The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation
Abstract
1. Introduction
2. Results
2.1. Characterization of Nanoparticles
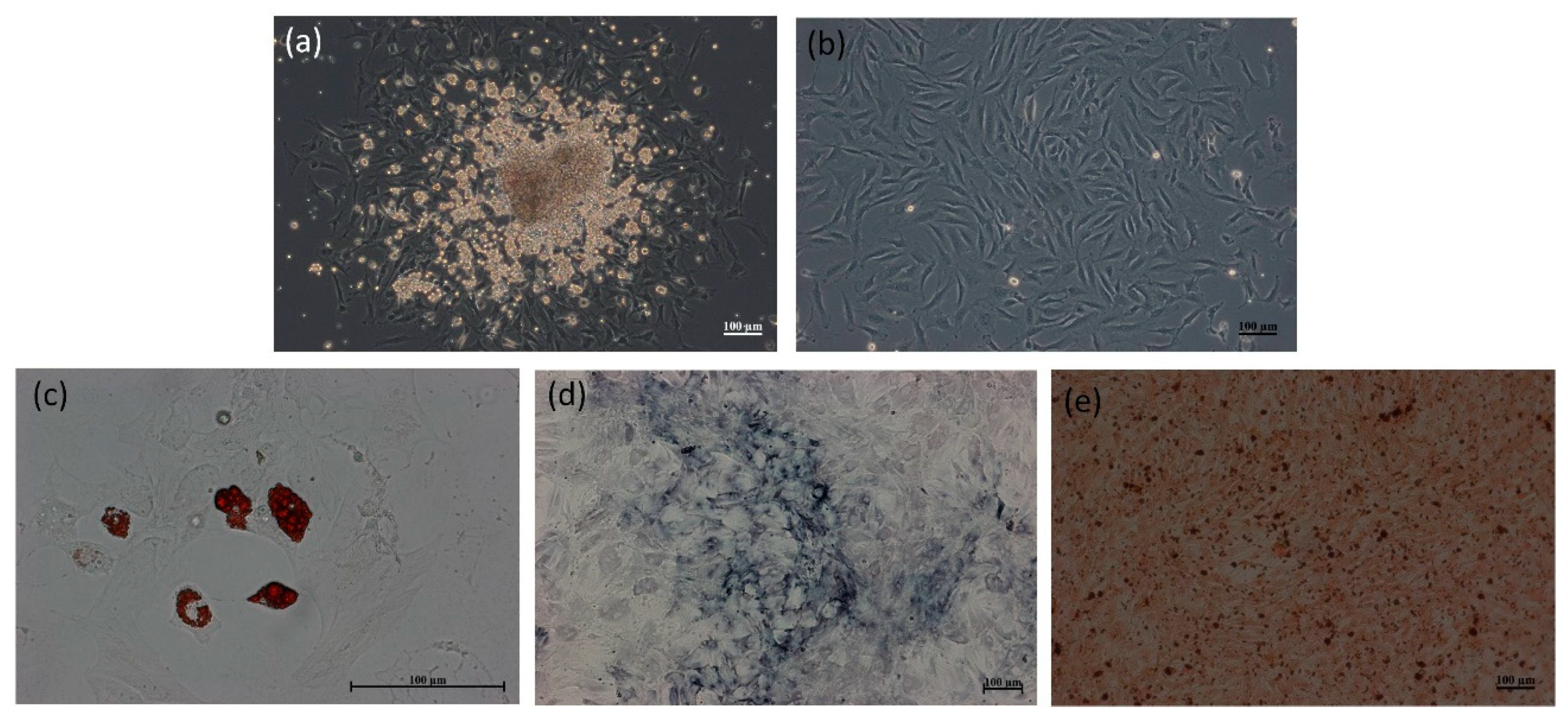
2.2. Morphology and Differentiation Potential of the Rat MSCs
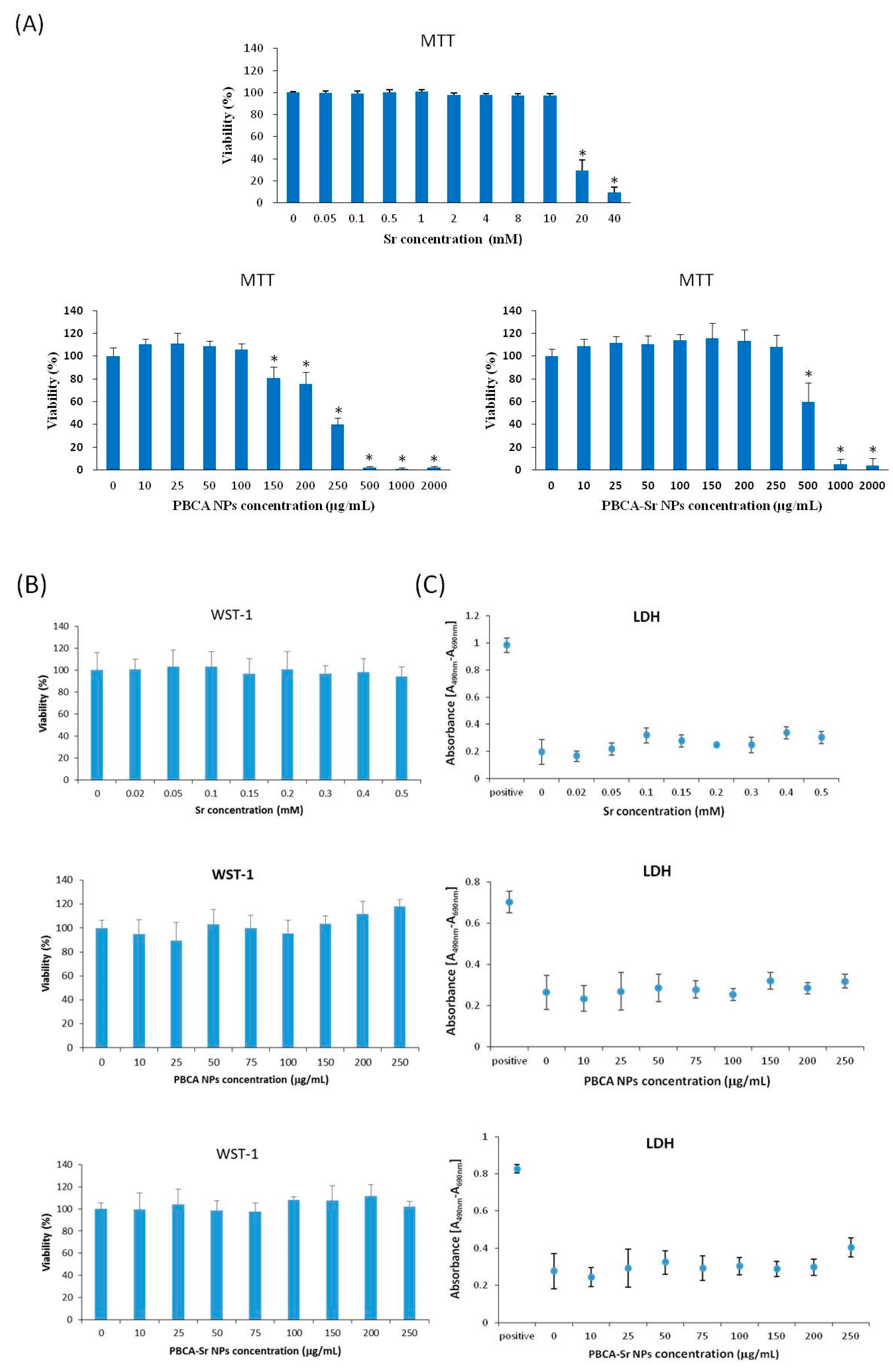
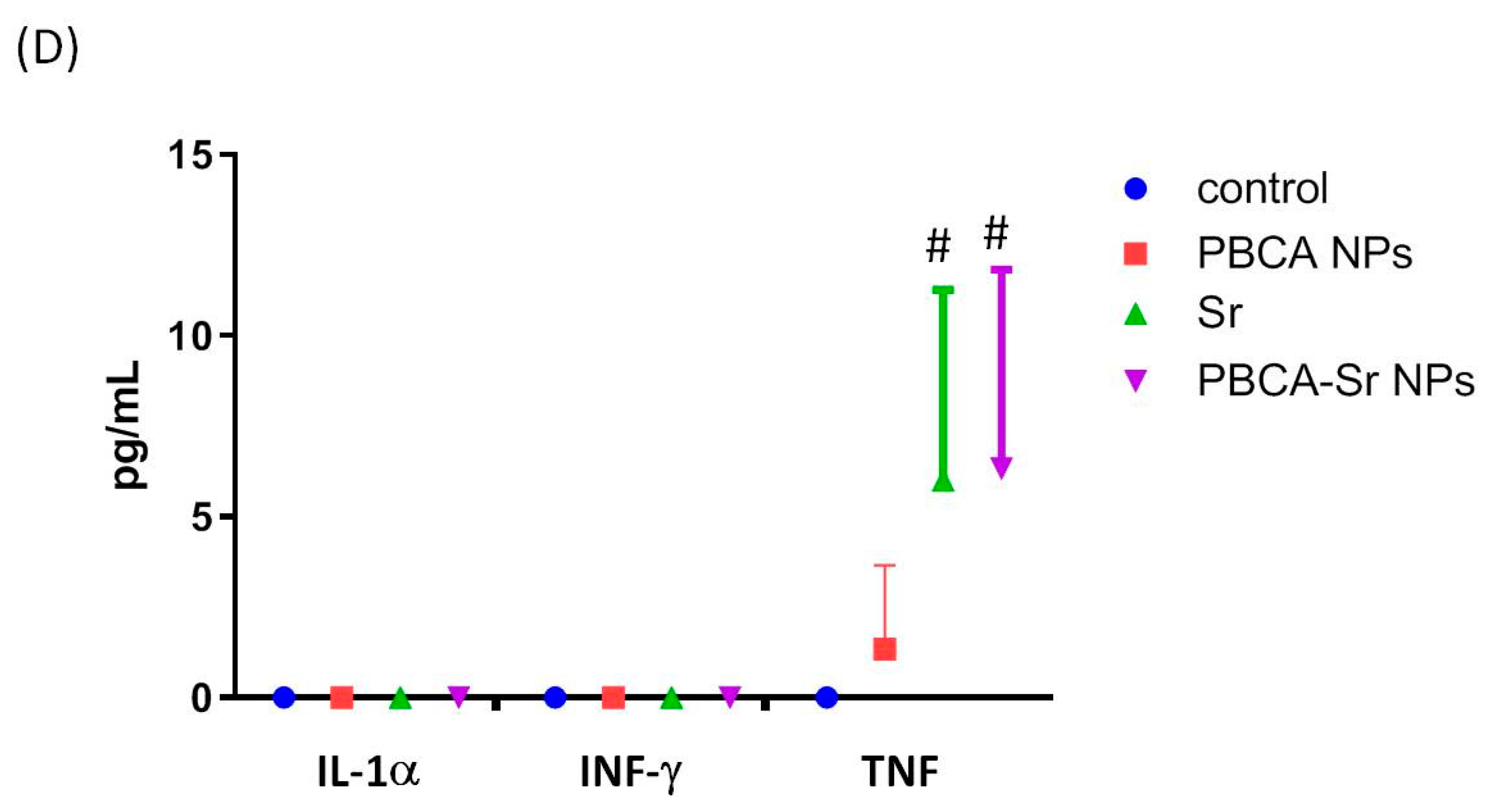
2.3. Assessment of Cytotoxicity
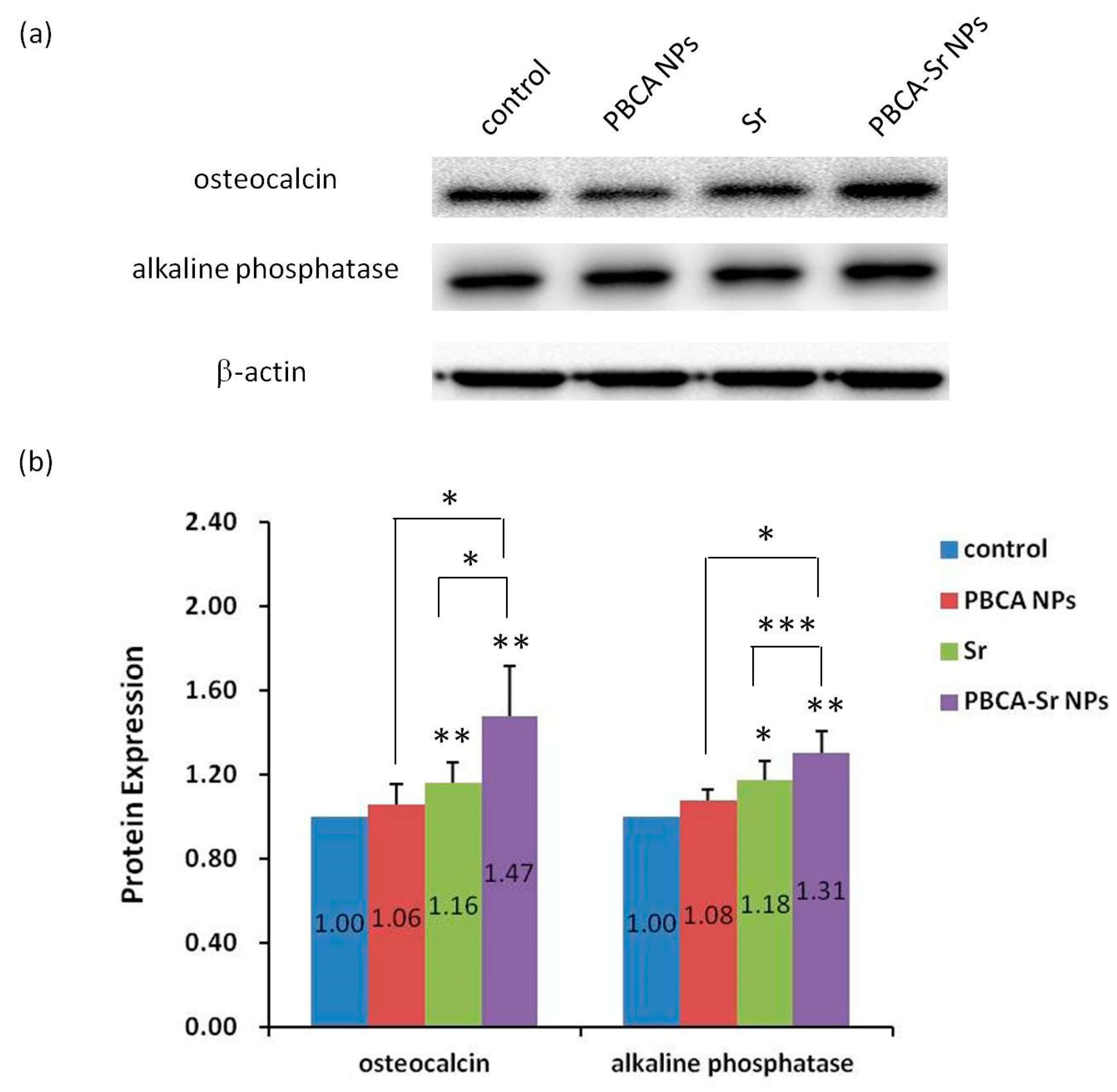
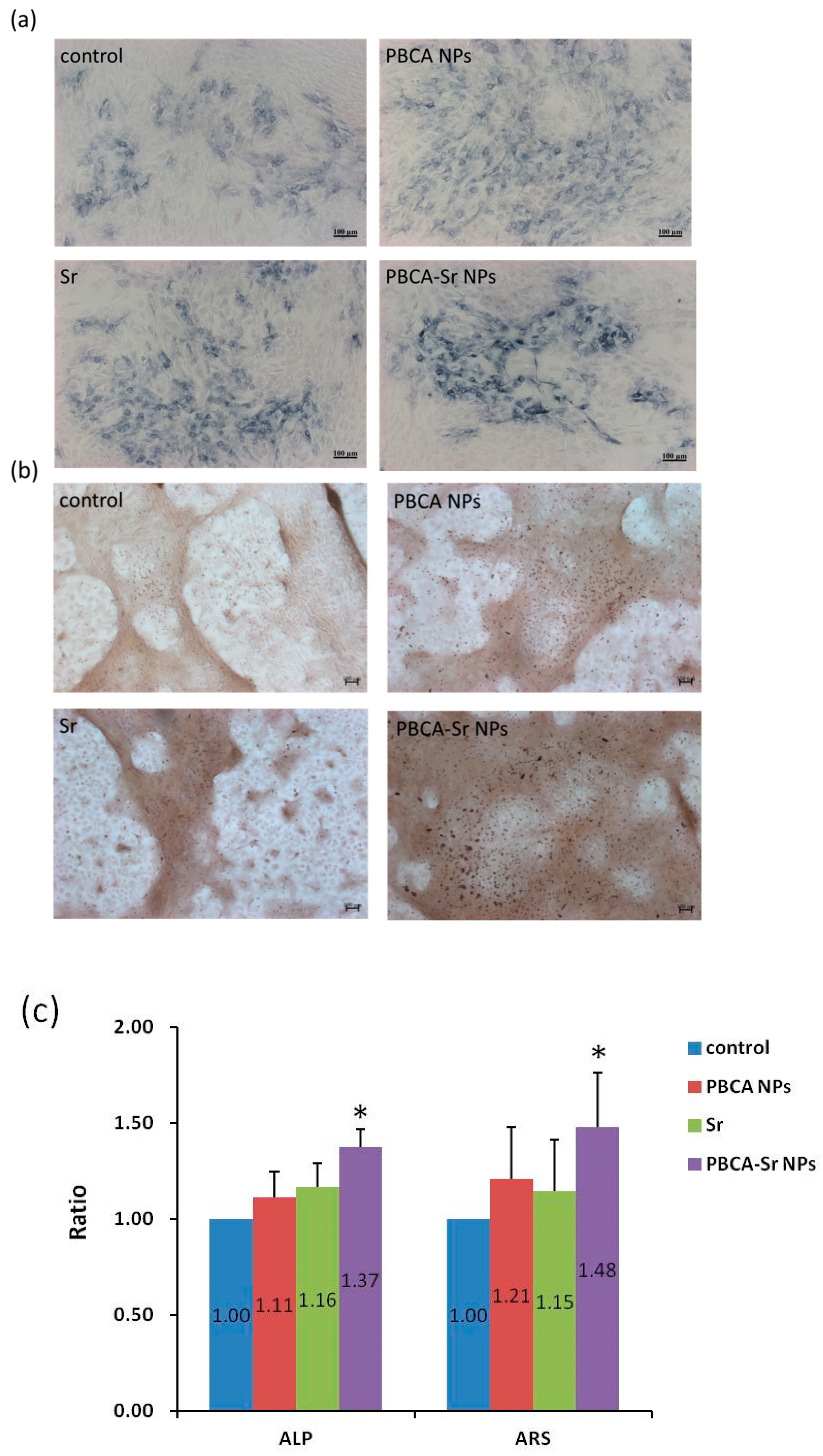
2.4. The effect of Osteogenesis and Mineralization of PBCA-Sr NPs on Committed MSCs
2.4.1. 2D Culture
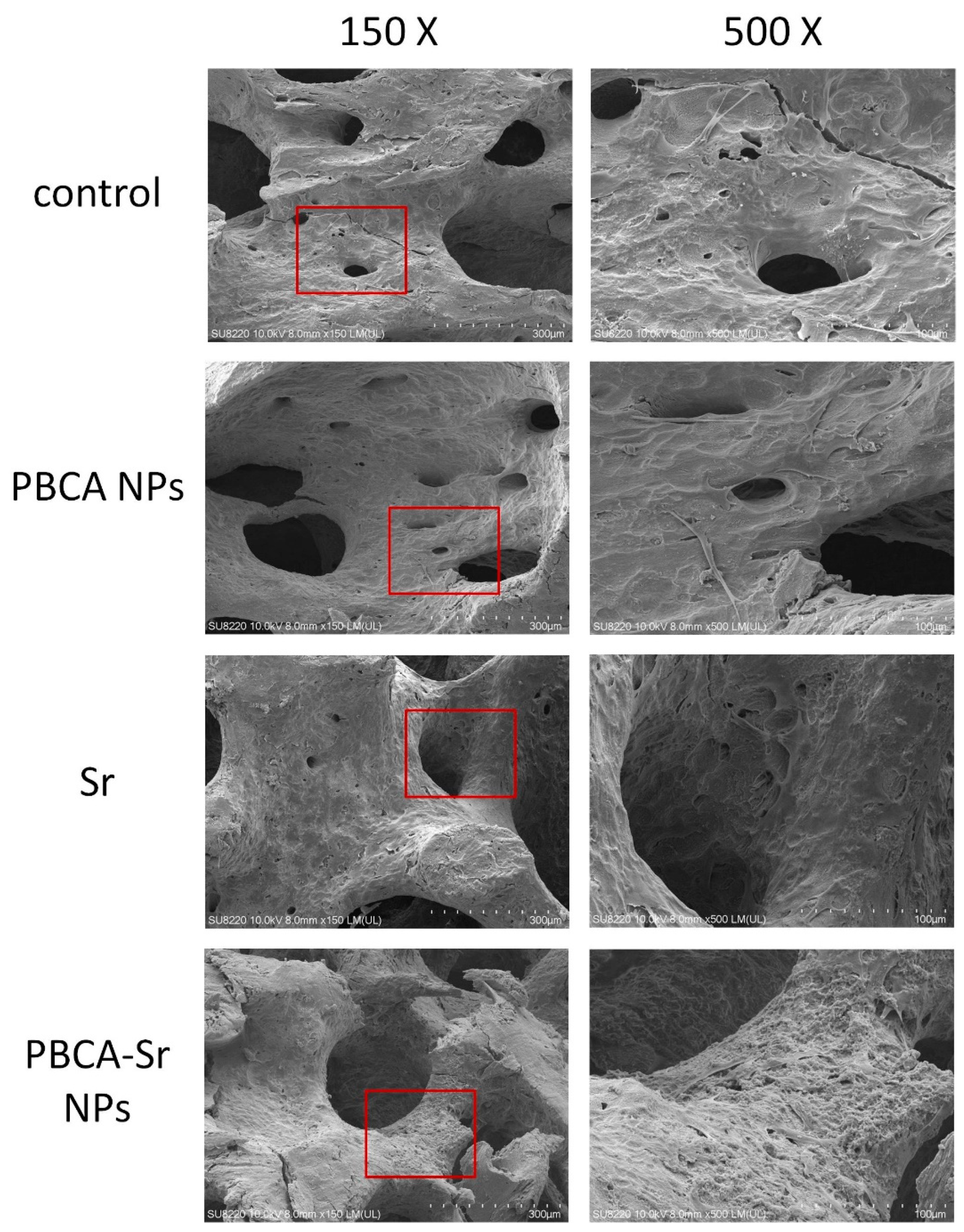
2.4.2. 3D Culture
3. Discussion
4. Materials and Methods
4.1. Synthesis of PBCA-Sr NPs
4.2. Characterization
4.3. Evaluation of Entrapment Efficiency
4.4. Isolation of Rat Bone Marrow Stromal Cells
4.5. MSC Differentiation Potential
4.6. Differentation of Rat Committed MSCs and Cell Culture
4.7. Evaluate the Effect of PBCA-Sr NPs on Osteoblasts in Vitro
4.7.1. Cell Viability Test
MTT Assay
WST-1 Assay
LDH Assay
Determination of Inflammation
4.7.2. ALP Staining
4.7.3. Analysis of Calcium Deposition
4.7.4. Western Blot Analysis
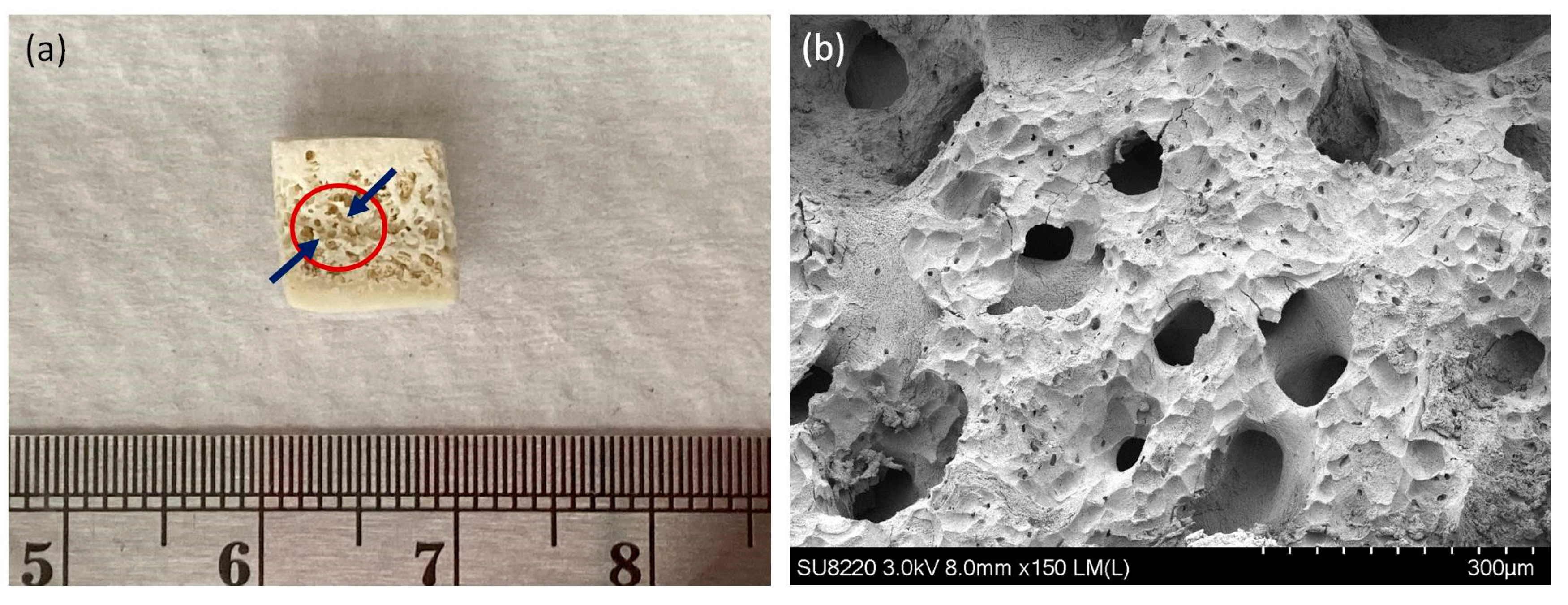
4.8. Generation of Procaine Mandibular Bone Block by Decellularization
4.9. The Morphology of Osteoblasts Attached on Bone Block
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone—A systematic review and meta-analysis. Eur. J. Oral Implantol. 2014, 7 (Suppl. 2), S219–S234. [Google Scholar]
- Kassim, B.; Ivanovski, S.; Mattheos, N. Current perspectives on the role of ridge (socket) preservation procedures in dental implant treatment in the aesthetic zone. Aust. Dent. J. 2014, 59, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Araujo, M.G.; Simion, M.; Osteology Consensus, G. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implant. Res. 2012, 23 (Suppl. 5), 80–82. [Google Scholar] [CrossRef] [PubMed]
