Interaction between the Renin–Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis
Abstract
1. Introduction
2. Results
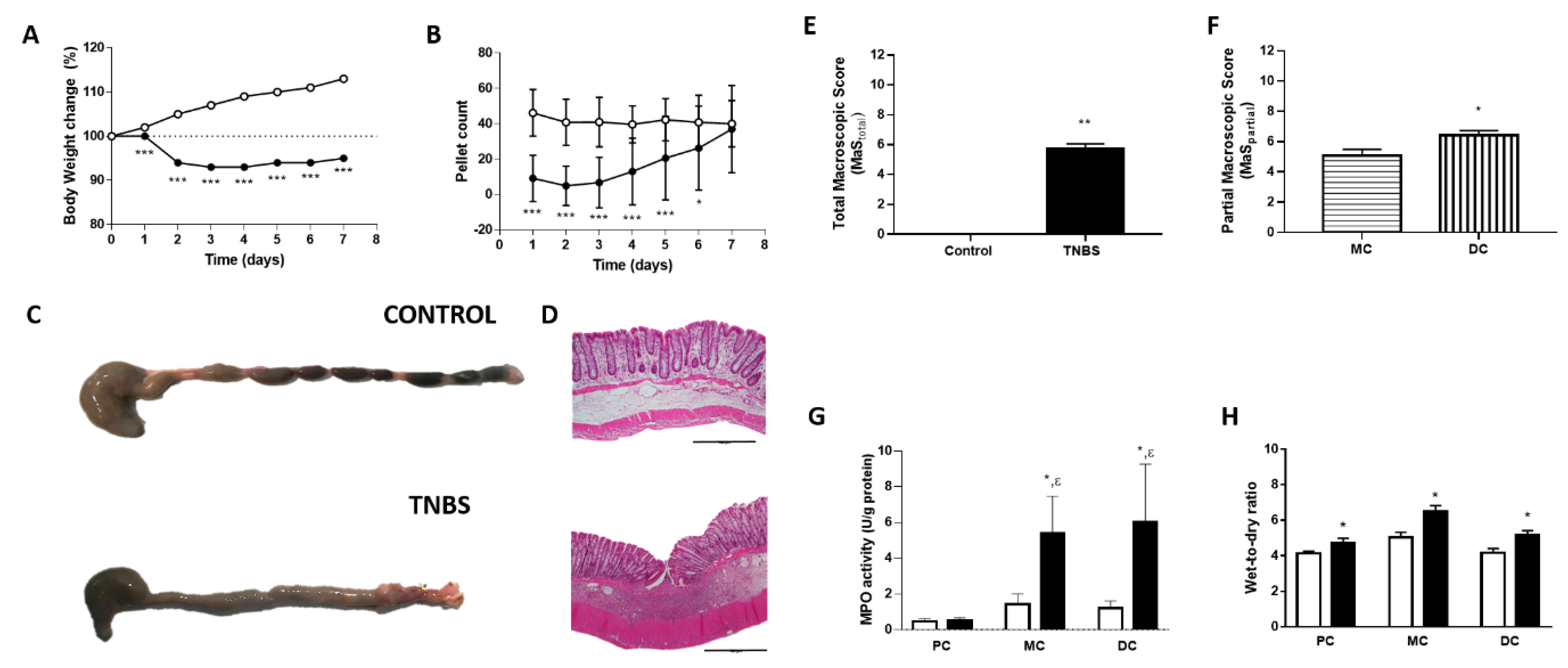
2.1. Assessment of Colitis
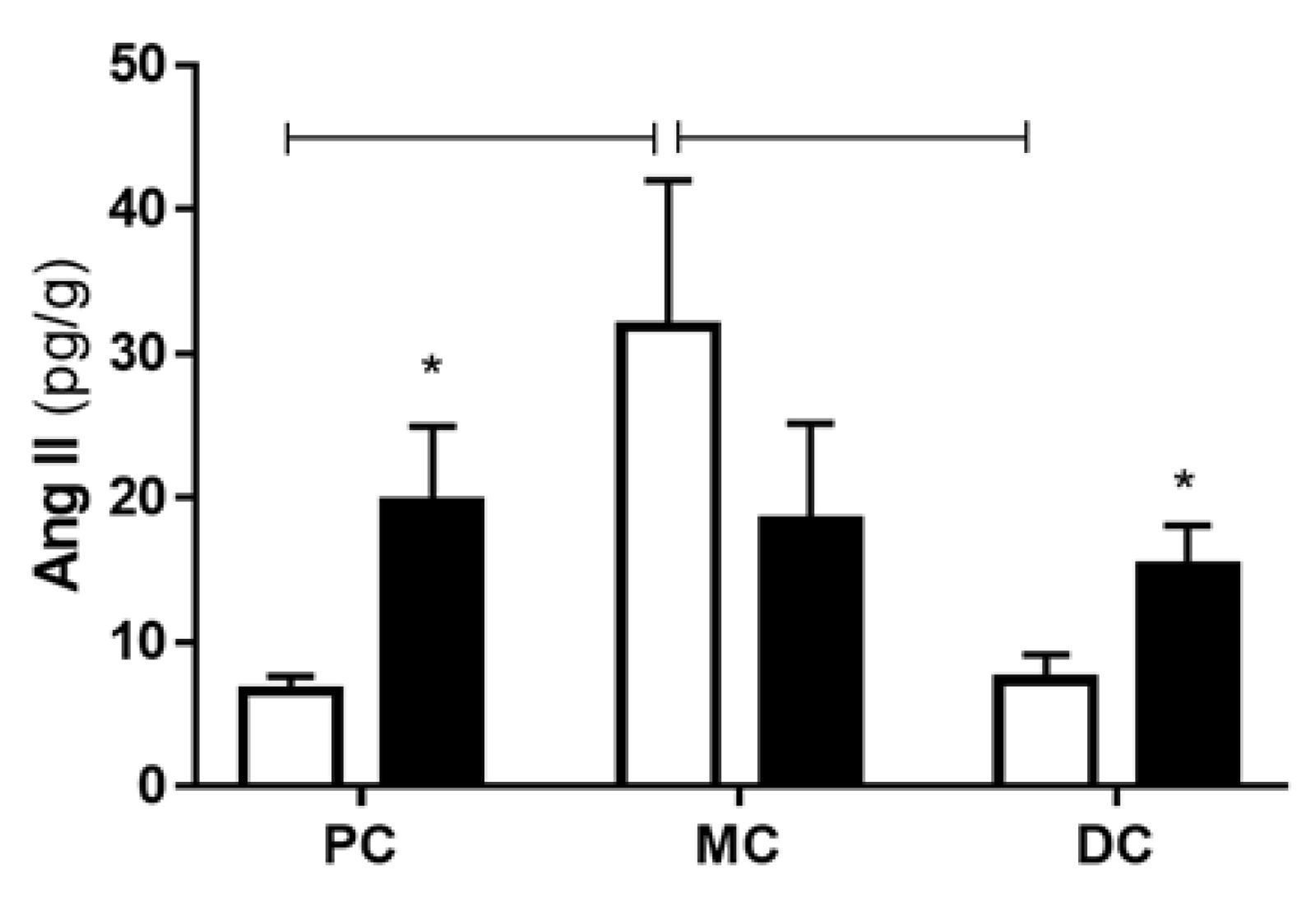
2.2. Colonic Portions Reactivity to Angiotensin II and Peptide Levels
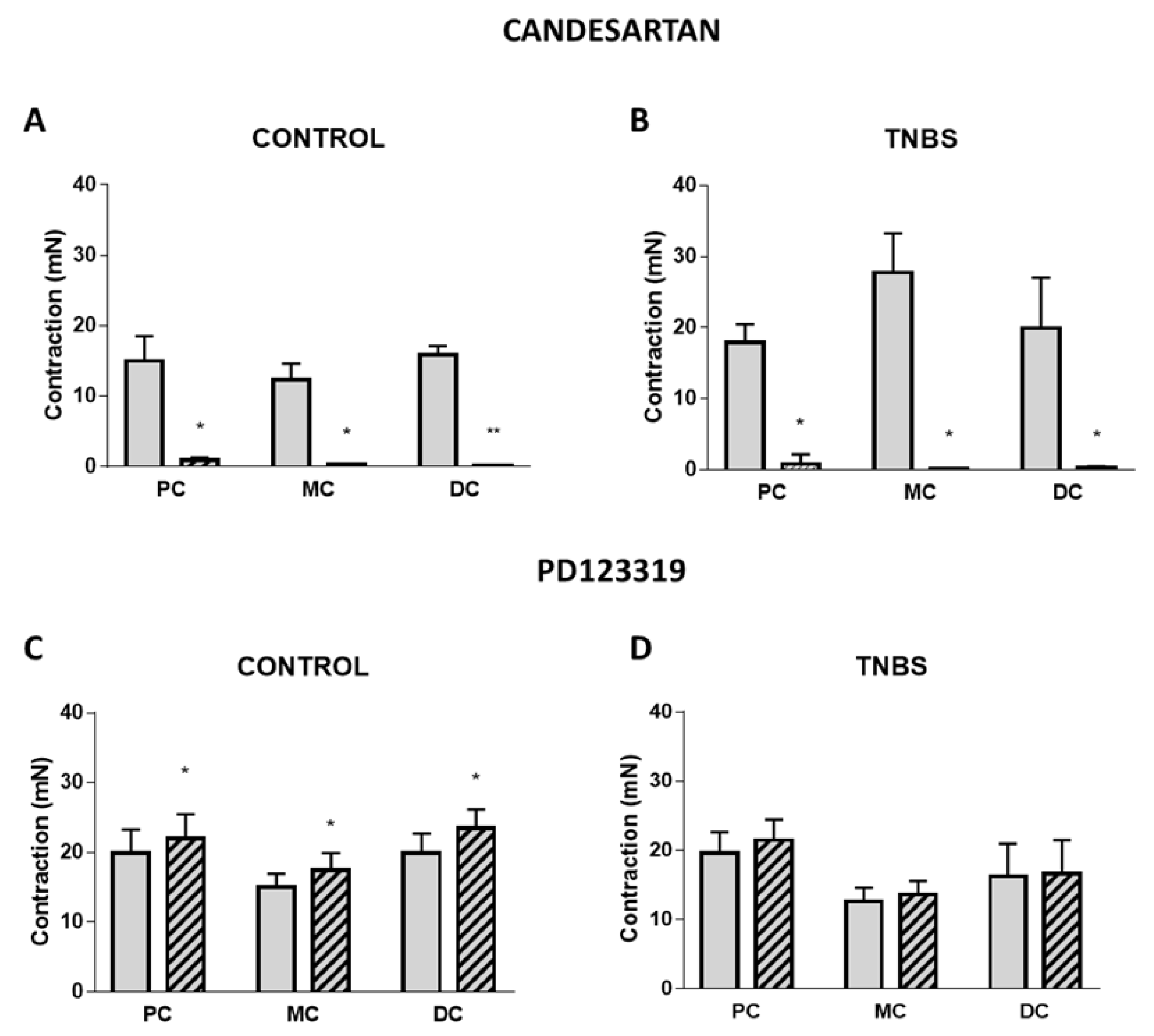
2.3. Role and Expression of Angiotensin II Type I and Type II Receptors
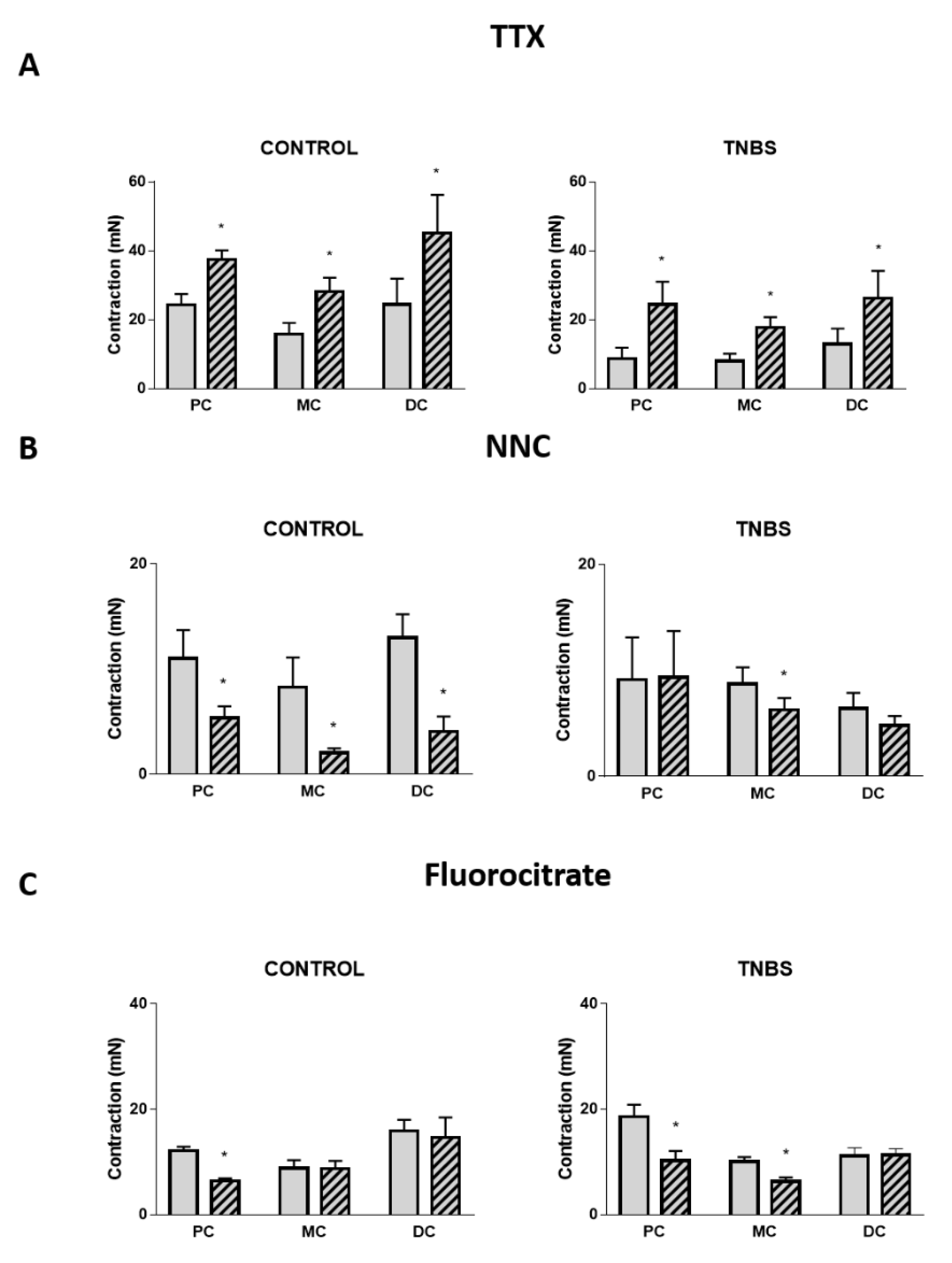
2.4. Neural and Non-Neural Cells Mediating Angiotensin II-Induced Colonic Contraction
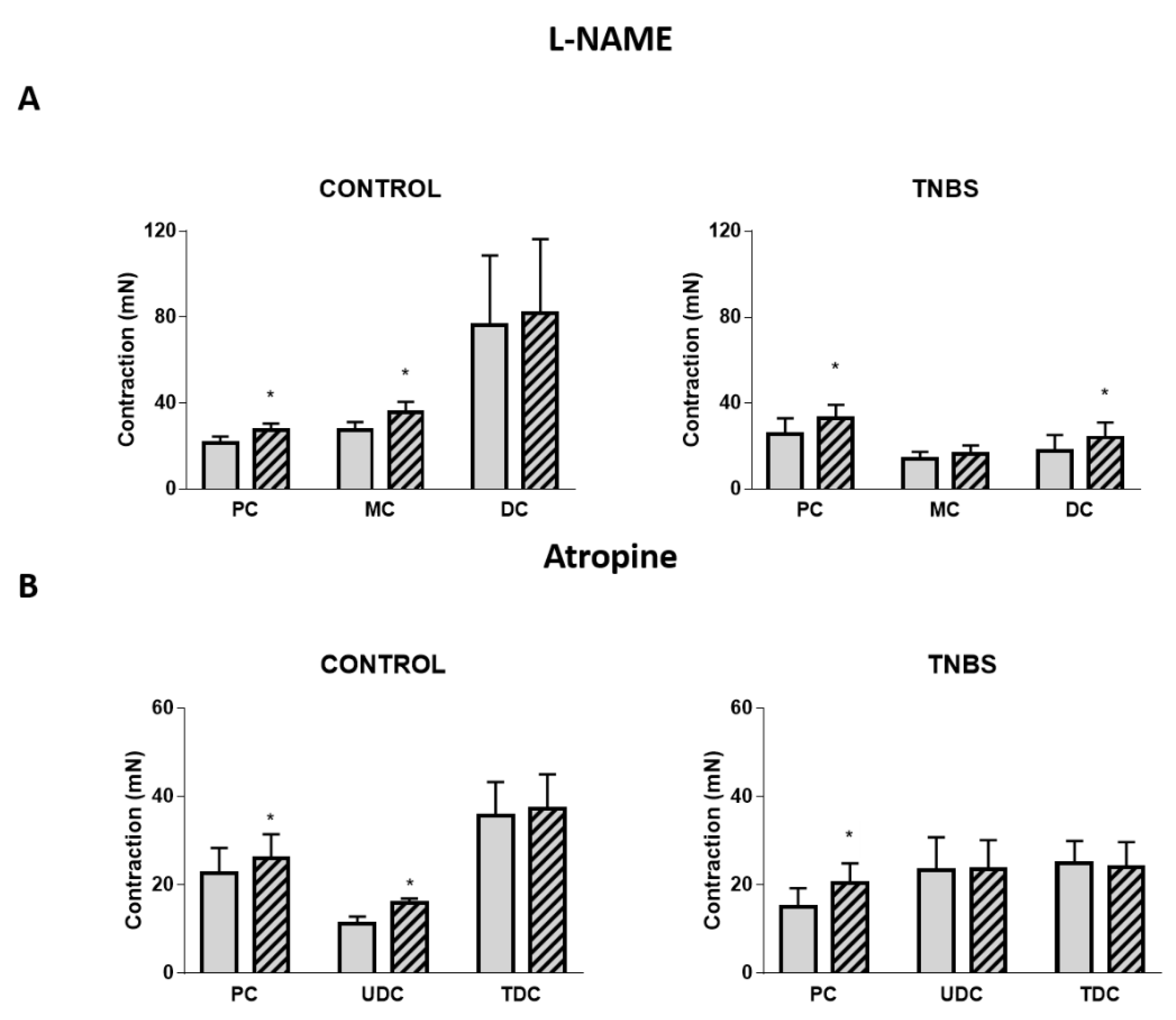
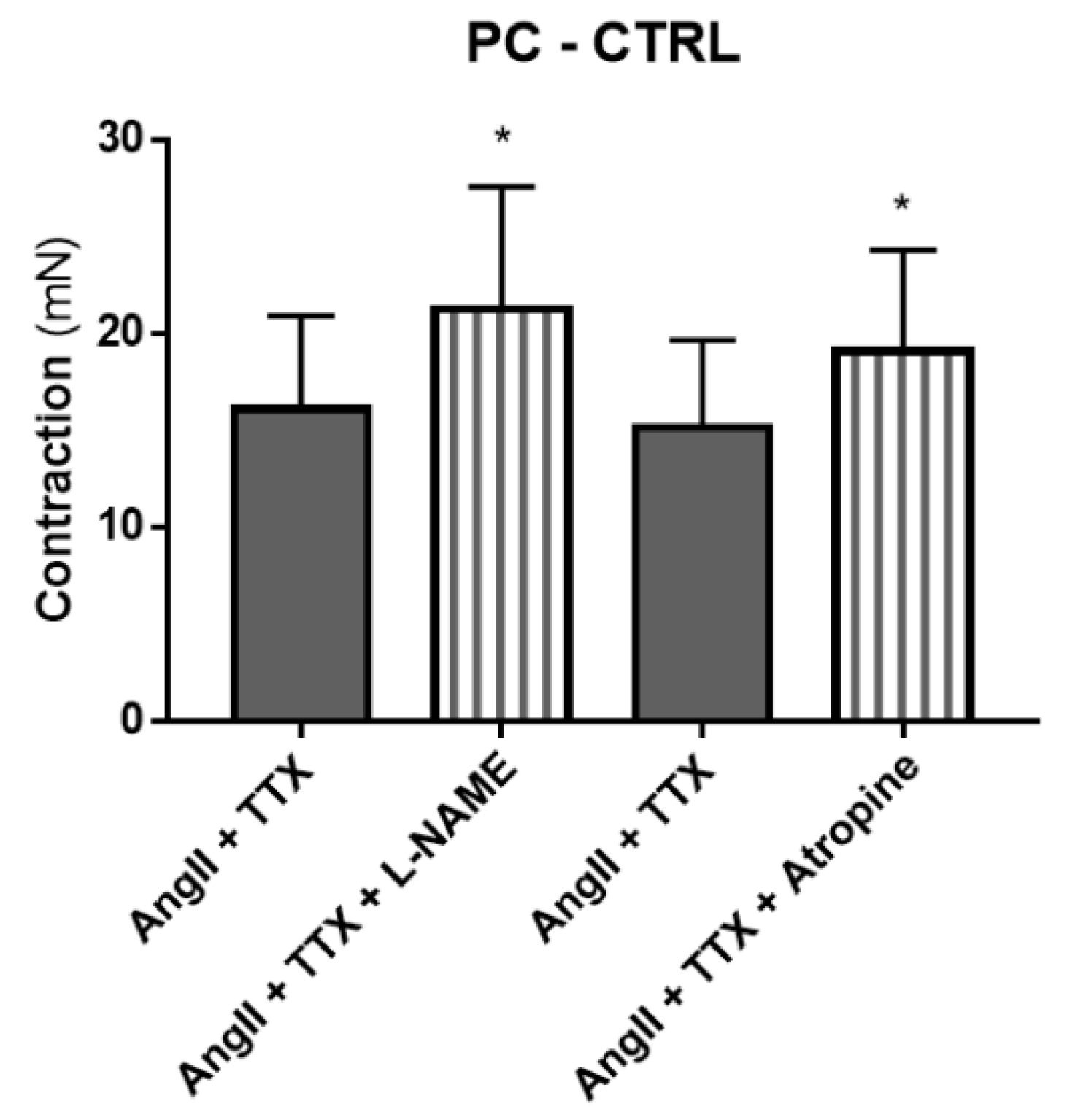
2.5. Mediators Involved in Angiotensin II-Induced Colonic Contraction
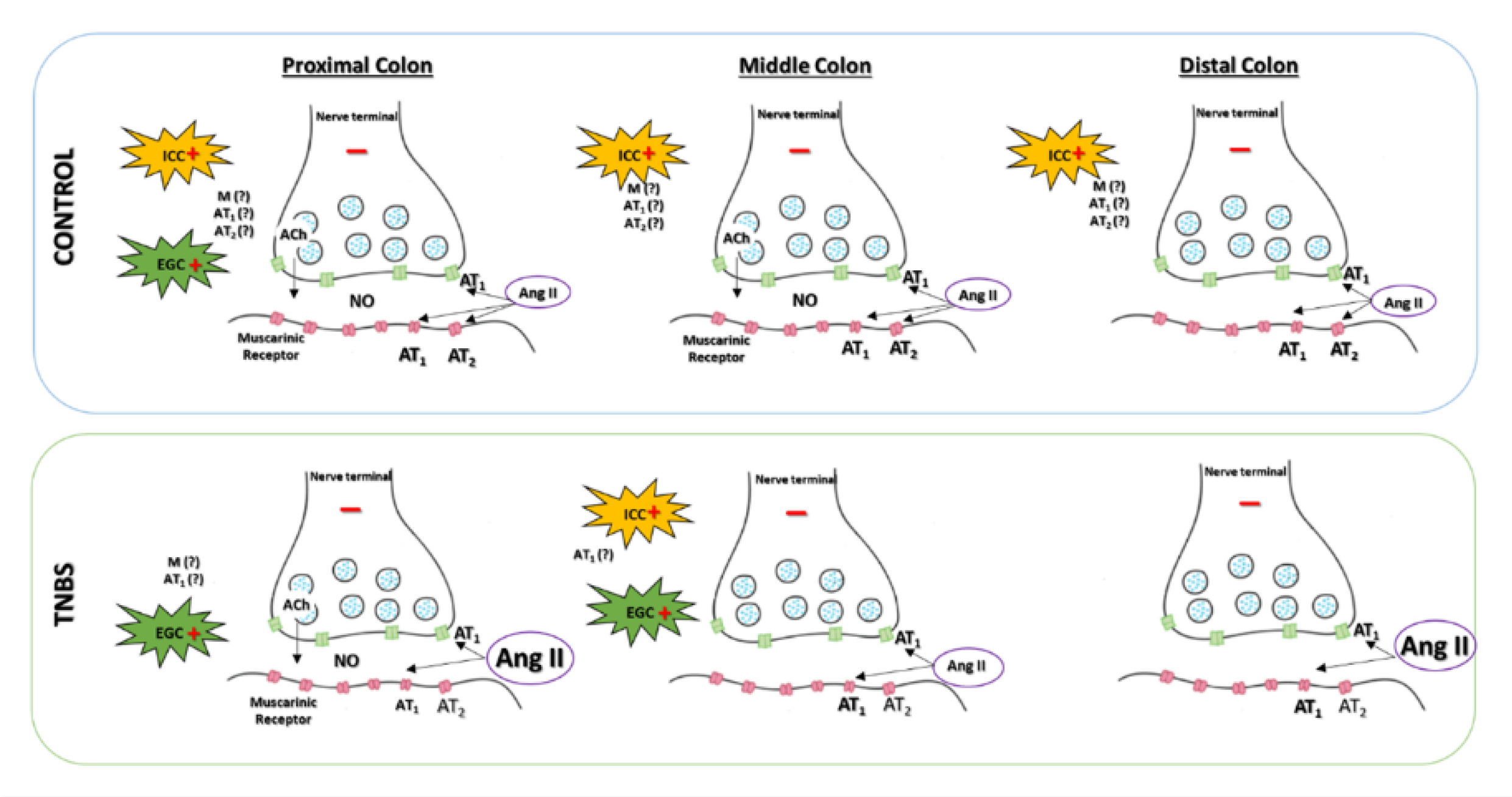
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Colitis Induction
4.3. Evaluation of Macroscopic and Microscopic Inflammatory Damage
4.4. Myeloperoxidase Assay
4.5. Characterization of the Reactivity of Colonic Tissue to Angiotensin II
4.6. Extraction and Quantification of Angiotensin II Levels in Colonic Samples from PC, MC and DC
4.7. Expression of Angiotensin II Type I and II Receptors in Colonic Samples from PC, MC and DC by qPCR
4.8. Data Collection and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; E Underwood, F.; Tang, W.; I Benchimol, E.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Strohl, M.; Gonczi, L.; Kurt, Z.; Bessissow, T.; Lakatos, P.L. Quality of care in inflammatory bowel diseases: What is the best way to better outcomes? World J. Gastroenterol. 2018, 24, 2363–2372. [Google Scholar] [CrossRef]
- Büsch, K.; Sonnenberg, A.; Bansback, N. Impact of Inflammatory Bowel Disease on Disability. Curr. Gastroenterol. Rep. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nat. Cell Biol. 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Leitner, G.C.; Vogelsang, H. Pharmacological- and non-pharmacological therapeutic approaches in inflammatory bowel disease in adults. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 5–20. [Google Scholar] [CrossRef]
