Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse Model of Ovarian Cancer
Abstract
1. Introduction
2. Results
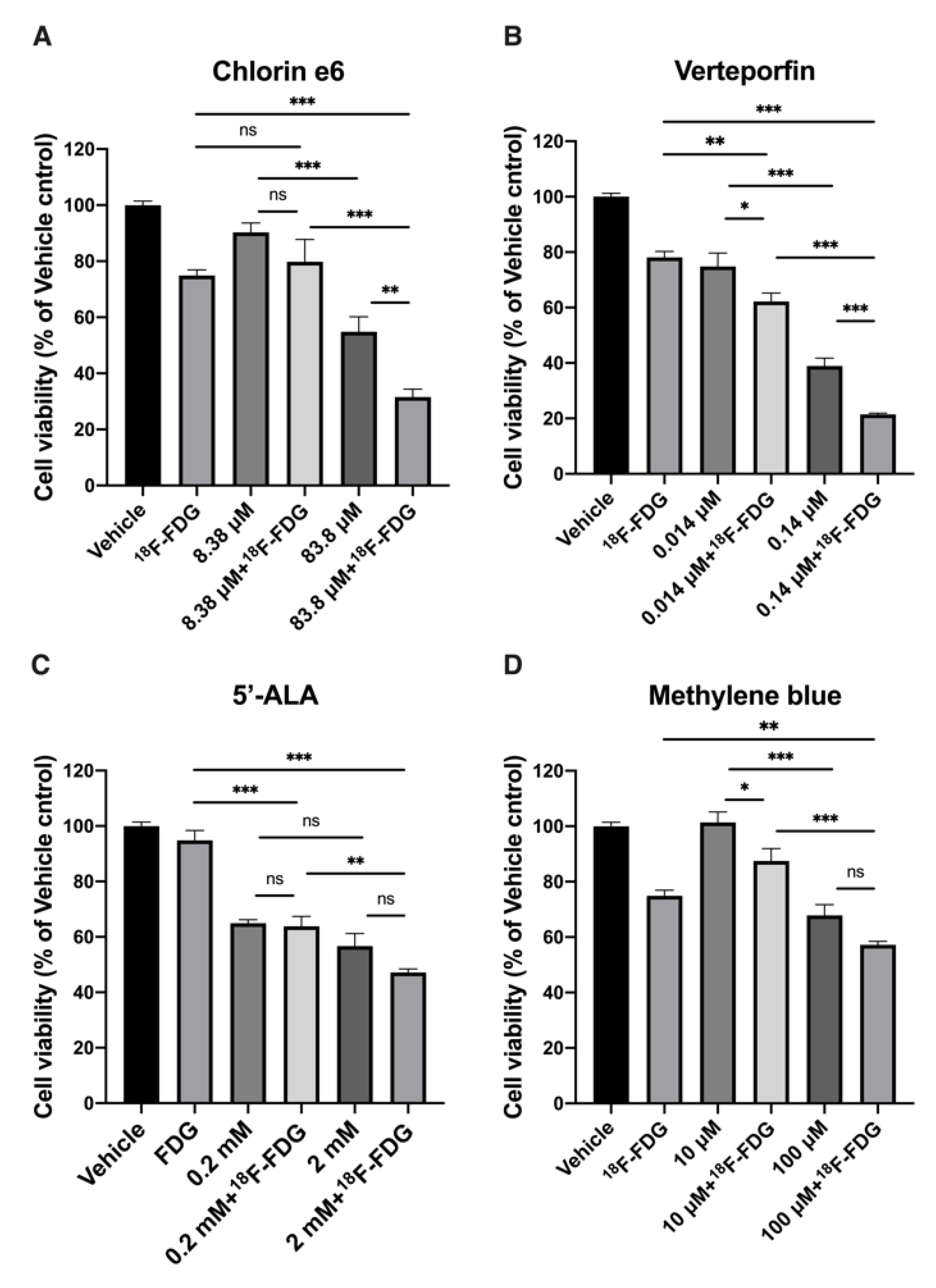
2.1. In Vitro Effect of CR-Induced PDT with 18F-FDG
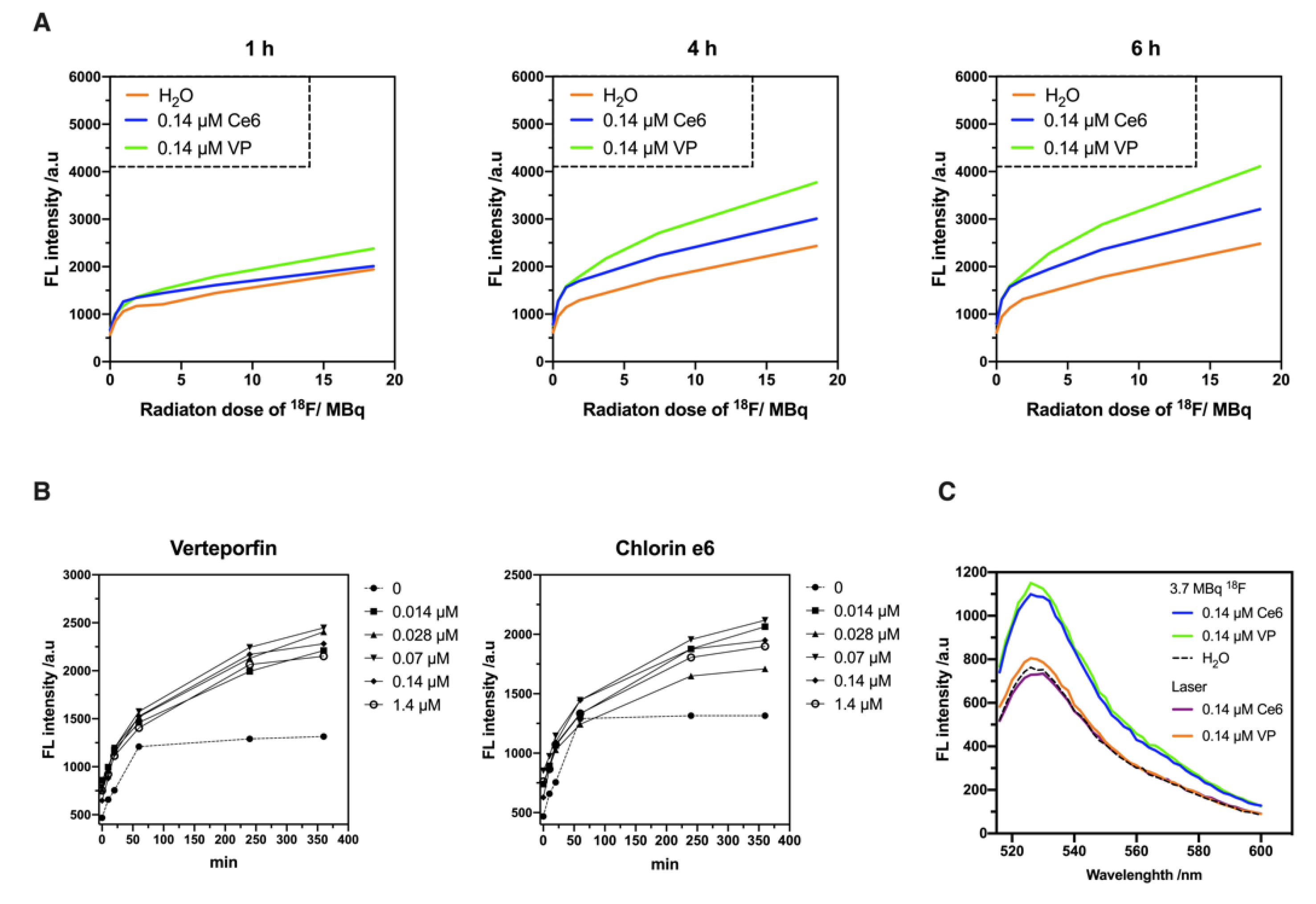
2.2. Evaluation of 18F-Emitted CR-Induced Photoexcitation
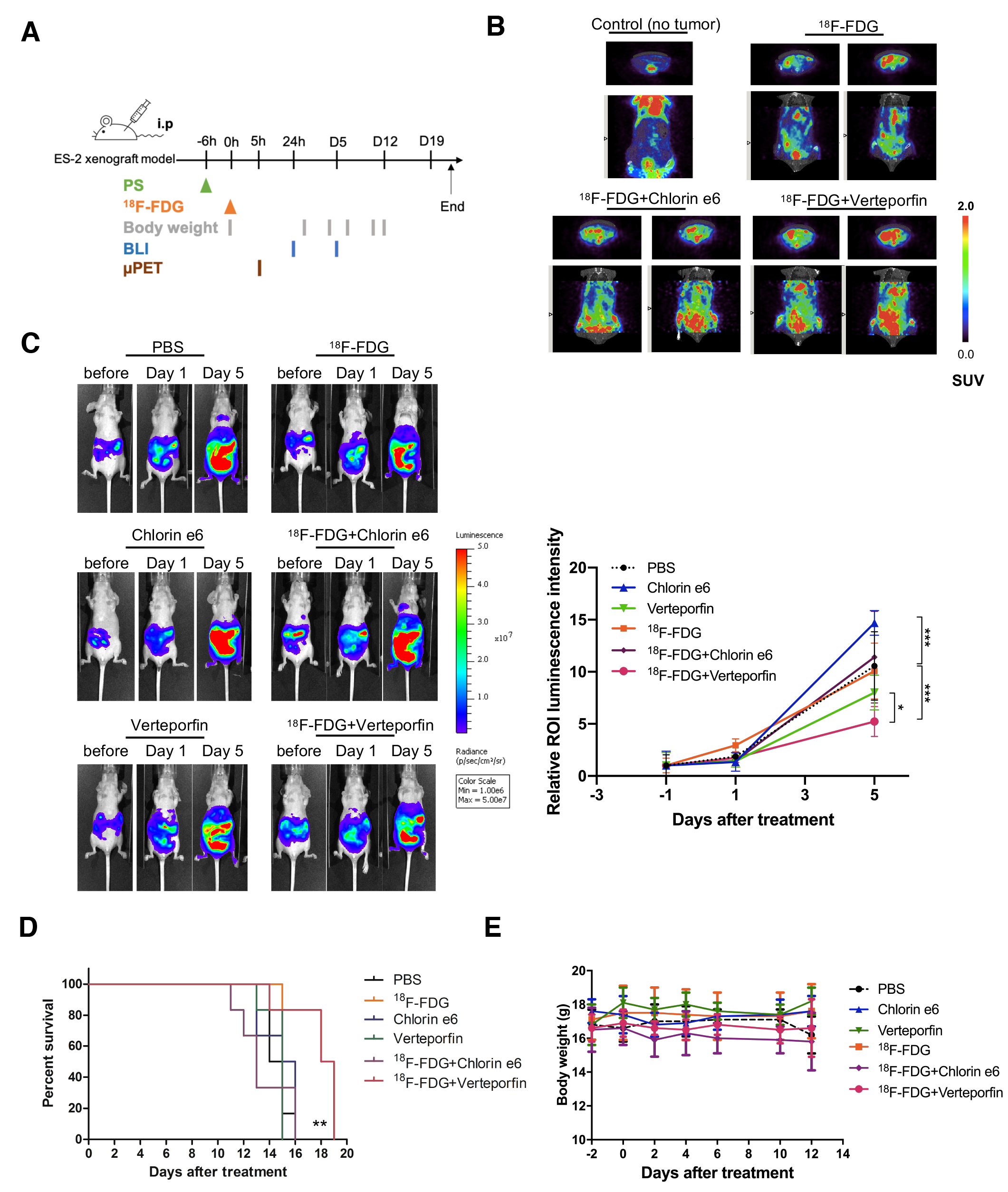
2.3. Biologic Uptake and CLI of 18F-FDG in the ES2-Luc Xenograft Model
2.4. In Vivo Effect of CR-Induced Photodynamic Therapy on Ovarian Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. In Vitro CR-Induced Photodynamic Therapy
4.3. Detection of Singlet Oxygen
4.4. Animal Model and In Vivo CR-PDT
4.5. MicroPET Imaging and Imaging Analysis
4.6. In Vivo Bioluminescence Image
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Cherenkov, P.A. Radiation from high-speed particles. Science 1960, 131, 136–142. [Google Scholar] [CrossRef]
- Thorek, D.; Robertson, R.; Bacchus, W.A.; Hahn, J.; Rothberg, J.; Beattie, B.J.; Grimm, J. Cerenkov imaging-A new modality for molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 163–173. [Google Scholar]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Lheureux, S.; Karakasis, K.; Kohn, E.C.; Oza, A.M. Ovarian cancer treatment: The end of empiricism? Cancer 2015, 121, 3203–3211. [Google Scholar] [CrossRef]
