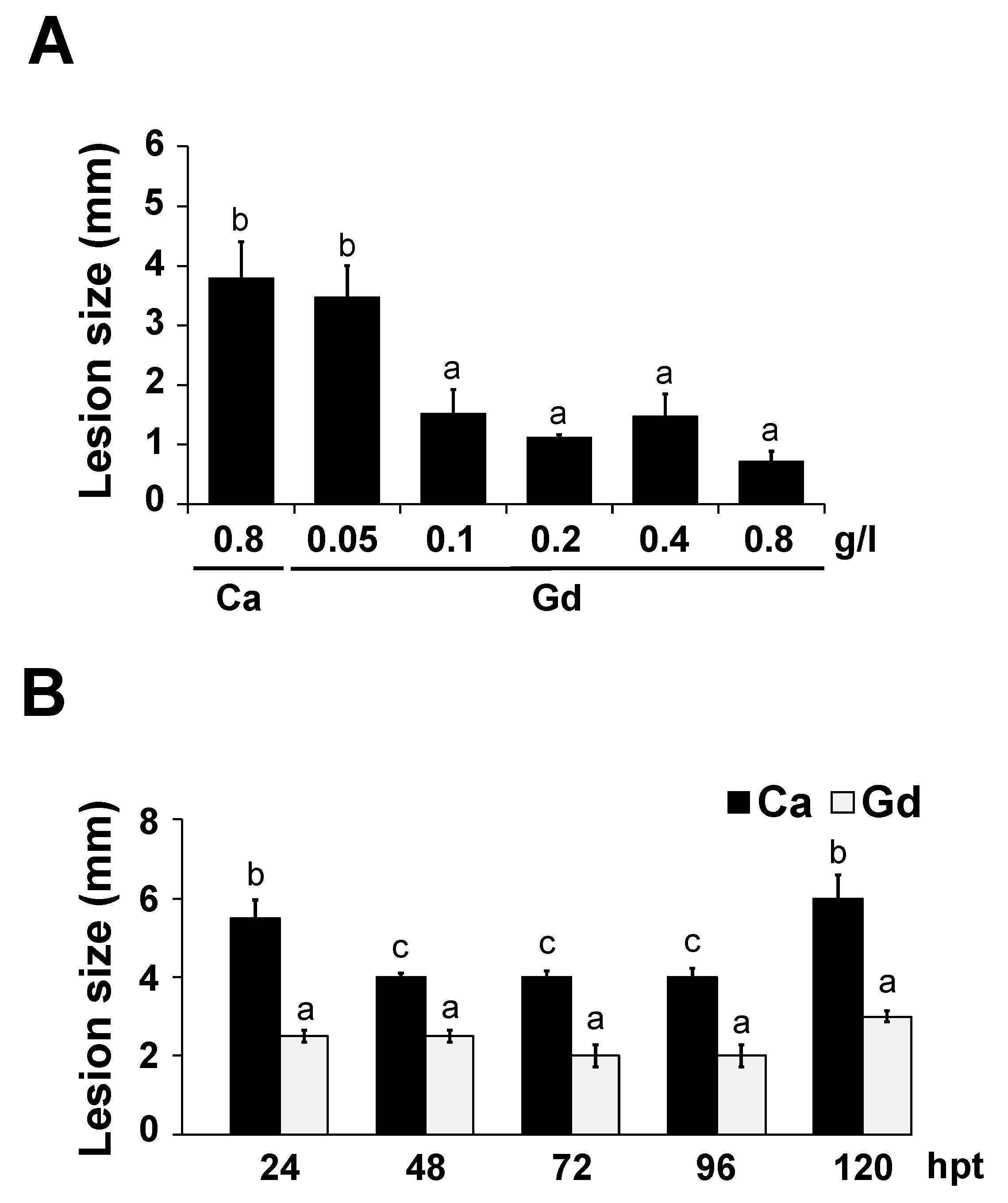
2.4. Gd-Induced Protection against B. cinerea Is Dose-Dependent and Long-Lasting
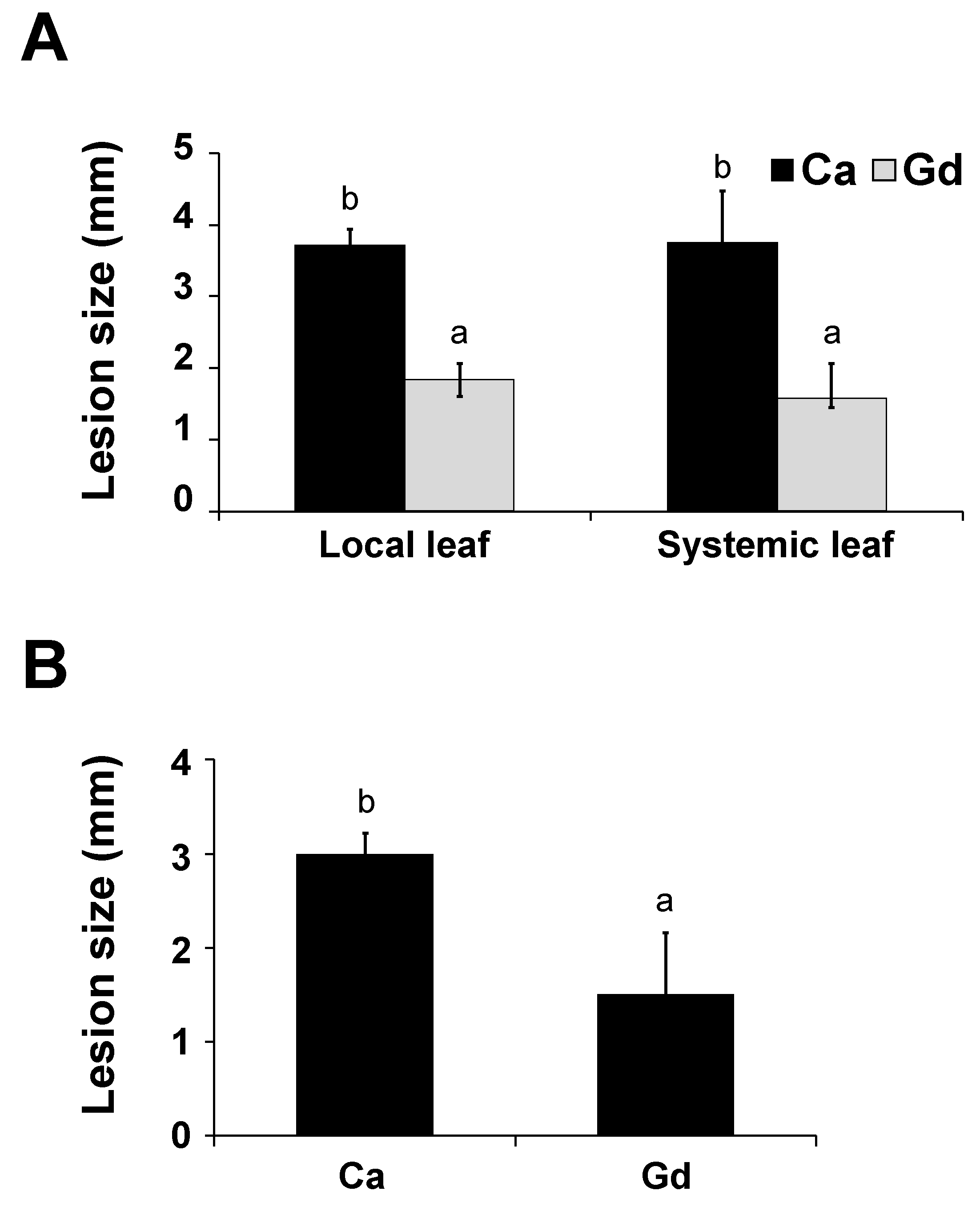
2.5. Gd Triggers a Systemic Defense Response to B. cinerea
2.6. Gd Does Not Modify the Cuticle Permeability but Triggers a ROS Burst
2.7. Gadolinium Up-Regulates the Responses to Biotic Stimulus and Represses the Responses to Abiotic Stimulus
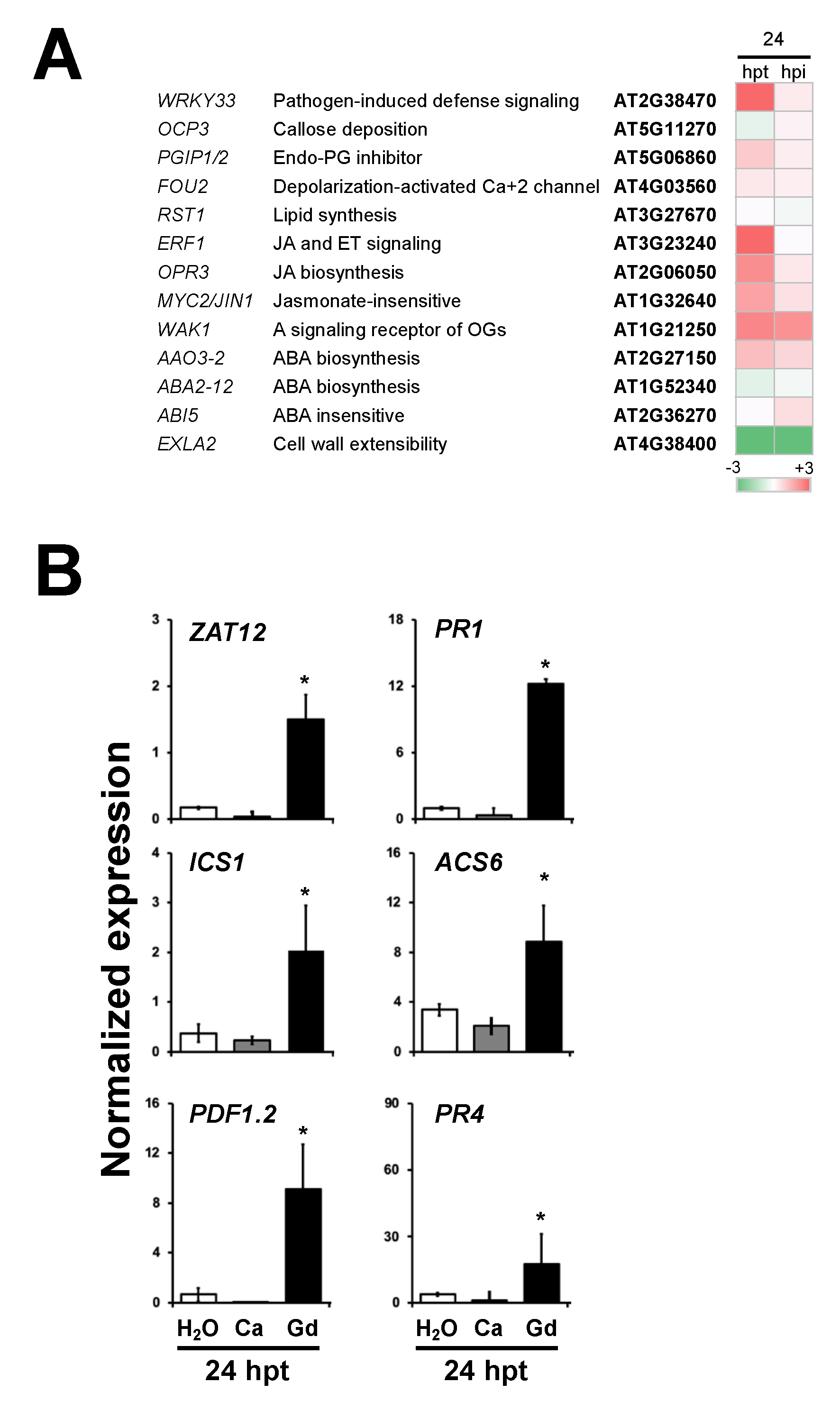
2.8. Plant Defense Response Genes Are Induced by Gadolinium Treatment and during the Interaction with B. cinerea
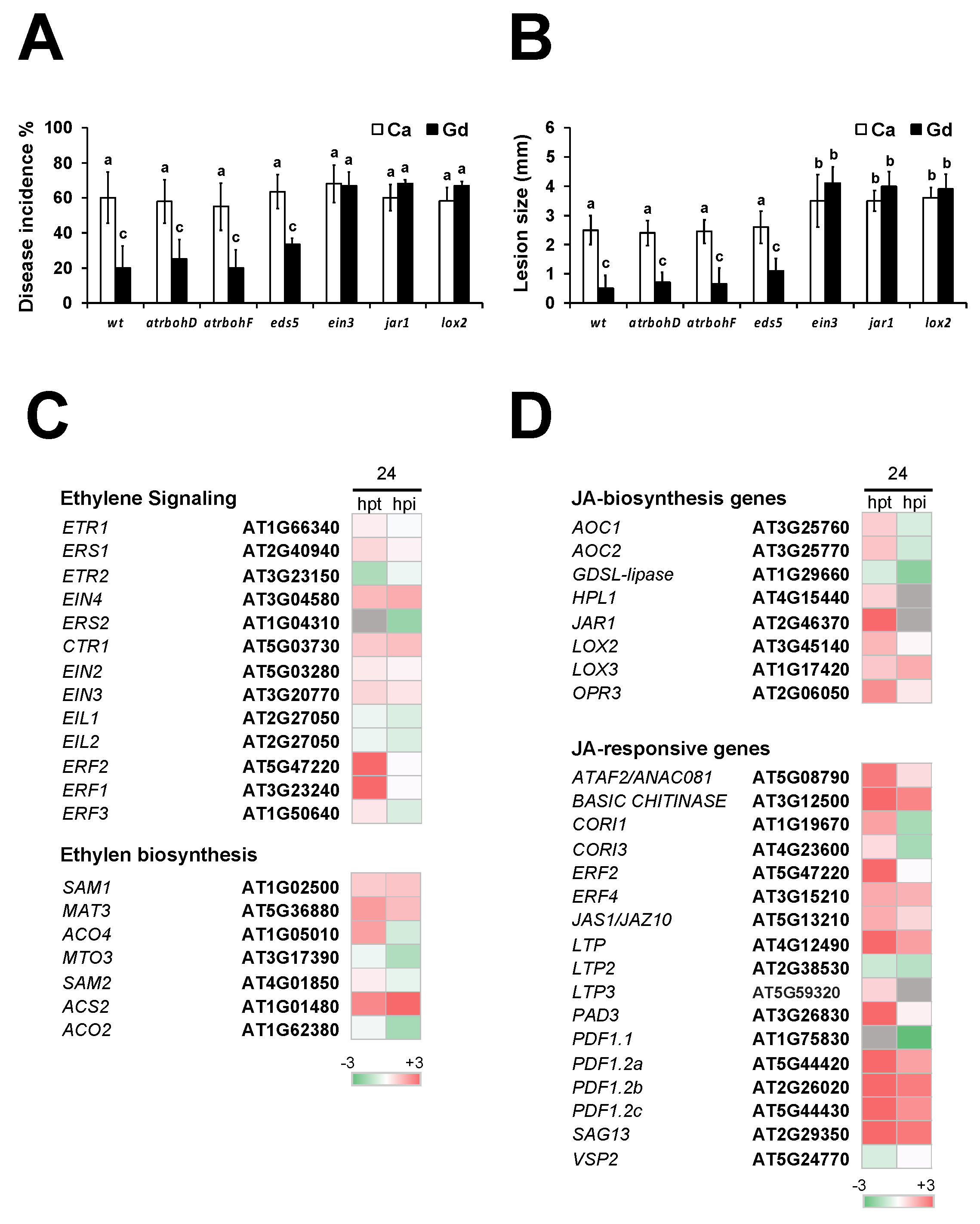
2.9. Gd-Induced Defense Response Against B. cinerea Is Dependent of JA and ET
3. Discussion
3.1. A Transcriptome Analysis Provides New Insights into the Molecular Mechanisms Involved in the Gd Response in A. thaliana
3.2. Gd Is a Novel Biocontrol against the Broad Host-Range Necrotrophic Fungus B. cinerea
3.3. Gd Induces Protection against B. cinerea by Activating Early and Late Defense Responses, in Particular the JA- and ET-Induced Signaling Pathway
3.4. Ca might Elicit the Defense Responses but Not as Strongly as Gd
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Gadolinium Treatment and B. cinerea Plant Inoculation
4.3. Systemic Defense Response Analysis
4.4. In Planta B. cinerea Growth Analysis
4.5. In Vitro Inhibitory Assay of B. cinerea Growth
4.6. Cuticle Permeability Analysis
4.7. Detection of ROS
4.8. RNA Extraction and Genome-Wide Transcriptomic Analysis
4.9. Quantitative Real Time RT-PCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Chapter 2—Postharvest disease initiation. In Postharvest Diseases of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3–24. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef]
- Katagiri, F.; Tsuda, K. Understanding the Plant Immune System. Mol. Plant Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef]