- Barndt, P.; Zhang, H.; Liu, F. Immediate loading: From biology to biomechanics. Report of the Committee on Research in fixed Prosthodontics of the American Academy of fixed Prosthodontics. J. Prosthet. Dent. 2015, 113, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Stafford, G.L. Different loading times for dental implants—No clinically important differences? Evid. Based Dent. 2013, 14, 109–110. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Wang, X.; Zhang, Q.; Yang, W.; Zhu, W.; Zhao, K. Immediate versus early loading of flapless placed dental implants: A systematic review. J. Prosthet. Dent. 2014, 112, 760–769. [Google Scholar] [CrossRef]
- Valeria De Risi, M.C. Gianluca Vittorini, Alice Mannocci, Massimo De Sanctis. Alveolar ridge preservation techniques: A systemic review and meta-analysis of histological and histomorphometrical data. Clin. Oral Implant. Res. 2013, 1–19. [Google Scholar]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Iolascon, L.F.G.; Di Pietro, G.; Capaldo, A.; Luciano, F.; Gimigliano, F. Bone quality and bone strength: Benefits of the bone-forming approach. Clin. Cases Miner. Bone Metab. 2014, 11, 20–24. [Google Scholar] [CrossRef]
- Subhajit Das, J.C.C. Osteoporosis—A current view of pharmacological prevention and treatment. Drug Design Dev. Ther. 2013, 7, 435–448. [Google Scholar]
- Conserva, E.; Pisciotta, A.; Borghi, F.; Nasi, M.; Pecorini, S.; Bertoni, L.; de Pol, A.; Consolo, U.; Carnevale, G. Titanium Surface Properties Influence the Biological Activity and FasL Expression of Craniofacial Stromal Cells. Stem Cells Int. 2019, 2019, 4670560. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion implantation on human bone cell interaction with titanium. Biomaterials 2005, 26, 4717–4727. [Google Scholar] [CrossRef]