- Hahn, S.M.; Fraker, D.L.; Mick, R.; Metz, J.; Busch, T.M.; Smith, D.; Zhu, T.; Rodriguez, C.; Dimofte, A.; Spitz, F.; et al. A phase II trial of intraperitoneal photodynamic therapy for patients with peritoneal carcinomatosis and sarcomatosis. Clin. Cancer Res. 2006, 12, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Jones, H.; Burock, M.; Smith, D.; Fraker, D.L.; Metz, J.; Glatstein, E.; Hahn, S.M. Patterns of recurrence in patients treated with photodynamic therapy for intraperitoneal carcinomatosis and sarcomatosis. Int. J. Oncol. 2004, 24, 711–717. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Agress, H., Jr.; Goldsmith, S.J. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003, 23, 315–340, quiz 533. [Google Scholar] [CrossRef]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Lai, X.; Wang, X.; Leung, A.W.; Zhang, H.; Xu, C. Photodynamic action of methylene blue in osteosarcoma cells in vitro. Photodiagnosis Photodyn. Ther. 2014, 11, 13–19. [Google Scholar] [CrossRef]
- Lu, J.; Roy, B.; Anderson, M.; Leggett, C.L.; Levy, M.J.; Pogue, B.; Hasan, T.; Wang, K.K. Verteporfin- and sodium porfimer-mediated photodynamic therapy enhances pancreatic cancer cell death without activating stromal cells in the microenvironment. J. Biomed. Opt. 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, R.S.; Zhu, J.G.; Li, Y.C.; Liu, H.C. Subcellular location and photodynamic therapeutic effect of chlorin e6 in the human tongue squamous cell cancer Tca8113 cell line. Oncol. Lett. 2015, 9, 551–556. [Google Scholar] [CrossRef]
- El-Khatib, M.; Tepe, C.; Senger, B.; Dibue-Adjei, M.; Riemenschneider, M.J.; Stummer, W.; Steiger, H.J.; Cornelius, J.F. Aminolevulinic acid-mediated photodynamic therapy of human meningioma: An in vitro study on primary cell lines. Int. J. Mol. Sci. 2015, 16, 9936–9948. [Google Scholar] [CrossRef] [PubMed]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef]