- Mengiste, T.; Laluk, K.; AbuQamar, S. Mechanisms of induced resistance against B. cinerea. In Postharvest Pathology; Prusky, D., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 13–30. [Google Scholar]
- AbuQamar, S.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Chien, S.S. Peculiar Effects of Barium, Strontium, and Cerium on Spirogyra. Bot. Gaz. 1917, 63, 406–409. [Google Scholar] [CrossRef]
- Hu, Z.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and Biochemical Effects of Rare Earth Elements on Plants and Their Agricultural Significance: A Review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Kerstin, R. Rare Earth Elements in Agriculture with Emphasis on Animal Husbandry. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2006. [Google Scholar]
- Wang, L.; Cheng, M.; Chu, Y.; Li, X.; Chen, D.D.Y.; Huang, X.; Zhou, Q. Responses of plant calmodulin to endocytosis induced by rare earth elements. Chemosphere 2016, 154, 408–415. [Google Scholar] [CrossRef]
- Kovarikova, M.; Tomaskova, I.; Soudek, P. Rare earth elements in plants. Biol. Plant 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Zhang, M.; Zhang, N.; Li, M. Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharm. Sin. B 2013, 3, 20–24. [Google Scholar] [CrossRef]
- Jia, Y.; Chunyan, L.; Guozheng, Q.; Kaoshan, C. The Effect of Nd~(3+) and Burdock Oligosaccharide on the Germination of Cassia obtusifolia Seeds. Chin. Agric. Sci. Bull. 2008, 11. [Google Scholar]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Chapter 92 Rare earth elements in biological systems. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1990; Volume 13, pp. 423–452. [Google Scholar]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef]
- Ismail, A.; Riemann, M.; Nick, P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012, 63, 2127–2139. [Google Scholar] [CrossRef]
