Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models
Abstract
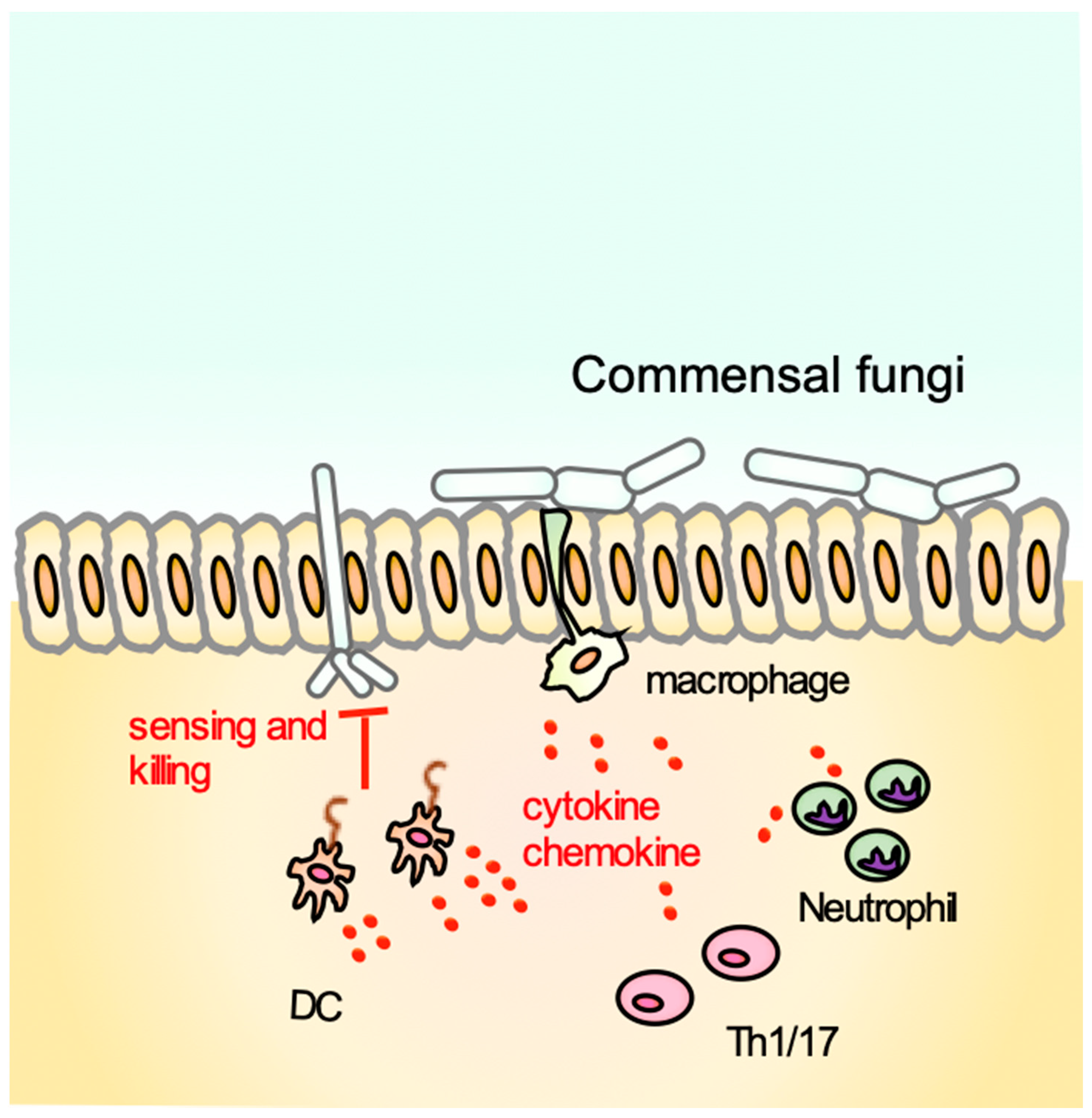
1. Introduction
2. Major Receptor
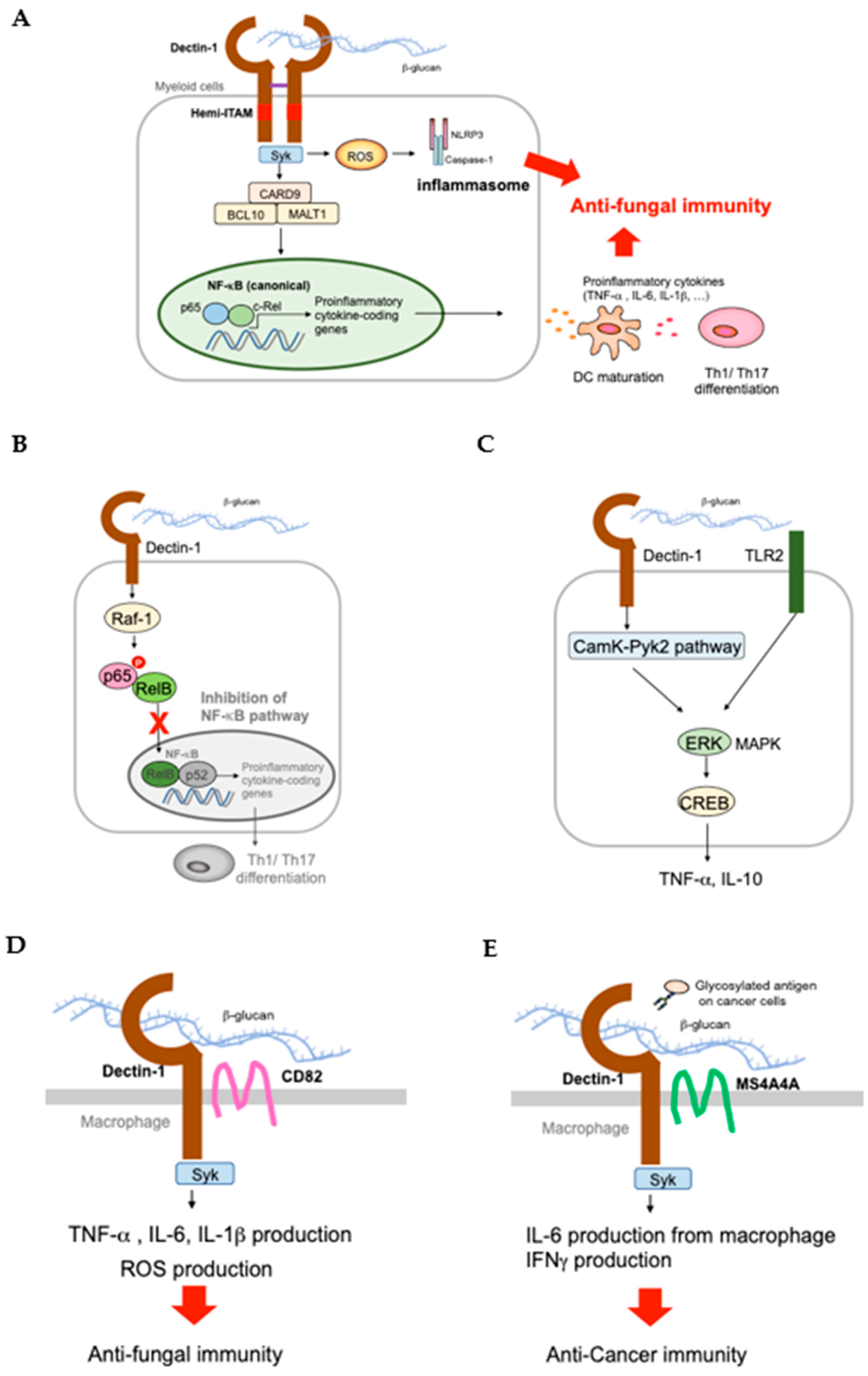
2.1. Dectin-1 and Signaling Cascade
2.2. Dectin-1 KO (Clec7a−/−) Mice
3. Alternative Receptors
3.1. CR3 KO Mice
3.2. EphA2 KO Mice
4. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMDC | bone marrow-derived dendritic cells |
| BMDM | bone marrow-derived macrophages |
| CaMK | calmodulin-dependent kinase II |
| CARD | caspase recruitment domain family member 9 |
| CBM | CARD9-BCL10-MALT1 |
| CLEC | C-type lectin receptor |
| CRD | carbohydrate recognition domain |
| CREB | cAMP-response element-binding protein |
| CR3 | complement receptor 3 |
| DC | dendritic cells |
| DCIR | dendritic cell immunoreceptor |
| DSS | dextran sulfate sodium |
| EphA2 | ephrin type-A receptor 2 |
| ITAM | immunoreceptor tyrosine-based activation motif |
| LacCer | lactosylceramide |
| MALT1 | mucosa-associated lymphoid tissue 1 |
| MAPK | mitogen-associated protein kinase |
| NaClO | sodium hypochlorite |
| NK | natural killer |
| OPC | oropharyngeal candidiasis |
| PAMP | pathogen associated molecular pattern |
| PRR | pathogen recognition receptor |
| PYK2 | proline-rich tyrosine kinase II |
| ROS | reactive oxygen species |
| RTK | receptor tyrosine kinase |
| SH2 | SRC homology 2 |
| SNP | single nucleotide polymorphism |
| SPG | Sparassis crispa glucan |
| STAT3 | signal transducer and activator of transcription 3 |
| TLR | toll-like receptor |
References
- Vetvicka, V.; Vetvicka, J. Glucan supplementation enhances the immune response againstan influenza challenge in mice. Ann. Transl. Med. 2015, 3, 22. [Google Scholar] [PubMed]
- Kuda, T.; Kosaka, M.; Hirano, S.; Kawahara, M.; Sato, M.; Kaneshima, T.; Nishizawa, M.; Takahashi, H.; Kimura, B. Effect of sodium-alginate and laminaran on Salmonella typhimurium infection in human enterocyte-like HT-29-Luc cells and balb/c mice. Carbohydr. Polym. 2015, 125, 113–119. [Google Scholar] [CrossRef]
- Bobek, P.; Galbavy, S. Effect of pleuran (β-glucan from Pleurotus ostreatus) on the antioxidant status of the organism and on dimethylhydrazine-induced precancerous lesions in rat colon. Br. J. Biomed. Sci. 2001, 58, 164–168. [Google Scholar]
- Shen, R.-L.; Wang, Z.; Dong, J.-L.; Xiang, Q.-S.; Liu, Y.-Q. Effects of oat soluble and insoluble β-glucan on 1,2-dimethylhydrazine-induced early colon carcinogenesis in mice. Food Agric. Immunol. 2016, 27, 657–666. [Google Scholar] [CrossRef]
- Desamero, M.J.; Kakuta, S.; Chambers, J.K.; Uchida, K.; Hachimura, S.; Takamoto, M.; Nakayama, J.; Nakayama, H.; Kyuwa, S. Orally administered brown seaweed-derived β-glucan effectively restrained development of gastric dysplasia in A4gnt KO mice that spontaneously develop gastric adenocarcinoma. Int. Immunopharmacol. 2018, 60, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Carbonero, E.R.; Cordova, M.M.; Baggio, C.H.; Santos, A.R.S.; Sassaki, G.L.; Cipriani, T.R.; Gorin, P.A.J.; Lacomini, M. Lactarius rufus (1->3), (1->6)-β-D-glucans: Structure, antinociceptive and anti-inflammatory effects. Carbohydr. Polym. 2013, 94, 1236–1291. [Google Scholar] [CrossRef]
- Silveira, M.L.L.; Smiderle, F.R.; Moraes, C.P.; Borato, D.G.; Baggio, C.H.; Ruthers, A.R.; Wisbeck, E.; Sassaki, G.L.; Cipriani, T.R.; Furlan, S.A.; et al. Structural characterization and anti-inflammatory activity of linear β-D-glucan isolated from Pleurotus sajor-caju. Carbohydr. Polym. 2014, 113, 588–596. [Google Scholar] [CrossRef]
- Snart, J.; Bibiloni, R.; Grayson, T.; Lay, C.; Zhang, H.; Allison, G.E.; Laverdiere, J.K.; Temelli, F.; Vasanthan, T.; Bell, R.; et al. Supplementation of the diet with high-viscosity beta-glucan results in enrichment for Lactobacilli in the rat cecum. Appl. Environ. Microbiol. 2006, 72, 1925–1931. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant activity of β-glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar]