- Zacchetti, G.; Dayer, R.; Rizzoli, R.; Ammann, P. Systemic treatment with strontium ranelate accelerates the filling of a bone defect and improves the material level properties of the healing bone. Biomed Res. Int. 2014, 2014, 549785. [Google Scholar] [CrossRef]
- Karakan, N.C.; Akpinar, A.; Goze, F.; Poyraz, O. Investigating the Effects of Systemically Administered Strontium Ranelate on Alveolar Bone Loss Histomorphometrically and Histopathologically on Experimental Periodontitis in Rats. J. Periodontol. 2017, 88, e24–e31. [Google Scholar] [CrossRef]
- Tsai, T.T.; Tai, C.L.; Ho, N.Y.; Lai, P.L.; Fu, T.S.; Niu, C.C.; Chen, L.H.; Chen, W.J. Effects of Strontium Ranelate on Spinal Interbody Fusion Surgery in an Osteoporotic Rat Model. PLoS ONE 2017, 12, e0167296. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y. Cardiac concerns associated with strontium ranelate. Expert Opin. Drug Saf. 2014, 13, 1209–1213. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Brandi, M.L.; Cannata-Andia, J.; Cooper, C.; Cortet, B.; Feron, J.M.; Genant, H.; Palacios, S.; Ringe, J.D.; Rizzoli, R. The position of strontium ranelate in today’s management of osteoporosis. Osteoporos. Int. 2015, 26, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, J.; Li, Z.; Sajjan, S.; O’Regan, C.; Modi, A.; Sazonov, V. History of cardiovascular events and cardiovascular risk factors among patients initiating strontium ranelate for treatment of osteoporosis. Int. J. Womens Health 2015, 7, 913–918. [Google Scholar]
- Zapolski, T.; Wysokinski, A. Current views on the interaction between the treatment of osteoporosis and cardiovascular diseases. Wiad. Lek. 2016, 69, 665–674. [Google Scholar]