- Qiao, F.; Chang, X.-L.; Nick, P. The cytoskeleton enhances gene expression in the response to the Harpin elicitor in grapevine. J. Exp. Bot. 2010, 61, 4021–4031. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Val’kov, A.V.; Stepanov, S.I.; Sergievskii, V.V.; Chekmarev, A.M. Monazite raw material for the production of highly effective fertilizers. Theor. Found. Chem. Eng. 2010, 44, 497–499. [Google Scholar] [CrossRef]
- Sabiha, J.; Waheed, S.; Siddique, N.; Shakoor, R.; Tufail, M. Measurement of rare earths elements in Kakul phosphorite deposits of Pakistan using instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2010, 284, 397–403. [Google Scholar] [CrossRef]
- Adisa, I.O.; Reddy Pullagurala, V.L.; Rawat, S.; Hernandez-Viezcas, J.A.; Dimkpa, C.O.; Elmer, W.H.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Role of Cerium Compounds in Fusarium Wilt Suppression and Growth Enhancement in Tomato (Solanum lycopersicum). J. Agric. Food Chem. 2018, 66, 5959–5970. [Google Scholar] [CrossRef]
- Guohong, W.; Yucai, C. Study on the application of rare earths to rubber trees. In New Frontiers in Rare Earth Science and Applications; Guangxian, X., Jimei, X., Eds.; Academic Press: Cambridge, MA, USA, 1985; p. 1510. [Google Scholar]
- Majumdar, S.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Castillo-Michel, H.; Hernandez-Viezcas, J.-A.; Sahi, S.; Gardea-Torresdey, J.L. Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defense mechanisms. J. Hazard. Mater. 2014, 278, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Batista-Oliveira, J.S. Elementos Terras Raras na Indução de Resistência em Soja. Ph.D. Thesis, Universidade Estadual de Maringá, Maringá, Brazil, 2018. [Google Scholar]