- Capettini, L.S.; Montecucco, F.; Mach, F.; Stergiopulos, N.; Santos, R.; da Silva, R.F. Role of renin-angiotensin system in inflammation, immunity and aging. Curr. Pharm. Des. 2012, 18, 963–970. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, T.; He, L.; Dougherty, U.; Chen, L.; Adhikari, S.; Alpert, L.; Zhou, G.; Lui, W.; Wang, J.; et al. Activation of the Renin-Angiotensin System Promotes Colitis Development. Sci. Rep. 2016, 6, 27552. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, L.; Li, F.; Zhang, H.; Li, Q.; Liu, H.; Fu, S.; Li, C.; Zhang, X.; Wang, J.; et al. Generation of outbred Ace2 knockout mice by RNA transfection of TALENs displaying colitis reminiscent pathophysiology and inflammation. Transgenic Res. 2014, 24, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Uchida, Y.; Yamamoto, K.; Nakayama, T.; Cheng, X.W.; Matsushita, T.; Nakamura, S.; Murohara, T.; Takeshita, K. Angiotensin receptor blocker irbesartan reduces stress-induced intestinal inflammation via AT1a signaling and ACE2-dependent mechanism in mice. Brain Behav. Immun. 2018, 69, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Serio, R. PD123319, angiotensin II type II receptor antagonist, inhibits oxidative stress and inflammation in 2, 4-dinitrobenzene sulfonic acid-induced colitis in rat and ameliorates colonic contractility. Inflammopharmacology 2020, 28, 187–199. [Google Scholar] [CrossRef]
- Garg, M.; Angus, P.W.; Burrell, L.M.; Herath, C.; Gibson, P.R.; Lubel, J.S. Review article: The pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2012, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Ewert, S.; Spak, E.; Olbers, T.; Johnsson, E.; Edebo, A.; Fändriks, L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol. 2006, 188, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mastropaolo, M.; Zizzo, M.G.; Mule, F.; Serio, R. Angiotensin II contractile effects in mouse colon: Role for pre- and post-junctional AT 1A receptors. Acta Physiol. 2013, 207, 337–345. [Google Scholar] [CrossRef]
- Hawcock, A.B.; Barnes, J.C. Pharmacological characterization of the contractile responses to angiotensin analogues in guinea-pig isolated longitudinal muscle of small intestine. Br. J. Pharmacol. 1993, 108, 1150–1155. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Auteri, M.; Amato, A.; Caldara, G.; Nuzzo, D.; Di Carlo, M.; Serio, R. Angiotensin II type II receptors and colonic dysmotility in 2,4-dinitrofluorobenzenesulfonic acid-induced colitis in rats. Neurogastroenterol. Motil. 2017, 29, e13019. [Google Scholar] [CrossRef] [PubMed]