- Sener, G.; Eksioglu-Demiralp, E.; Cetiner, M.; Ercan, F.; Yegen, B.C. β-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immunomodulatory effects. Eur. J. Pharmacol. 2006, 542, 170–178. [Google Scholar] [CrossRef]
- Yamanaka, D.; Tada, R.; Adachi, Y.; Ishibashi, K.-I.; Motoi, M.; Iwakura, Y.; Ohno, N. Agarius brasiliensis-derived β-glucans exert immunoenhaning effects via a dectin-1 –dependent pathway. Int. Immunopharmacol. 2012, 14, 311–319. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Jenkins, D.J.A.; Zdravkovic, U.; Wursch, P.; Vuksan, V. Depression of the glycemic index by high levels β-glucan fiber in two functional foods tested in type 3 diabetes. Eur. J. Clin. Nutr. 2002, 56, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Vetvicka, V. β-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef]
- Silva, V.O.; Moura, N.O.; de Oliveira, L.J.R.; Peconick, A.P.; Pereira, L.J. Promising effects of beta-glucans in metabolism and on the immune responses: Review article. Am. J. Immunol. 2017, 13, 62–72. [Google Scholar] [CrossRef]
- Samuelsen, A.B.C.; Schrezenmeir, J.; Knutsen, S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2014, 58, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Steimbach, L.; Borgmann, A.V.; Gomar, G.G.; Hoffmann, L.V.; Rutckeviski, R.; de Andrade, D.P.; Smiderle, F.R. Fungal beta-glucans as adjuvants for treating cancer patients- A systematic review of clinical trials. Clin. Nutr. 2020, S0261–S5614, 30650–30656. [Google Scholar]
- Figdor, C.G.; van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and langerhan cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Roger, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Wiliams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by decti-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507. [Google Scholar] [CrossRef]
- Mambula, S.S.; Sau, K.; Henneke, P.; Golenbock, D.T.; Levitz, S.M. Toll-like receptor (tlr) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 2002, 277, 39320–39326. [Google Scholar] [CrossRef]
- Capecchi, M.R. Gene targeting in mice: Functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 2005, 6, 507–512. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Saijo, S.; Fujikado, N.; Furuta, T.; Chung, S.-H.; Kotaki, H.; Seki, K.; Sudo, K.; Akira, A.; Adachi, Y.; Ohno, N.; et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williamns, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, K.; Shen, G.L.; Shikano, S.; Xu, S.; Ritter, R., 3rd; Kumamoto, T.; Edelbaum, D.; Morita, A.; Bergstresser, P.R.; Takashima, A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by substractive cDNA cloning. J. Biol. Chem. 2000, 275, 20157–20167. [Google Scholar] [CrossRef] [PubMed]
- Saijo, S.; Iwakura, Y. Dectin-1 and dectin-2 in innate immunity against fungi. Int. Immunol. 2011, 23, 467–472. [Google Scholar] [CrossRef]
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018. [Google Scholar] [CrossRef]
- Yokota, K.; Takashima, A.; Bergstresser, P.R.; Ariizumi, K. Identification of a human homologue of the dendritic cell-associated C-type lectin, dectin-1. Gene 2001, 272, 51–60. [Google Scholar] [CrossRef]
- Ariizumi, K.; Shen, G.-L.; Shikano, S.; Ritter, R., III; Zukas, P.; Edelbaum, D.; Morita, A.; Takashima, A. Cloning of a second dendritic-cell associated c-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 2000, 275, 11957–11963. [Google Scholar] [CrossRef]
- Willment, J.A.; Gordon, S.; Brown, G.D. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 2001, 276, 43818–43823. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef]
- Cohen-Kedar, S.; Baram, L.; Elad, H.; Brazowski, E.; Guzner-Gur, H.; Dotan, I. Human intestinal epithelial cells respond to β-glucans via dectin-1 and syk. Eur. J. Immunol. 2014, 44, 3729–3740. [Google Scholar] [CrossRef]
- Volman, J.L.; Mensink, R.P.; Buurman, W.A.; Onning, G.; Plat, J. The absence of functional dectin-1 on enterocytes may serve to prevent intestinal damage. Eur. J. Gastroenterol. Hepatol. 2020, 22, 88–94. [Google Scholar] [CrossRef]