- Guo, J.; Cai, J.; Zhang, Y.; Zhu, Y.; Yang, P.; Wang, Z. Establishment of two ovarian cancer orthotopic xenograft mouse models for in vivo imaging: A comparative study. Int. J. Oncol. 2017, 51, 1199–1208. [Google Scholar] [CrossRef]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Riechelmann, H.; Dudas, J. Photodynamic Effect of Methylene Blue and Low Level Laser Radiation in Head and Neck Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef]
- Teshigawara, T.; Mizuno, M.; Ishii, T.; Kitajima, Y.; Utsumi, F.; Sakata, J.; Kajiyama, H.; Shibata, K.; Ishizuka, M.; Kikkawa, F. Novel potential photodynamic therapy strategy using 5-Aminolevulinic acid for ovarian clear-cell carcinoma. Photodiagnosis Photodyn. Ther. 2018, 21, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kostenich, G.A.; Zhuravkin, I.N.; Zhavrid, E.A. Experimental grounds for using chlorin e6 in the photodynamic therapy of malignant tumors. J. Photochem. Photobiol. B 1994, 22, 211–217. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, R.; Liu, D.; Li, B.; Lin, J.; Zhang, W. The tumor affinity of chlorin e6 and its sonodynamic effects on non-small cell lung cancer. Ultrason Sonochem. 2013, 20, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 2020, 204, 111787. [Google Scholar] [CrossRef]