- Valès, S.; Touvron, M.; Van Landeghem, L. Enteric glia: Diversity or plasticity? Brain Res. 2018, 1693, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Aguiar, J.M.; Bon-Frauches, A.C.; Gomes, A.L.; Verissimo, C.P.; Aguiar, D.P.; Matias, D.; Thomasi, B.B.; Gomes, A.S.; Brito, G.A.; Moura-Neto, V. The enteric glia: Identity and functions. Glia 2015, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Christofi, F.L. Are We Close to Targeting Enteric Glia in Gastrointestinal Diseases and Motility Disorders? Gastroenterology 2018, 155, 245–251. [Google Scholar] [CrossRef]
- Nasser, Y.; Fernandez, E.; Keenan, C.M.; Ho, W.; Oland, L.D.; Tibbles, L.A.; Schemann, M.; Macnaughton, W.K.; Rühl, A.; Sharkey, K.A. Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Liver Physiol. 2006, 291, G912–G927. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Chow, A.K.; Gulbransen, B.D. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am. J. Physiol. Liver Physiol. 2017, 312, G145–G152. [Google Scholar] [CrossRef]
- Bradley, J.S., Jr.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461. [Google Scholar] [CrossRef]
- Lies, B.; Beck, K.; Keppler, J.; Saur, D.; Groneberg, D.; Friebe, A. Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J. Physiol. 2015, 593, 4589–4601. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Beck, K.; Friebe, A.; Voussen, B. Nitrergic signaling via interstitial cells of Cajal and smooth muscle cells influences circular smooth muscle contractility in murine colon. Neurogastroenterol. Motil. 2018, 30, e13300. [Google Scholar] [CrossRef]
- Antonioli, L.; Colucci, R.; Pellegrini, C.; Giustarini, G.; Tuccori, M.; Blandizzi, C.; Fornai, M. The role of purinergic pathways in the pathophysiology of gut diseases: Pharmacological modulation and potential therapeutic applications. Pharmacol. Ther. 2013, 139, 157–188. [Google Scholar] [CrossRef]
- Bernardini, N.; Segnani, C.; Ippolito, C.; de Giorgio, R.; Colucci, R.; Faussone-Pellegrini, M.S.; Chiarugi, M.; Campani, D.; Castagna, M.; Mattii, L.; et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J. Cell. Mol. Med. 2011, 16, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rumessen, J.J.; Vanderwinden, J.-M.; Horn, T. Crohn’s disease of the colon: Ultrastructural changes in submuscular interstitial cells of Cajal. Cell Tissue Res. 2010, 343, 421–428. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tanović, A.; Fernández, E.; Jiménez, M. Alterations in intestinal contractility during inflammation are caused by both smooth muscle damage and specific receptor-mediated mechanisms. Croat. Med. J. 2006, 47, 318–326. [Google Scholar] [PubMed]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Jaszewski, R.; Tolia, V.; Ehrinpreis, M.N.; Bodzin, J.H.; Peleman, R.R.; Korlipara, R.; Weinstock, J.V. Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology 1990, 98, 1543–1548. [Google Scholar] [CrossRef]
- Matsuda, T.; Suzuki, J.; Furuya, K.; Masutani, M.; Kawakami, Y. Serum angiotensin I-converting enzyme is reduced in Crohn’s disease and ulcerative colitis irrespective of genotype. Am. J. Gastroenterol. 2001, 96, 2705–2710. [Google Scholar] [CrossRef]
- Mastropaolo, M.; Zizzo, M.G.; Auteri, M.; Caldara, G.; Liotta, R.; Mule, F.; Serio, R. Activation of angiotensin II type 1 receptors and contractile activity in human sigmoid colon in vitro. Acta Physiol. 2015, 215, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Duarte, M.; Pinto-Rodrigues, T.; Menezes-Pinto, D.; Esteves-Monteiro, M.; Gonçalves-Monteiro, S.; Capas-Peneda, S.; Magro, F.; Dias-Pereira, P.; Morato, M.; Duarte-Araújo, M. TNBS-induced colitis in Rattus norgevicus: A categorization proposal. Exp. Anim. 2021. [Google Scholar] [CrossRef] [PubMed]
- Katada, K.; Yoshida, N.; Suzuki, T.; Okuda, T.; Mizushima, K.; Takagi, T.; Ichikawa, H.; Naito, Y.; Cepinskas, G.; Yoshikawa, T. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm. Res. 2008, 57, 84–91. [Google Scholar] [CrossRef]
- Mizushima, T.; Sasaki, M.; Ando, T.; Wada, T.; Tanaka, M.; Okamoto, Y.; Ebi, M.; Hirata, Y.; Murakami, K.; Mizoshita, T. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G255–G266. [Google Scholar] [CrossRef]
- Morato, M.; Pinho, D.; Sousa, T.; Guimarães, S.; Moura, D.; Albino-Teixeira, A. Pre- and postjunctional effects of angiotensin II in hypertension due to adenosine receptor blockade. Eur. J. Pharmacol. 2006, 531, 209–216. [Google Scholar] [CrossRef]
- Balt, J.C.; Mathy, M.-J.; Nap, A.; Pfaffendorf, M.; Van Zwieten, P.A. Prejunctional and Postjunctional Inhibitory Actions of Eprosartan and Candesartan in the Isolated Rabbit Mesenteric Artery. J. Cardiovasc. Pharmacol. 2002, 40, 50–57. [Google Scholar] [CrossRef][Green Version]