- Czop, J.K.; Fearon, D.T.; Austen, K.F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J. Immunol. 1978, 120, 1132–1138. [Google Scholar]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67. [Google Scholar] [CrossRef]
- Hara, H.; Ishihara, C.; Takeuchi, A.; Imanishi, T.; Xue, L.; Morris, S.W.; Inui, M.; Takai, T.; Shibuya, A.; Saijo, S. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and toll-like receptors. Nat. Immunol. 2007, 8, 619. [Google Scholar] [CrossRef]
- Mocsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Microbiol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Herre, J.; Marshall, A.S.J.; Caron, E.; Edwards, A.D.; Williams, D.L.; Schweighoffer, E.; Tybulewicz, V.; Sousa, C.R.; Gordon, S.; Brown, G.D. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 2004, 104, 4038–4045. [Google Scholar] [CrossRef]
- Bertin, J.; Guo, Y.; Wang, L.; Srinivasula, S.M.; Jacobson, M.D.; Poyet, J.-L.; Merriam, S.; Du, M.-Q.; Dyer, M.J.S.; Robinson, K.E.; et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J. Biol. Chem. 2000, 275, 41082. [Google Scholar] [CrossRef]
- Gross, O.; Gewies, A.; Finger, K.; Schafer, M.; Sparwasser, T.; Peschel, C.; Forster, I.; Ruland, J. CARD9 controls a non-TLR signaling pathway for innate anti-fungal immunity. Nature 2006, 442, 651. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.M.; Geijtenbeek, T.B.H. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009, 10, 203. [Google Scholar] [CrossRef]
- Drummond, R.A.; Saijo, S.; Iwakura, Y.; Brown, G.D. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 2011, 41, 276. [Google Scholar] [CrossRef]
- Dennehy, K.M.; Willment, J.A.; Williams, D.L.; Brown, G.D. Reciprocal regulation of IL-23 and IL-12 following co-activation of dectin-1 and TLR signaling pathways. Eur. J. Immunol. 2009, 39, 1379–1386. [Google Scholar] [CrossRef]
- Kelly, E.K.; Wang, L.; Ivashkiv, L.B. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J. Immunol. 2010, 184, 5545–5552. [Google Scholar] [CrossRef]
- Tam, J.M.; Reedy, J.L.; Lukason, D.P.; Kuna, S.G.; Acharya, M.; Khan, N.S.; Negoro, P.E.; Xu, S.; Ward, R.A.; Feldman, M.B.; et al. Tetraspanin CD82 organizes dectin-1 into signaling domains to mediate cellular responses to Candida albicans. J. Immunol. 2019, 202, 3256–3266. [Google Scholar] [CrossRef]
- Mattiola, I.; Tomay, F.; De Pizzol, M.; Silva-Gomes, R.; Savino, B.; Gulic, T.; Doni, A.; Lonardi, S.; Boutet, M.A.; Nerviani, A.; et al. The macrophage tetraspananMS4A4A enhances dectin-1-dependent NK cell-mediated resistance to metastasis. Nat. Immunol. 2019, 20, 1012–1022. [Google Scholar] [CrossRef]
- Nakamura, K.; Kinjo, T.; Saijo, S.; Miyamoto, A.; Adachi, Y.; Ohno, N.; Fujita, J.; Kaku, M.; Iwakura, Y.; Kawakami, K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol. Immunol. 2007, 51, 1115–1119. [Google Scholar] [CrossRef]
- Werner, J.L.; Metz, A.E.; Horn, D.; Schoeb, T.R.; Hewitt, M.M.; Schwiebert, L.M.; Faro-Trindade, I.; Brown, G.D.; Steele, C. Requisite role for dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009, 182, 4938. [Google Scholar] [CrossRef] [PubMed]
- Galès, A.; Conduché, A.; Bernad, J.; Lefevre, L.; Olagnier, D.; Béraud, M.; Martin-Blondel, G.; Linas, M.D.; Auwerx, J.; Coste, A.; et al. PPARγ controls Dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog. 2010, 6, e1000714. [Google Scholar] [CrossRef]
- Rivera, A.; Hohl, T.M.; Collins, N.; Leiner, I.; Gallegos, A.; Saijo, S.; Coward, J.W.; Iwakura, Y.; Pamer, E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011, 208, 369–381. [Google Scholar] [CrossRef]
- Bhatia, S.; Fei, M.; Yarlagadda, M.; Qi, Z.; Akira, S.; Saijo, S.; Iwakura, Y.; van Rooijen, N.; Gibson, G.A.; St Croix, C.M.; et al. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS ONE 2011, 6, e15943. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interaction between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Viriyakosol, S.; Jimenez, M.D.P.; Gurney, M.A.; Ashbaugh, M.E.; Fierer, J. Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio 2013, 4, e00597-12. [Google Scholar] [CrossRef]