- Park, J.W.; Kang, D.G.; Hanawa, T. New bone formation induced by surface strontium-modified ceramic bone graft substitute. Oral Dis. 2016, 22, 53–61. [Google Scholar] [CrossRef]
- Santocildes-Romero, M.E.; Crawford, A.; Hatton, P.V.; Goodchild, R.L.; Reaney, I.M.; Miller, C.A. The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses. J. Tissue Eng. Regen. Med. 2015, 9, 619–631. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018. [Google Scholar] [CrossRef]
- Masalskas, B.F.; Martins Junior, W.; Leoni, G.B.; Faloni, A.P.S.; Marcaccini, A.M.; Silva Sousa, Y.T.C.; Castro-Raucci, L.M.S. Local delivery of strontium ranelate promotes regeneration of critical size bone defects filled with collagen sponge. J. Biomed. Mater. Res. A 2018, 106, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Suleimenova, D.; Hashimi, S.M.; Li, M.; Ivanovski, S.; Mattheos, N. Gene expression profiles in guided bone regeneration using combinations of different biomaterials: A pilot animal study. Clin. Oral Implant. Res. 2017, 28, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin. Oral Implant. Res. 2015, 27, e206–e214. [Google Scholar] [CrossRef]
- Nahass, H.E.; Din, N.N.E.; Nasry, S.A. The Effect of Strontium Ranelate Gel on Bone Formation in Calvarial Critical Size Defects. Open Access Maced. J. Med. Sci. 2017, 5, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Lai, Z.T.; Hong, D.W.; Chu, I.M.; Lai, P.L. Controlled release of strontium through neutralization reaction within a methoxy(polyethylene glycol)-polyester hydrogel. J. Appl. Biomater. Funct. Mater. 2017, 15, e162–e169. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; Kedia, G.; Bhat, A.; Law, R.V.; Radecka, I.; Hill, R.G. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J. R. Soc. Interface 2012. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Yang, Y.; Fang, Z.; Chen, X.; Wang, Y.; Kang, J.; Qu, X.; Yuan, W.; Dai, K.; et al. Osteoinductivity and Antibacterial Properties of Strontium Ranelate-Loaded Poly(Lactic-co-Glycolic Acid) Microspheres With Assembled Silver and Hydroxyapatite Nanoparticles. Front. Pharmacol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Munad, J. Al-Duliamy, N.H.G. Omar, A.; Kader, Bashar, H.; Abdullah, Enhancement of orthodontic anchorage and retention by the local injection of strontium: An experimental study in rats. Saudi Dent. J. 2015, 27, 22–29. [Google Scholar]
- Marie, P.J.; Garba, M.T.; Hott, M.; Miravet, L. Effect of low doses of stable strontium on bone metabolism in rats. Min. Electrolyte Metab 1985, 11, 5–13. [Google Scholar]
- Morohashi, T.; Sano, T.; Yamada, S. Effects of strontium on calcium metabolism in rats. I. A distinction between the pharmacological and toxic doses. Jpn. J. Pharmacol. 1994, 64, 155–162. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. The effects of strontium on skeletal development in zebrafish embryo. J. Trace Elem Med. Biol. 2013, 27, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.B.; Bertassoli, B.M.; Sousa, C.A.; Albergaria, J.D.; de Paula, R.S.; Jorge, E.C. Effects of strontium ranelate treatment on osteoblasts cultivated onto scaffolds of trabeculae bovine bone. J. Bone Miner. Metab. 2018, 36, 73–86. [Google Scholar] [CrossRef]
- Fatemeh, D.R.; Ebrahimi Shahmabadi, H.; Abedi, A.; Alavi, S.E.; Movahedi, F.; Koohi Moftakhari Esfahani, M.; Zadeh Mehrizi, T.; Akbarzadeh, A. Polybutylcyanoacrylate nanoparticles and drugs of the platinum family: Last status. Indian J. Clin. Biochem. 2014, 29, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Lin, S. Effect of surfactant on fabrication and characterization of paclitaxel-loaded polybutylcyanoacrylate nanoparticulate delivery systems. J. Pharm. Pharmacol. 2003, 55, 895–902. [Google Scholar] [CrossRef]