- Moura, D.; Pinheiro, H.; Paiva, M.Q.; Guimaraes, S. Prejunctional effects of angiotensin II and bradykinin in the heart and blood vessels. J. Auton. Pharmacol. 1999, 19, 321–325. [Google Scholar] [CrossRef]
- Mota, A.; Guimarães, S. Interaction between α2-autoreceptors and receptors mediating the effects of angiotensin II and bradykinin in the heart of newborn rats. Eur. J. Pharmacol. 2002, 453, 265–270. [Google Scholar] [CrossRef]
- Shetty, S.S.; Delgrande, D. Differential inhibition of the prejunctional actions of angiotensin II in rat atria by valsartan, irbesartan, eprosartan, and losartan. J. Pharmacol. Exp. Ther. 2000, 294, 179–186. [Google Scholar] [PubMed]
- Cox, S.L.; Trendelenburg, A.U.; Starke, K. Prejunctional angiotensin receptors involved in the facilitation of noradrenaline release in mouse tissues. Br. J. Pharmacol. 1999, 127, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Rump, L.; Schuster, M.; Wilde, K.; Schollmeyer, P. Modulation of noradrenaline release from rat cortical kidney slices: Effects of angiotensin I and II. Br. J. Clin. Pharmacol. 1990, 30, 168S–170S. [Google Scholar] [CrossRef][Green Version]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Kinoshita, K.; Horiguchi, K.; Fujisawa, M.; Kobirumaki, F.; Yamato, S.; Hori, M.; Ozaki, H. Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem. Cell Biol. 2006, 127, 41–53. [Google Scholar] [CrossRef]
- Altdorfer, K.; Bagameri, G.; Donath, T.; Feher, E. Nitric oxide synthase immunoreactivity of interstitial cells of Cajal in experimental colitis. Inflamm. Res. 2002, 51, 569–571. [Google Scholar] [CrossRef]
- Vieira, C.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Silva, I.; Marques, P.; Correia-De-Sá, P. Post-inflammatory Ileitis Induces Non-neuronal Purinergic Signaling Adjustments of Cholinergic Neurotransmission in the Myenteric Plexus. Front. Pharmacol. 2017, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Jabbur, S.J.; El-Kak, F.H.; Nassar, C.F. The enteric nervous system—an overview. Med. Res. Rev. 1988, 8, 459–469. [Google Scholar] [CrossRef]