- Liu, H.; Carter, P.J.H.; Laan, A.C.; Eelkema, R.; Denkova, A.G. Singlet Oxygen Sensor Green is not a Suitable Probe for (1)O2 in the Presence of Ionizing Radiation. Sci. Rep. 2019, 9, 8393. [Google Scholar] [CrossRef]
- Baier, J.; Maisch, T.; Maier, M.; Landthaler, M.; Baumler, W. Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin. J. Invest. Dermatol. 2007, 127, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.M.; Ray, P.; Willmann, J.K.; Drescher, C.; Gambhir, S.S. 2-deoxy-2-[F-18]fluoro-D-glucose accumulation in ovarian carcinoma cell lines. Mol. Imaging Biol. 2007, 9, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nagaya, T.; Sato, K.; Okuyama, S.; Ogata, F.; Wong, K.; Adler, S.; Choyke, P.L.; Kobayashi, H. Cerenkov Radiation-Induced Photoimmunotherapy with (18)F-FDG. J. Nucl. Med. 2017, 58, 1395–1400. [Google Scholar] [CrossRef][Green Version]
- Taylor, K.; Lemon, J.A.; Boreham, D.R. Radiation-induced DNA damage and the relative biological effectiveness of 18F-FDG in wild-type mice. Mutagenesis 2014, 29, 279–287. [Google Scholar] [CrossRef]
- Mir, Y.; Elrington, S.A.; Hasan, T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine 2013, 9, 1114–1122. [Google Scholar] [CrossRef]
- Chang, C.M.; Lan, K.L.; Huang, W.S.; Lee, Y.J.; Lee, T.W.; Chang, C.H.; Chuang, C.M. (188)Re-Liposome Can Induce Mitochondrial Autophagy and Reverse Drug Resistance for Ovarian Cancer: From Bench Evidence to Preliminary Clinical Proof-of-Concept. Int. J. Mol. Sci. 2017, 18, 903. [Google Scholar] [CrossRef]
| Animal Groups | SUVmean | SUVmax |
|---|---|---|
| Control (no tumor) | 6.71 ± 0.32 | 6.25 ± 1.49 |