- Lu, B.; Feng, J.F.; Yang, X.C. Human recombinant interferon-alpha 2a polybutylcyanoacrylate sustained release lyophilized nanospheres for liver-targeting. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 1–4. [Google Scholar] [PubMed]
- Yang, Y.X.; Zhu, L.; He, X.; Bao, D.Y.; Bao, X. Antitumor activity of mitoxantrone-nanosphere against murine liver tumor H22. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 68–70. [Google Scholar]
- Gao, H.; Wang, J.Y.; Shen, X.Z.; Deng, Y.H.; Zhang, W. Preparation of magnetic polybutylcyanoacrylate nanospheres encapsulated with aclacinomycin A and its effect on gastric tumor. World J. Gastroenterol. 2004, 10, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Kolter, M.; Ott, M.; Hauer, C.; Reimold, I.; Fricker, G. Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo. J. Control. Release 2015, 197, 165–179. [Google Scholar] [CrossRef]
- Lissarrague, M.H.; Fascio, M.L.; Goyanes, S.; D’Accorso, N.B. Acrylic bone cements: The role of nanotechnology in improving osteointegration and tunable mechanical properties. J. Biomed. Nanotechnol. 2014, 10, 3536–3557. [Google Scholar] [CrossRef]
- Bagad, M.; Khan, Z.A. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int. J. Nanomed. 2015, 10, 3921–3935. [Google Scholar]
- Lin, M.H.; Chung, C.Y.; Chen, K.T.; Yeh, J.C.; Lee, T.H.; Lee, M.H.; Lee, I.N.; Huang, W.C.; Yang, J.T. Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs. Int. J. Mol. Sci. 2019, 20, 182. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Chen, M.; Xu, Y.; Ping, J.; Chen, W.; Liang, W. Recent developments in strontium-based biocomposites for bone regeneration. J. Artif. Organs 2020, 23, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Catalano, A.; Santoro, D.; Lasco, A.; Benvenga, S. Effects of strontium ranelate on markers of cardiovascular risk in postmenopausal osteoporotic women. Endocrine 2016, 53, 305–312. [Google Scholar] [CrossRef]
- Ali, M.S.; Berencsi, K.; Marinier, K.; Deltour, N.; Perez-Guthann, S.; Pedersen, L.; Rijnbeek, P.; Lapi, F.; Simonetti, M.; Reyes, C.; et al. Comparative cardiovascular safety of strontium ranelate and bisphosphonates: A multi-database study in 5 EU countries by the EU-ADR Alliance. Osteoporos. Int. 2020, 31, 2425–2438. [Google Scholar] [CrossRef]
- Chung, C.Y.; Yang, J.T.; Kuo, Y.C. Polybutylcyanoacrylate nanoparticles for delivering hormone response element-conjugated neurotrophin-3 to the brain of intracerebral hemorrhagic rats. Biomaterials 2013, 34, 9717–9727. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Quade, M.; Vater, C.; Schlootz, S.; Bolte, J.; Langanke, R.; Bretschneider, H.; Gelinsky, M.; Goodman, S.B.; Zwingenberger, S. Strontium enhances BMP-2 mediated bone regeneration in a femoral murine bone defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 174–182. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Jiang, Y.; Zhou, W.; Liu, S.; Liu, Y.; Rausch-Fan, X.; Liu, Z. Strontium ion attenuates lipopolysaccharide-stimulated proinflammatory cytokine expression and lipopolysaccharide-inhibited early osteogenic differentiation of human periodontal ligament cells. J. Periodontal Res. 2018, 53, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Berksoy Hayta, S.; Durmus, K.; Altuntas, E.E.; Yildiz, E.; Hisarciklio, M.; Akyol, M. The reduction in inflammation and impairment in wound healing by using strontium chloride hexahydrate. Cutan. Ocul. Toxicol. 2018, 37, 24–28. [Google Scholar] [CrossRef]