- Shiina, T.; Gurung, Y.B.; Suzuki, Y.; Takewaki, T.; Shimizu, Y. Alteration of neuromuscular transmissions in the hamster colon following the resolution of TNBS-induced colitis. J. Physiol. Sci. 2013, 63, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.-S.; La, J.-H.; Kim, T.-W.; Yang, I.-S. Alteration of nitrergic neuromuscular transmission as a result of acute experimental colitis in rat. J. Vet. Sci. 2006, 7, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Morrison, K.J.; Vanhoutte, P.M. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br. J. Pharmacol. 1994, 112, 519–524. [Google Scholar] [CrossRef]
- Tangsucharit, P.; Takatori, S.; Zamami, Y.; Goda, M.; Pakdeechote, P.; Kawasaki, H.; Takayama, F. Muscarinic acetylcholine receptor M1 and M3 subtypes mediate acetylcholine-induced endothelium-independent vasodilatation in rat mesenteric arteries. J. Pharmacol. Sci. 2016, 130, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.D.; Kendig, D.M.; Al-Qudah, M.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Role of various kinases in muscarinic M3 receptor-mediated contraction of longitudinal muscle of rat colon. J. Smooth Muscle Res. 2014, 50, 103–119. [Google Scholar] [CrossRef]
- Barnes, J.; Costall, B.; Horovitz, Z.; Naylor, R. Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res. 1989, 491, 136–143. [Google Scholar] [CrossRef]
- Harrington, A.M.; Peck, C.J.; Liu, L.; Burcher, E.; Hutson, J.M.; Southwell, B.R. Localization of muscarinic receptors M1R, M2R and M3R in the human colon. Neurogastroenterol. Motil. 2010, 22, 999–1008. [Google Scholar] [CrossRef]
- Kodama, Y.; Iino, S.; Shigemasa, Y.; Suzuki, H. Properties of acetylcholine-induced relaxation of smooth muscle isolated from the proximal colon of the guinea-pig. J. Smooth Muscle Res. 2010, 46, 185–200. [Google Scholar] [CrossRef]
- Porter, A.J.; A Wattchow, D.; Brookes, S.J.H.; Costa, M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut 2002, 51, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kortezova, N.; Shikova, L.; Papasova, M. Participation of M1 receptors in NO pathway in cat ileum. Acta Physiol. Pharmacol. Bulg. 1998, 23, 1–4. [Google Scholar] [PubMed]
- Li, M.; Johnson, C.P.; Adams, M.B.; Sarna, S.K. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am. J. Physiol. Liver Physiol. 2002, 283, G544–G552. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136. [Google Scholar]
- Green, C.L.; Ho, W.; Sharkey, K.A.; McKay, D.M. Dextran sodium sulfate-induced colitis reveals nicotinic modulation of ion transport via iNOS-derived NO. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G706–G714. [Google Scholar] [CrossRef]