- Zhang, H.; Feng, J.; Zhu, W.; Liu, C.; Gu, J. Bacteriostatic effects of cerium-humic acid complex. Biol. Trace Elem. Res. 2000, 73, 29–36. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, H.; Gao, Q.; Zhang, Z.; Liu, Q. Effect of lanthanum on growth and biochemical property of Sclerotinia sclerotiorum. Chin. J. Appl. Ecol. 2000, 11, 382–384. [Google Scholar]
- L’Haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E.; Balet, F.; Schoonbeek, H.-J.; Hess, S.; Mir, R.; Léon, J.; et al. A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef]
- Blanc, C.; Coluccia, F.; L’Haridon, F.; Torres, M.; Ortiz-Berrocal, M.; Stahl, E.; Reymond, P.; Schreiber, L.; Nawrath, C.; Métraux, J.-P.; et al. The Cuticle Mutant eca2 Modifies Plant Defense Responses to Biotrophic and Necrotrophic Pathogens and Herbivory Insects. Mol. Plant Microbe Interact. 2018, 31, 344–355. [Google Scholar] [CrossRef]
- Aragón, W.; Reina-Pinto, J.J.; Serrano, M. The intimate talk between plants and microorganisms at the leaf surface. J. Exp. Bot. 2017, 68, 5339–5350. [Google Scholar] [CrossRef]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. mapman: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Davletova, S.; Liang, H.; Mittler, R. The Zinc Finger Protein Zat12 Is Required for Cytosolic Ascorbate Peroxidase 1 Expression during Oxidative Stress in Arabidopsis. J. Biol. Chem. 2004, 279, 11736–11743. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 417, 562–565. [Google Scholar] [CrossRef]
- Uknes, S.; Mauch-Mani, B.; Moyer, M.; Potter, S.; Williams, S.; Dincher, S.; Chandler, D.; Slusarenko, A.; Ward, E.; Ryals, J. Acquired resistance in Arabidopsis. Plant Cell 1992, 4, 645. [Google Scholar]