- Loures, F.V.; Araújo, E.F.; Feriotti, C.; Bazan, S.; Costa, T.A.; Brown, G.D.; Calich, V.L. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary Paracoccidioidomycosis. J. Infect. Dis. 2014, 210, 762–773. [Google Scholar] [CrossRef]
- Tang, C.E.; Kamiya, T.; Liu, Y.; Kadoki, M.; Kakuta, S.; Oshima, K.; Hattori, M.; Takeshita, K.; Kanai, T.; Saijo, S.; et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing Lactobaccilus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 2015, 18, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Lin, C.-Y.; Wu, -Y.; Chen, W.-Y.; Chu, C.-L.; Brown, G.D.; Chuu, C.-P.; Wu-Hsieh, B.A. CR3 and dectin-1 collaborate in macrophage cytokine response through association on lipid rafts and activation of Syk-JNK-AP-1 pathway. PLoS Pathog. 2015, 11, e1004985. [Google Scholar] [CrossRef]
- Higashino-Kameda, M.; Yabe-Wada, T.; Matsuba, S.; Takeda, K.; Anzawa, K.; Mochizuki, T.; Makimura, K.; Saijo, S.; Iwakura, Y.; Toga, H.; et al. A critical role of Dectin-1 in hypersensitivity pneumonitis. Inflamm. Res. 2016, 65, 235–244. [Google Scholar] [CrossRef]
- Yoshikawa, F.S.Y.; Yabe, R.; Iwakura, Y.; de Almeida, S.R.; Saijo, S. Dectin-1 and dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis. Innate Immun. 2016, 22, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J.; Galles, K.; Filutowicz, H.; Warner, T.; Evans, M.; et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Zou, Z.; Qiu, X.R.; Hou, W.T.; Zhang, Y.; Fang, W.; Chen, Y.L.; Wang, Y.D.; Jiang, Y.Y.; Shen, H.; et al. The critical role of Dectin-1 in host controlling systemic Candida krusei infection. Am. J. Transl. Res. 2019, 11, 721–732. [Google Scholar]
- Brandhorst, T.T.; Wüthrich, M.; Finkel-Jimenez, B.; Warner, T.; Klein, B.S. Exploiting type 3 complement receptor for TNF-α suppression, immune evasion, and progressive pulmonary fungal infection. J. Immunol. 2004, 173, 7444–7453. [Google Scholar] [CrossRef]
- Li, X.; Utomo, A.; Cullere, X.; Choi, M.M.; Milner, D.A., Jr.; Venkatesh, D.; Yun, S.-H.; Mayadas, T.N. The β-glucan receptor dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe 2011, 10, 603–615. [Google Scholar] [CrossRef]
- Soloviev, D.A.; Jawhara, S.; Fonzi, W.A. Regulation of innate immune response to Candida albicans infections by αMβ2-Pra1p interaction. Infect. Immun. 2011, 79, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.M., Jr.; Vareechon, C.; Cowden, S.; Cobb, B.A.; Latgé, J.P.; Momany, M.; Pearlman, E. Fungal antioxidant pathways promote survival against neutrophils during infection. J. Clin. Investig. 2012, 122, 2482–2498. [Google Scholar] [CrossRef]
- Sun, D.; Sun, P.; Li, H.; Zhang, M.; Liu, G.; Strickland, A.B.; Chen, Y.; Fu, Y.; Xu, J.; Yosri, M.; et al. Fungal dissemination is limited by liver macrophage filtration of the blood. Nat. Commun. 2019, 10, 4566. [Google Scholar] [CrossRef]
- Clark, H.L.; Abbondante, S.; Minns, M.S.; Greenberg, E.N.; Sun, Y.; Pearlman, E. Protein deiminase 4 and CR3 regulate Aspergillus fumigatusi and β-glucan-induced neutrophil extracellular trap formation, but hyphal killing is I dependent only in CR3. Front. Immunol. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Solis, N.V.; Lionakis, M.S.; Filler, S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat. Microbiol. 2018, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef]
- Plantinga, T.S.; van der Velden, W.J.F.M.; Ferwerda, B.; van Spriel, A.B.; Adema, G.; Feuth, T.; Donnelly, J.P.; Brown, G.D.; Kullberg, B.-J.; Blijlevens, N.M.A.; et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2009, 49, 724. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, W.J.; Plantinga, T.S.; Feuth, T.; Donnelly, J.P.; Netea, M.G.; Blijlevens, N.M. The incidence of acute graft-versus-host increases with Candida colonization depending the dectin-1 gene status. Clin. Immunol. 2010, 136, 302. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Dambuza, I.M.; Vautier, S.; Taylor, J.A.; Reid, D.M.; Bain, C.C.; Underhill, D.M.; Masopust, D.; Kaplan, D.H.; Brown, G.D. CD4+ t-cell survival in the GI tract requires dectin-1 during fungal infection. Mucosal Immunol. 2016, 9, 492–502. [Google Scholar] [CrossRef]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human pathogen, Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef]