| [18F]FDG | 7.49 ± 0.54 | 8.52 ± 1.78 |
| [18F]FDG + Verteporfin | 9.98 ± 3.10 | 7.98 ± 3.68 |
| [18F]FDG + Chlorin e6 | 9.61 ± 5.69 | 8.91 ± 2.11 |
| N = 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Li, J.-J.; Lin, S.-L.; Lu, C.-H.; Chiu, S.-J.; Jeng, F.-S.; Chang, C.-W.; Yang, B.-H.; Chang, M.-C.; Ke, C.-C.; et al. Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse Model of Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 4934. https://doi.org/10.3390/ijms22094934
Chen Y-A, Li J-J, Lin S-L, Lu C-H, Chiu S-J, Jeng F-S, Chang C-W, Yang B-H, Chang M-C, Ke C-C, et al. Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse Model of Ovarian Cancer. International Journal of Molecular Sciences. 2021; 22(9):4934. https://doi.org/10.3390/ijms22094934
Chicago/Turabian StyleChen, Yi-An, Jia-Je Li, Syue-Liang Lin, Cheng-Hsiu Lu, Sain-Jhih Chiu, Fong-Shya Jeng, Chi-Wei Chang, Bang-Hung Yang, Ming-Cheng Chang, Chien-Chih Ke, and et al. 2021. "Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse Model of Ovarian Cancer" International Journal of Molecular Sciences 22, no. 9: 4934. https://doi.org/10.3390/ijms22094934
APA StyleChen, Y.-A., Li, J.-J., Lin, S.-L., Lu, C.-H., Chiu, S.-J., Jeng, F.-S., Chang, C.-W., Yang, B.-H., Chang, M.-C., Ke, C.-C., & Liu, R.-S. (2021). Effect of Cerenkov Radiation-Induced Photodynamic Therapy with 18F-FDG in an Intraperitoneal Xenograft Mouse Model of Ovarian Cancer. International Journal of Molecular Sciences, 22(9), 4934. https://doi.org/10.3390/ijms22094934