- Manners, J.M.; Penninckx, I.A.; Vermaere, K.; Kazan, K.; Brown, R.L.; Morgan, A.; Maclean, D.J.; Curtis, M.D.; Cammue, B.P.; Broekaert, W.F. The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol. Biol. 1998, 38, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Arteca, J.M.; Arteca, R.N. A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsis leaves. Plant Mol. Biol. 1999, 39, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kravchuk, Z.; Vicedo, B.; Flors, V.; Camañes, G.; González-Bosch, C.; García-Agustín, P. Priming for JA-dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea. J. Plant Physiol. 2011, 168, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hael-Conrad, V.; Abou-Mansour, E.; Díaz-Ricci, J.C.; Métraux, J.P.; Serrano, M. The novel elicitor AsES triggers a defense response against Botrytis cinerea in Arabidopsis thaliana. Plant Sci. 2015, 241, 120–127. [Google Scholar] [CrossRef]
- Serrano, M.; Guzman, P. Isolation and Gene Expression Analysis of Arabidopsis thaliana Mutants with Constitutive Expression of ATL2, an Early Elicitor-Response RING-H2 Zinc-Finger Gene. Genetics 2004, 167, 919–929. [Google Scholar] [CrossRef]
- Windram, O.; Madhou, P.; McHattie, S.; Hill, C.; Hickman, R.; Cooke, E.; Jenkins, D.J.; Penfold, C.A.; Baxter, L.; Breeze, E.; et al. Arabidopsis Defense against Botrytis cinerea: Chronology and Regulation Deciphered by High-Resolution Temporal Transcriptomic Analysis. Plant Cell Online 2012, 24, 3530–3557. [Google Scholar] [CrossRef]
- Méndez-Bravo, A.; Calderón-Vázquez, C.; Ibarra-Laclette, E.; Raya-González, J.; Ramírez-Chávez, E.; Molina-Torres, J.; Guevara-García, A.A.; López-Bucio, J.; Herrera-Estrella, L. Alkamides Activate Jasmonic Acid Biosynthesis and Signaling Pathways and Confer Resistance to Botrytis cinerea in Arabidopsis thaliana. PLoS ONE 2011, 6, e27251. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 2002, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, L.; Zhou, Q.; Huang, X. Toxic effects of heavy metal terbium ion on the composition and functions of cell membrane in horseradish roots. Ecotoxicol. Environ. Saf. 2015, 111, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186. [Google Scholar] [CrossRef]