- Vautier, S.; Drummond, R.A.; Redelinghuys, P.; Murray, G.I.; MacCallum, D.M.; Brown, G.D. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect. Immun. 2012, 80, 4216–4222. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Vautier, S.; Potrykus, J.; Walker, L.A.; Shepardson, K.M.; Hopke, A.; Mora-Montes, H.M.; Kerrigan, A.; Netea, M.G.; Murray, G.I.; et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013, 9, e1003315. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Eissenberg, L.G.; Goldman, W.E. Histoplasma capsulatum alpha-(1,3) glucan blocks innate immune recognition by the beta-glucan receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Giovannini, G.; De Luca, A.; D’Angelo, C.; Casagrande, A.; Iannitti, R.G.; Ricci, G.; Cunha, C.; Romani, L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 2012, 9, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Del Pilar Jimenez-A, M.; Viriyakosol, S.; Walls, L.; Datta, S.K.; Kirkland, T.; Heinsbroek, S.E.M. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of dectin-1 (Clec7a). Genes Immun. 2008, 9, 338–348. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Inbred mouse strains differ in resistance to lethal Coccidiodes immitis infection. Infect. Immun. 1983, 40, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Heinsbroek, S.E.M.; Taylor, P.R.; Rosas, M.; Wilment, J.A.; Williams, D.L.; Gordon, S.; Brown, G.D. Expression of functionally different dectin-1 isoforms by murine macrophages. J. Immunol. 2006, 176, 5513–5518. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Muller, J.P.; Spies-Weisshart, B.; Grafe, C.; Kurzai, O.; Hunniger, K.; Hochhaus, A.; Scholl, S.; Schnetzke, U. Isoform localization of dectin-1 regulates the signaling quality of anti-fungal immunity. Eur. J. Immunol. 2017, 47, 848–859. [Google Scholar] [CrossRef]
- Ashman, R.; Fulurija, A.; Papadimitrou, J.M. Strain-dependent difference in host response to Candida albicans infection in mice are related to organ susceptibility and infectious load. Infect. Immun. 1996, 64, 1866–1869. [Google Scholar] [CrossRef]
- Steele, C.; Rapaka, R.R.; Metz, A.; Pop, S.M.; Williams, D.L.; Gordon, S.; Kolls, J.K.; Brown, G.D. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005, 1, e42. [Google Scholar] [CrossRef]
- Hohl, T.M.; Van Epps, H.L.; Rivera, A.; Morgan, L.A.; Chen, P.L.; Feldmesser, M.; Pamer, E.G. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 2005, 1, e30. [Google Scholar] [CrossRef]
- Lewis, L.E.; Bain, J.M.; Lowes, C.; Gillespie, C.; Rudkin, F.M.; Gow, N.A.R.; Erwig, L.-P. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012, 8, e1002578. [Google Scholar] [CrossRef]
- Wheeler, R.T.; Fink, G.R. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006, 2, e35. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans β-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1671. [Google Scholar] [CrossRef]
- Klis, F.M.; de Groot, P.; Hellingwerf, K. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 2001, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Borges-Walmsley, M.E.; Chen, D.; Shu, X.; Walmsley, A.R. The pathobiology of Paracococcidioides brasiliensis. Trends Microbiol. 2002, 10, 80–87. [Google Scholar] [CrossRef]
- Kozel, T.R. Virulence factors of Cryptococcus neoformans. Trends Microbiol. 1995, 3, 295–299. [Google Scholar] [CrossRef]
- Miura, N.M.; Miura, T.; Ohno, N.; Adachi, Y.; Watanabe, M.; Tamura, H.; Tanaka, S.; Yadomae, T. Gradual solubilization of Candida cell wall β-glucan by oxidative degradation in mice. FEMS Immunol. Med. Microbiol. 1998, 21, 123–129. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohno, N.; Oshima, Y.; Yadomae, T. Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol. Med. Microbiol. 1998, 21, 223–230. [Google Scholar] [PubMed]