- Lundberg, S.; Holst, M.; Hellström, P.M. Expression of iNOS mRNA associated with suppression of colonic contraction in rat colitis. Acta Physiol. 2006, 187, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.; Remião, F.; Duarte, J.; Ferreira, R.; Navarro, A.S.; Bastos, M.; Carvalho, F. P-glycoprotein induction: An antidotal pathway for paraquat-induced lung toxicity. Free Radic. Biol. Med. 2006, 41, 1213–1224. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Mechanism of the Oxidation of 3,5,3‘,5‘-Tetramethylbenzidine by Myeloperoxidase Determined by Transient- and Steady-State Kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.L.; Ruben, P.C. Interaction between Voltage-Gated Sodium Channels and the Neurotoxin, Tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar]
- Paulsen, R.E.; Contestabile, A.; Villani, L.; Fonnum, F. An In Vivo Model for Studying Function of Brain Tissue Temporarily Devoid of Glial Cell Metabolism: The Use of Fluorocitrate. J. Neurochem. 1987, 48, 1377–1385. [Google Scholar] [CrossRef]
- Kraus, R.L.; Li, Y.; Gregan, Y.; Gotter, A.L.; Uebele, V.N.; Fox, S.V.; Doran, S.M.; Barrow, J.C.; Yang, Z.-Q.; Reger, T.S.; et al. In Vitro Characterization of T-Type Calcium Channel Antagonist TTA-A2 and In Vivo Effects on Arousal in Mice. J. Pharmacol. Exp. Ther. 2010, 335, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Costagli, C.; Lapi, D.; Del Seppia, C.; Federighi, G.; Balzan, S.; Colantuoni, A.; Iervasi, G.; Scuri, R. Renin-Angiotensin System Responds to Prolonged Hypotensive Effect Induced by Mandibular Extension in Spontaneously Hypertensive Rats. Front. Physiol. 2018, 9, 1613. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
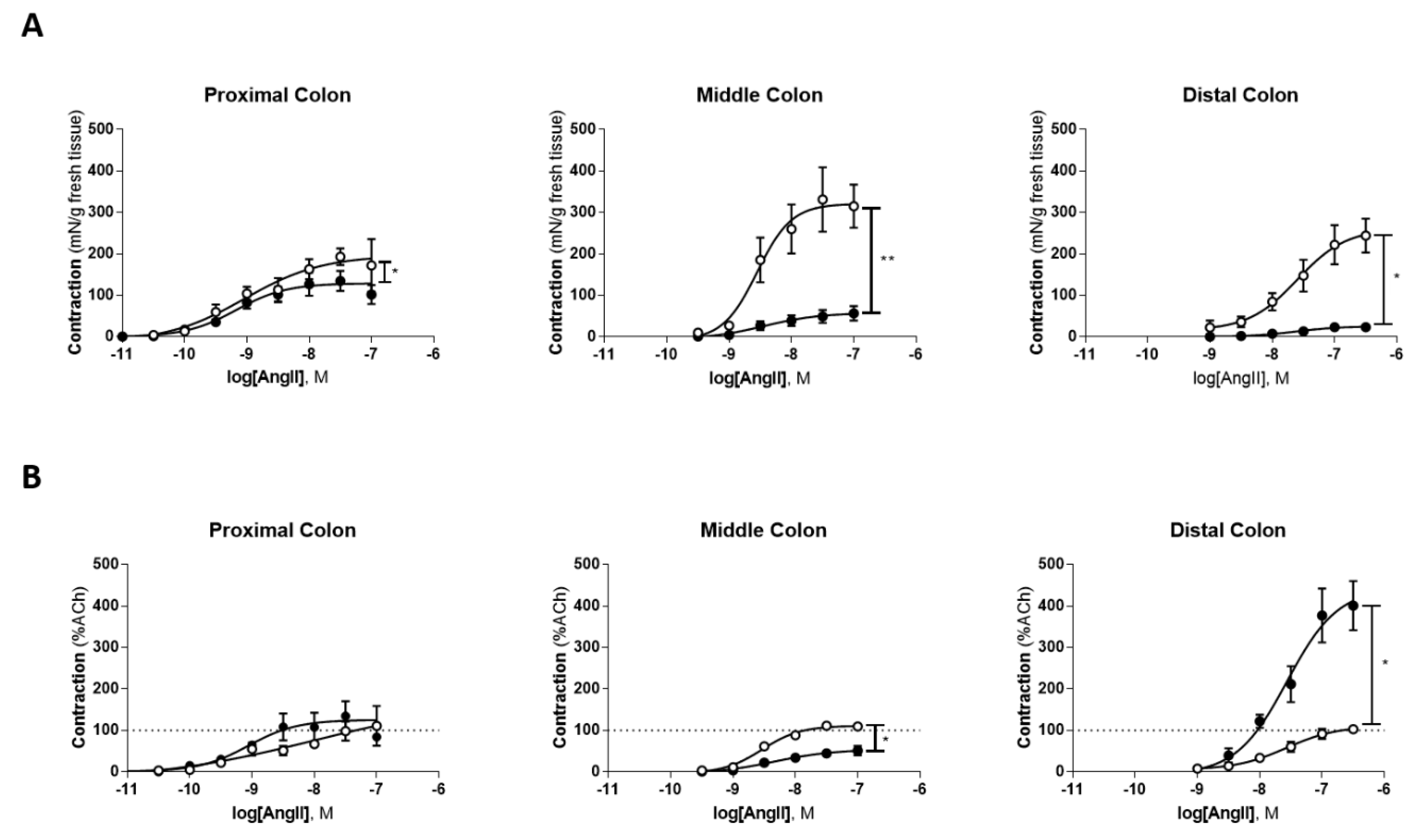

| PC | MC | DC | |
|---|---|---|---|
| Emax (mN/g) | Emax (mN/g) | Emax (mN/g) | |
| Control | 199.7 ± 27.38 | 334.3 ± 61.72 | 329.8 ± 51.59 |
| TNBS | 103.9 ± 25.05 * | 43.76 ± 12.86 ** | 27.12 ± 7.295 * |
| Emax (% ACh) | Emax (% ACh) | Emax (% ACh) | |
| Control | 100.2 ± 21.42 | 115.2 ± 5.009 | 142.1 ± 18.98 |
| TNBS | 102.8 ± 29.95 | 43.11 ± 9.518 * | 467.3 ± 80.05 * |
| Macroscopic Score (MaS) | Adhesions to Adjacent Organs | Colon Thickness | Mucosal Edema/ Hyperemia | Mucosal Ulcers | |