- Sasanuma, I.; Suzuki, T. Effect of calcium on cell-wall degrading enzymes of Botrytis cinerea. Biosci. Biotechnol. Biochem. 2016, 80, 1730–1736. [Google Scholar] [CrossRef]
- Benikhlef, L.; L’Haridon, F.; Abou-Mansour, E.; Serrano, M.; Binda, M.; Costa, A.; Lehmann, S.; Metraux, J.-P. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 2013, 13, 133. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, S.; Wang, X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci. Rep. 2016, 6, 31860. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol. Biol. 2002, 48, 267–276. [Google Scholar] [CrossRef]
- Halim, V.A.; Altmann, S.; Ellinger, D.; Eschen-Lippold, L.; Miersch, O.; Scheel, D.; Rosahl, S. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J. 2009, 57, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.-C.; Kauffmann, S. beta-1,3 Glucan Sulfate, but Not beta-1,3 Glucan, Induces the Salicylic Acid Signaling Pathway in Tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J.; Penninckx, I.A.M.A.; Broekaert, W.F.; Cammue, B.P.A. The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 2001, 13, 63–68. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Plotnikova, J.M.; De Lorenzo, G.; Ausubel, F.M. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003, 35, 193–205. [Google Scholar] [CrossRef]
- Choi, W.-G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Zachary, M.; Teaster, N.D.; Sparks, J.A.; Valster, A.H.; Motes, C.M.; Blancaflor, E.B. Fluorescence Resonance Energy Transfer Sensitized Emission of Yellow Cameleon 3.60 Reveals Root-Zone-Specific Calcium Signatures in Arabidopsis in Response to Aluminum and Other Trivalent Cations. Plant Physiol. 