- Thompson, A.; Griffiths, J.S.; Walker, L.; da Fonseca, D.M.; Lee, K.K.; Taylor, P.R.; Gow, N.A.R.; Orr, S.J. Dependence on dectin-1 varies with multiple Candida species. Front. Microbiol. 2019, 10, 1800. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell population. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.K.; Atkins, R.; Locksley, M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17 producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Osorio, F.; LeibundGut-Landmann, S.; Lochner, M.; Lahl, K.; Sparwasser, T.; Eberl, G.; Sousa, C.R. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 2008, 38, 3274–3281. [Google Scholar] [CrossRef]
- Campuzano, A.; Zhang, H.; Ostroff, G.R.; Dias, L.D.S.; Wuthrich, M.; Klein, B.S.; Yu, J.-J.; Lara, H.H.; Lopez-Ribot, J.L.; Hung, C.-Y. CARD9-associated dectin-1 and dectin-2 are required for protective immunity of a multivalent vaccine against Coccidioides posadii infection. J. Immunol. 2020, 204, 3296–3306. [Google Scholar] [CrossRef]
- Cunha, C.; Di Lanni, M.; Bozza, S.; Giovannini, G.; Zagarella, S.; Zelante, T.; D’Angelo, C.; Pierini, A.; Pitzurra, L.; Falzetti, F.; et al. Detin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010, 116, 5394–5402. [Google Scholar] [CrossRef]
- Ferwerda, B.; Ferwerda, G.; Plantinga, T.S.; Willment, J.A.; van Spriel, A.B.; Venselaar, H.; Elbers, C.C.; Johnson, M.D.; Cambi, A.; Huysamen, C.; et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009, 361, 1760. [Google Scholar] [CrossRef]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef]
- Huang, H.; Na, L.; Fidel, P.L.; Schwarzenberger, P. Requirement for interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004, 190, 624–631. [Google Scholar] [CrossRef]
- LeibunGut-Landmann, S.; Osorio, F.; Brown, G.D.; Sousa, C.R. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 2008, 112, 4971–4980. [Google Scholar] [CrossRef]
- Agrawal, S.; Gupta, S.; Agrawal, A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS ONE 2010, 5, e13418. [Google Scholar] [CrossRef]
- Lesley, R.; Kelly, L.M.; Xu, Y.; Cyster, J.G. Naïve CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc. Natl. Acad. Sci. USA 2006, 103, 10717–10722. [Google Scholar] [CrossRef]
- Sokol, H.; Conway, K.L.; Zhang, M.; Choi, M.; Morin, B.; Cao, Z.; Villablanca, E.; Li, C.; Wijmenga, C.; Yun, S.H.; et al. Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology 2013, 145, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Rahabi, M.; Jacquemin, G.; Prat, M.; Meunier, E.; AlaEddine, M.; Bertrand, B.; Lefevre, L.; Benmoussa, K.; Batigne, P.; Aubouy, A.; et al. Divergent roles for macrophages C-type lectin receptors, dectin-1 and mannose receptors, in the intestinal inflammatory response. Cell Rep. 2020, 30, 4386–4398. [Google Scholar] [CrossRef]
- Ross, G.D.; Cain, J.A.; Lachmann, P.J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin and function as a receptor for zymosan and rabbit erythrocytes as well as a receptor iC3b. J. Immunol. 1985, 134, 3307. [Google Scholar]
- Cain, J.A.; Newmann, S.L.; Ross, D.G. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complementary 1987, 4, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Vetvicka, V.; Yan, J.; Hanikyrova, M.; Mayadas, T.; Ross, D.G. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1999, 162, 2281–2290. [Google Scholar]
- Vetvicka, V.; Thornton, B.P.; Ross, D.G. Soluble beta-guan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Investig. 1996, 98, 50–61. [Google Scholar] [CrossRef]
- Qi, C.; Cai, Y.; Gunn, L.; Ding, C.; Li, B.; Kloecker, G.; Qian, K.; Vasilakos, J.; Saijo, S.; Iwakura, Y.; et al. Differential pathways regulating innate and adaptive antitumor immune response by particulate and soluble yeast-derived β-glucans. Blood 2011, 117, 6825–6836. [Google Scholar] [CrossRef]
- Van Bruggen, R.; Drewniak, A.; Jansen, M.; Van Houdt, M.; Roos, D.; Chapel, H.; Verhoeven, A.J.; Kuijpers, T.W. Complement receptor 3, not dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol. Immunol. 2009, 47, 575–581. [Google Scholar] [CrossRef]