|---|---|---|---|---|---|
| Score | 0 | Absent | Normal | Absent | Absent |
| 1 | Mild/focal | Mild | Mild | Single | |
| 2 | Moderate/zonal | Moderate | Moderate | At one site | |
| 3 | Severe/diffuse | Marked increased | Severe | At more sites | |
| Genes of Interest | ||
| AT1 receptor | Forward | 5′-TCTGGATAAATCACACAACCCTC-3′ |
| Reverse | 5′-GAGTTGGTCTCAGACACTATTCG-3′ | |
| AT2 receptor | Forward | 5′-CTGGCAAGCATCTTATGTAGTTC-3′ |
| Reverse | 5′-ACAAGCATTCACACCTAAGTATTC-3′ | |
| Housekeeping Genes | ||
| Papbn-1 | Forward | 5′-TATGGTGCGACAGCAGAAGA-3′ |
| Reverse | 5′-TATGCAAACCCTTTGGGATG-3′ | |
| Hprt-1 | Forward | 5′-CCCAGCGTCGTGATTAGTGATG-3′ |
| Reverse | 5′-TTCAGTCCTGTCCATAATCAGTCC-3′ | |
| Hmbs | Forward | 5′-TCTAGATGGCTCAGATAGCATGCA-3′ |
| Reverse | 5′-TGGACCATCTTCTTGCTGAACA-3′ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Duarte, M.; Rodrigues-Pinto, T.; Sousa, T.; Faria, M.A.; Rocha, M.S.; Menezes-Pinto, D.; Esteves-Monteiro, M.; Magro, F.; Dias-Pereira, P.; Duarte-Araújo, M.; et al. Interaction between the Renin–Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. Int. J. Mol. Sci. 2021, 22, 4836. https://doi.org/10.3390/ijms22094836
Ferreira-Duarte M, Rodrigues-Pinto T, Sousa T, Faria MA, Rocha MS, Menezes-Pinto D, Esteves-Monteiro M, Magro F, Dias-Pereira P, Duarte-Araújo M, et al. Interaction between the Renin–Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. International Journal of Molecular Sciences. 2021; 22(9):4836. https://doi.org/10.3390/ijms22094836
Chicago/Turabian StyleFerreira-Duarte, Mariana, Tiago Rodrigues-Pinto, Teresa Sousa, Miguel A. Faria, Maria Sofia Rocha, Daniela Menezes-Pinto, Marisa Esteves-Monteiro, Fernando Magro, Patrícia Dias-Pereira, Margarida Duarte-Araújo, and et al. 2021. "Interaction between the Renin–Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis" International Journal of Molecular Sciences 22, no. 9: 4836. https://doi.org/10.3390/ijms22094836
APA StyleFerreira-Duarte, M., Rodrigues-Pinto, T., Sousa, T., Faria, M. A., Rocha, M. S., Menezes-Pinto, D., Esteves-Monteiro, M., Magro, F., Dias-Pereira, P., Duarte-Araújo, M., & Morato, M. (2021). Interaction between the Renin–Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. International Journal of Molecular Sciences, 22(9), 4836. https://doi.org/10.3390/ijms22094836