2010. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.M.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Chen, Z. Effects of rare earth elements and REE-binding proteins on physiological responses in plants. Protein Pept. Lett. 2012, 19, 198–202. [Google Scholar] [CrossRef]
- Todorovsky, D.S.; Minkova, N.L.; Bakalova, D.P. Effect of the application of superphosphate on rare earths’ content in the soil. Sci. Total Environ. 1997, 203, 13–16. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef]
- Nawrath, C.; Métraux, J.-P. Salicylic Acid Induction–Deficient Mutants of Arabidopsis Express PR-2 and PR-5 and Accumulate High Levels of Camalexin after Pathogen Inoculation. Plant Cell 1999, 11, 1393. [Google Scholar]
- Chassot, C.; Nawrath, C.; Metraux, J.P. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007, 49, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.; Lubarsky, B.; Kieber, J.J.; Rothenberg, M.; Ecker, J.R. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics 1995, 139, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bautista, N.; Domínguez-Núñez, J.A.; Moreno, M.M.C.; Berrocal-Lobo, M. Plant Tissue Trypan Blue Staining During Phytopathogen Infection. Bio Protoc. 2016, 6, e2078. [Google Scholar] [CrossRef]
- Ferreira-Saab, M.; Formey, D.; Torres, M.; Aragón, W.; Padilla, E.A.; Tromas, A.; Sohlenkamp, C.; Schwan-Estrada, K.R.F.; Serrano, M. Compounds Released by the Biocontrol Yeast Hanseniaspora opuntiae Protect Plants Against Corynespora cassiicola and Botrytis cinerea. Front. Microbiol. 2018, 9, 1596. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Garcion, C.; Metraux, J. FiRe and microarrays: A fast answer to burning questions. Trends Plant Sci. 2006, 11, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.