- Wan, B.; Wang, R.; Sun, Y.; Cao, J.; Wang, H.; Guo, J.; Chen, D. Building Osteogenic Microenvironments With Strontium-Substituted Calcium Phosphate Ceramics. Front. Bioeng. Biotechnol. 2020, 8, 591467. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Alvarez-Perez, M.; Giugliano, D.; Zeppetelli, S.; Ambrosio, L. Properties of carbon nanotube-dispersed Sr-hydroxyapatite injectable material for bone defects. Regen. Biomater. 2016, 3, 13–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
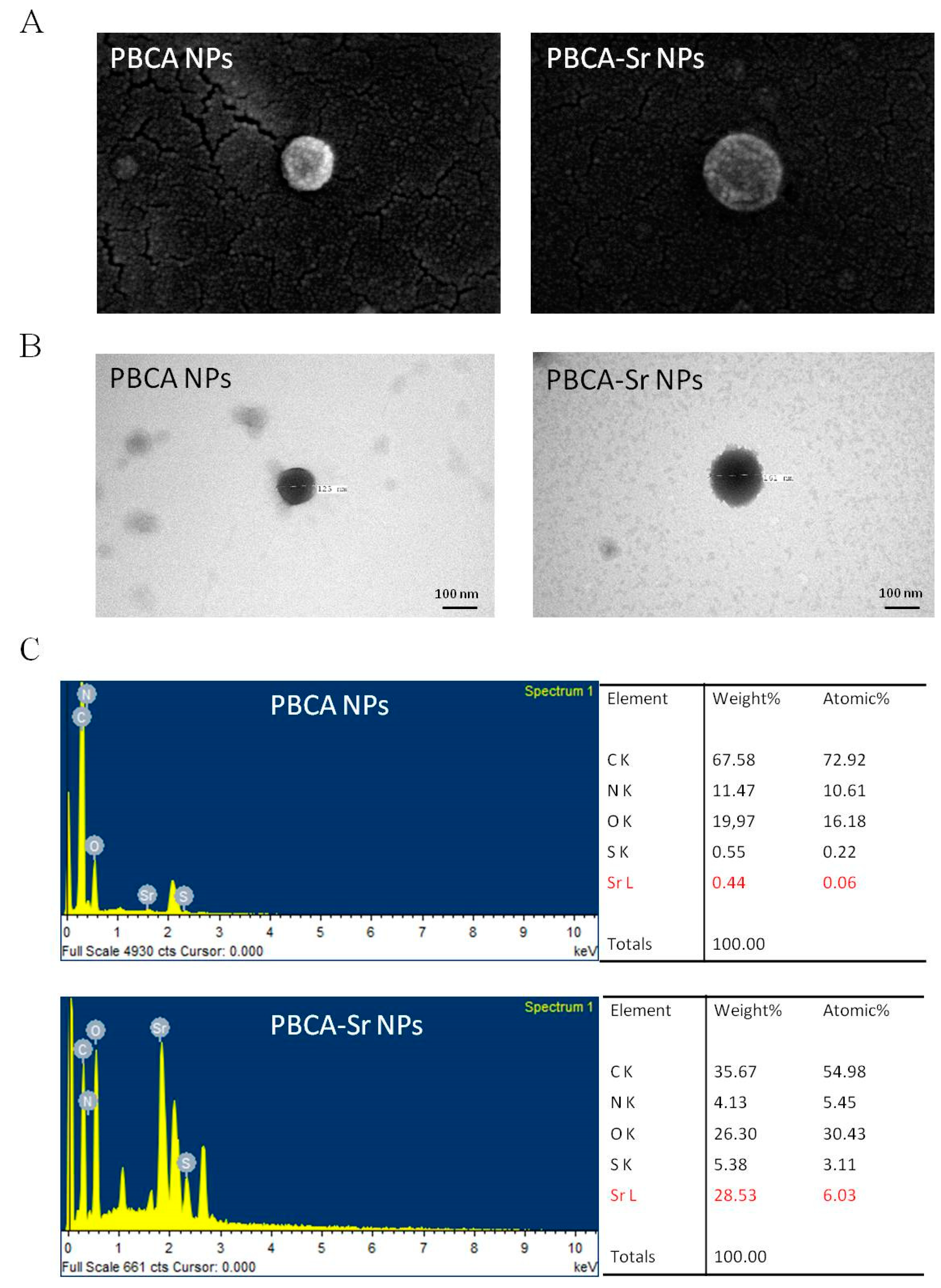
| Sample | Dav (nm) | Zeta Potential (mV) | PDI | EE Sr (%) |
|---|---|---|---|---|
| PBCA NPs | 126.1 ± 0.8 | −1.93 ± 0.24 | 0.044 ± 0.009 | - |
| PBCA-Sr NPs | 166.7 ± 2.3 | −1.15 ± 0.28 | 0.241 ± 0.019 | 90.04 ± 3.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-C.; Chung, C.-Y.; Chiu, C.-H.; Lin, M.H.-C.; Yang, J.-T. The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation. Int. J. Mol. Sci. 2021, 22, 4873. https://doi.org/10.3390/ijms22094873
Chang L-C, Chung C-Y, Chiu C-H, Lin MH-C, Yang J-T. The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation. International Journal of Molecular Sciences. 2021; 22(9):4873. https://doi.org/10.3390/ijms22094873
Chicago/Turabian StyleChang, Li-Ching, Chiu-Yen Chung, Chun-Hui Chiu, Martin Hsiu-Chu Lin, and Jen-Tsung Yang. 2021. "The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation" International Journal of Molecular Sciences 22, no. 9: 4873. https://doi.org/10.3390/ijms22094873
APA StyleChang, L.-C., Chung, C.-Y., Chiu, C.-H., Lin, M. H.-C., & Yang, J.-T. (2021). The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation. International Journal of Molecular Sciences, 22(9), 4873. https://doi.org/10.3390/ijms22094873