- Gandezam, R.P.; van Hamme, J.L.; Tool, A.T.; van Houdt, M.; Verkuijlen, P.J.J.H.; Herbst, M.; Liese, J.G.; van de Veerdonk, F.L.; Roos, D.; van de Berg, T.K. Two independent killing mechanisms of Candida albicans by human neutrophils: Evidence from innate immune defects. Blood 2014, 124, 590–597. [Google Scholar]
- Teschner, D.; Cholaszczyriska, A.; Ries, F.; Beckert, H.; Theobald, M.; Grabbe, S.; Radsak, M.; Bros, M. CD11b regulates fungal outgrowth but not neutrophil recruitment in a mouse model of invasive pulmonary aspergillosis. Front. Immunol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Li, D.; Bai, C.; Zhang, Q.; Li, Z.; Shao, D.; Li, X. β-1,3-glucan/CR3/SYK pathway-dependent LC3B-II accumulation enhanced the fungicidal activity in human neutrophils. J. Microbiol. 2019, 57, 263–270. [Google Scholar] [CrossRef]
- Pasquale, E.B. The Eph family of receptors. Curr. Opin. Cell. Biol. 1997, 9, 608–615. [Google Scholar] [CrossRef]
- Paavilainen, S.; Grandy, D.; Karelehto, E.; Chang, E.; Susi, P.; Erdjument-Bromage, H.; Nikolov, D.; Himanen, J. High-level expression of a full-length Eph receptor. Protein Expr. Purif. 2013, 92, 112–118. [Google Scholar] [CrossRef]
- Kania, A.; Klein, R. Mechanism of ephrin-eph signaling in development, physiology and disease. Nat. Rev. Mol. Cell. Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Hu, Z.; Kinch, M.S.; Pan, C.-X.; Flockhart, D.A.; Kao, C.; Gardner, T.A.; Zhang, S.; Li, L.; Baldridge, L.A.; et al. High-level expression of EphA2 receptor tyrosine kinase in prostatic intraepithelial neoplasia. Am. J. Pathol. 2003, 163, 2271–2276. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kato, H.; Fukuchi, M.; Nakajima, M.; Kuwano, H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int. J. Cancer 2003, 103, 657–663. [Google Scholar] [CrossRef]
- Charmsaz, S.; Beckett, K.; Smith, F.M.; Bruedigam, C.; Moore, A.S.; Al-Ejeh, F.; Lane, S.W.; Boyd, A.W. EphA2 is a therapy target in EphA2-positive leukemias but is not essential for normal hematopoiesis or leukemia. PLoS ONE 2015, 10, e0130692. [Google Scholar] [CrossRef]
- Kinch, M.S.; Moore, M.-B.; Harpole, D.H., Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin. Cancer. Res. 2003, 9, 613–618. [Google Scholar]
- Merritt, W.M.; Thaker, P.H.; Landen, C.N., Jr.; Deavers, M.T.; Fletcher, M.S.; Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Schmandt, R.; Gershenson, D.M.; et al. Analysis of EphA2 expression and mutant p53 in ovarian carcinoma. Cancer Biol. Ther. 2006, 5, 1357–1360. [Google Scholar] [CrossRef][Green Version]
- Herrem, C.J.; Tatsumi, T.; Olson, K.S.; Shirai, K.; Finke, J.H.; Bukowski, R.M.; Zhou, M.; Richmond, A.L.; Derweesh, I.; Kinch, M.S.; et al. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically treated patients with renal cell carcinoma. Clin. Cancer Res. 2005, 11, 226–231. [Google Scholar]
- Holm, R.; Van de Putte, G.; Suo, Z.; Lie, A.K.; Kristensen, G.B. Expressions of EphA2 and EphrinA-1 in early squamous cell cervical carcinomas and their relation to prognosis. Int. J. Med. Sci. 2008, 5, 121–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zelinski, D.P.; Zantek, N.D.; Stewart, J.C.; Irizarry, A.R.; Kinch, M.S. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001, 61, 2301–2306. [Google Scholar] [PubMed]
- Hong, J.Y.; Shin, M.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S.; Kim, S.K.; Chang, J.; Park, M.S. EphA2 receptor signaling mediates inflammatory responses in lipopolysaccharide-induced lung injury. Tuberc. Respir. Dis. 2015, 78, 218–226. [Google Scholar] [CrossRef]
- Thundyil, J.; Manzanero, S.; Pavlovski, D.; Cully, T.R.; Lok, K.Z.; Widiapradja, A.; Chunduri, P.; Jo, D.G.; Naruse, C.; Asano, M.; et al. Evidence that the EphA2 receptor exacerbates ischemic brain injury. PLoS ONE 2013, 8, e53528. [Google Scholar]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef]
- Abusleme, L.; Diaz, P.I.; Freeman, A.F.; Greenwell-Wild, T.; Brenchley, L.; Desai, J.V.; Ng, W.-I.; Holland, S.M.; Lionakis, M.S.; Segre, J.A.; et al. Human defects in STAT3 promote oral mucosal fungal and bacterial dysbiosis. JCI Insight 2018, 3, e122061. [Google Scholar] [CrossRef]