-P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Liu, W.; Karemera, N.J.U.; Wu, T.; Yang, Y.; Zhang, X.; Xu, X.; Wang, Y.; Han, Z. The ethylene response factor AtERF4 negatively regulates the iron deficiency response in Arabidopsis thaliana. PLoS ONE 2017, 12, e0186580. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista-Oliveira, J.S.; Formey, D.; Torres, M.; Aragón, W.; Romero-Contreras, Y.J.; Maruri-López, I.; Tromas, A.; Schwan-Estrada, K.R.F.; Serrano, M. Gadolinium Protects Arabidopsis thaliana against Botrytis cinerea through the Activation of JA/ET-Induced Defense Responses. Int. J. Mol. Sci. 2021, 22, 4938. https://doi.org/10.3390/ijms22094938
Batista-Oliveira JS, Formey D, Torres M, Aragón W, Romero-Contreras YJ, Maruri-López I, Tromas A, Schwan-Estrada KRF, Serrano M. Gadolinium Protects Arabidopsis thaliana against Botrytis cinerea through the Activation of JA/ET-Induced Defense Responses. International Journal of Molecular Sciences. 2021; 22(9):4938. https://doi.org/10.3390/ijms22094938
Chicago/Turabian StyleBatista-Oliveira, Juliana Santos, Damien Formey, Martha Torres, Wendy Aragón, Yordan Jhovani Romero-Contreras, Israel Maruri-López, Alexandre Tromas, Kátia Regina Freitas Schwan-Estrada, and Mario Serrano. 2021. "Gadolinium Protects Arabidopsis thaliana against Botrytis cinerea through the Activation of JA/ET-Induced Defense Responses" International Journal of Molecular Sciences 22, no. 9: 4938. https://doi.org/10.3390/ijms22094938
APA StyleBatista-Oliveira, J. S., Formey, D., Torres, M., Aragón, W., Romero-Contreras, Y. J., Maruri-López, I., Tromas, A., Schwan-Estrada, K. R. F., & Serrano, M. (2021). Gadolinium Protects Arabidopsis thaliana against Botrytis cinerea through the Activation of JA/ET-Induced Defense Responses. International Journal of Molecular Sciences, 22(9), 4938. https://doi.org/10.3390/ijms22094938