- Swidergall, M.; Solis, N.V.; Wang, Z.; Phan, Q.T.; Marshall, M.E.; Lionakis, M.S.; Pearlman, E.; Filler, S.G. EphA2 is a neutrophil receptor for Candida albicans that stimulates antifungal activity during oropharyngeal infection. Cell Rep. 2019, 28, 423–433. [Google Scholar] [CrossRef]
- Swidergall, M.; Solis, N.V.; Millet, N.; Huang, M.Y.; Lin, J.; Phan, Q.T.; Lazarus, M.D.; Wang, Z.; Yeaman, M.R.; Mitchell, A.P.; et al. Activation of EphA2-EGFR signaling in oral epithelial cells by Candida albicans virulence factors. PLoS Pathog. 2021, 17, e1009221. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Hupper, A.R.; Ramani, K.; McGreachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2, eaam8834. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate- glycosphingolipid interaction between a β-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014. [Google Scholar] [CrossRef]

| Receptor | Allele Symbol | Genetic Background | Fungus | Journal | Ref. |
|---|---|---|---|---|---|
| Dectin-1 | Clec7a <tm1Yiw> | C57BL/6J, BALB/c | Candida albicans, Pneumocystis carinii | Nat Immunol. 2007 | [25] |
| Clec7a <tm1Gdb> | B6;129 mix | C.albicans | Nat Immunol. 2007 | [24] | |
| Clec7a <tm1Yiw> | C57BL/6J | Cryptococcus neoformans | Microbiol Immunol. 2007 | [53] | |
| Clec7a <tm1Gdb> | 129/SvEv | Aspergillus fumigatus | J Immunol. 2009 | [54] | |
| Lyz2 <tm1(cre)Ifo>. Clec7a <tm1.1Bpip>: * | C57BL/6J | C.albicans | PLoS Pathog. 2010 | [55] | |
| Clec7a <tm1Yiw> | C57BL/6J | A. fumigatus | J Exp Med. 2011 | [56] | |
| Clec7a <tm1Yiw> | BALB/c | A. fumigatus | PLoS One 2011 | [57] | |
| Clec7a <tm1Gdb> | C57BL/6 | Candida tropicalis | Science 2012 | [58] | |
| Clec7a <tm1Gdb> | C57BL/6, (C57BL/6;DBA/2)F2 | Coccidioides immitis | mBio 2013 | [59] | |
| Clec7a <tm1Gdb> | C57BL/6 | Paracoccidioides brasiliensis | J Infect Dis. 2014 | [60] | |
| Clec7a <tm1Yiw> | C57BL/6J | C. tropicalis | Cell Host Microbe 2015 | [61] | |
| Clec7a <tm1Gdb>, Clec7a <tm1Gdb>. Itgam <tm1Myd> | C57BL/6 | Histoplasma capsulatum | PLoS Pathog. 2015 | [62] | |
| Clec7a <tm1Yiw> | C57BL/6J | Trichosporon asahii | Inflamm Res. 2016 | [63] | |
| Clec7a <tm1Yiw> | C57BL/6J | Trichophyton rubrum | Innate Immun. 2016 | [64] | |
| Clec7a <tmX>: ** | C57BL/6 | Blastomyces dermatitidis | J Clin Invest. 2016 | [65] | |
| Clec7a <tm1Gdb> | C57BL/6 | C.albicance, Candida krusei | Am J Transl Res. 2019 | [66] | |
| Itgam <tm1Myd> | C57BL/6 | B. dermatitidis | J Immunol. 2004 | [67] | |
| Itgam <tm1Myd> | C57BL/6 | C.albicans | Cell Host Microbe. 2011 | [68] | |
| Itgam <tm1Bll> | C57BL/6J | C.albicans | Infect Immun. 2011 | [69] | |
| CR3 | Itgb2 <tm2Bay> | C57BL/6J | A. fumigatus | J Clin Invest. 2012 | [70] |
| Itgam <tm1Myd>, Clec7a <tm1Gdb>. Itgam <tm1Myd> | C57BL/6 | H. capsulatum | PLoS Pathog. 2015 | [62] | |
| Itgam <tm1Myd> | C57BL/6 | C. neoformans | Nat Commun. 2019 | [71] | |
| Itgam <tm1Myd> | C57BL/6 | A. fumigatus | Front Immunol. 2019 | [72] | |
| EphA2 | Epha2 <tm1Jrui> | C57BL/6 | C.albicans | Nat Microbiol. 2018 | [73] |
| CD82 | Cd82 <tm1.1Cmir> | C57BL/6 | C.albicans | J Immunol. 2019 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desamero, M.J.M.; Chung, S.-H.; Kakuta, S. Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models. Int. J. Mol. Sci. 2021, 22, 4778. https://doi.org/10.3390/ijms22094778
Desamero MJM, Chung S-H, Kakuta S. Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models. International Journal of Molecular Sciences. 2021; 22(9):4778. https://doi.org/10.3390/ijms22094778
Chicago/Turabian StyleDesamero, Mark Joseph Maranan, Soo-Hyun Chung, and Shigeru Kakuta. 2021. "Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models" International Journal of Molecular Sciences 22, no. 9: 4778. https://doi.org/10.3390/ijms22094778
APA StyleDesamero, M. J. M., Chung, S.-H., & Kakuta, S. (2021). Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models. International Journal of Molecular Sciences, 22(9), 4778. https://doi.org/10.3390/ijms22094778






