Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta
Abstract
1. Introduction
2. Results
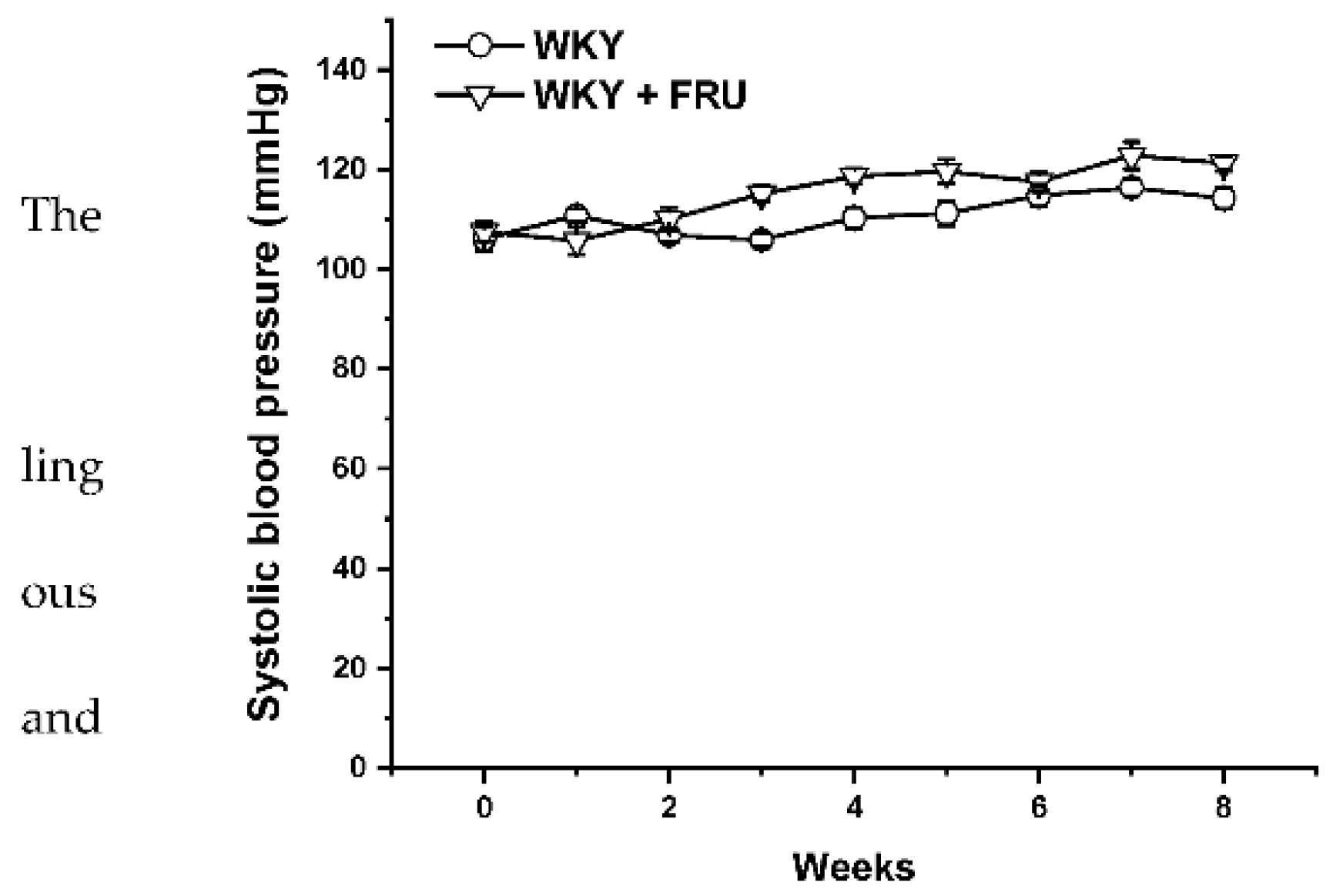
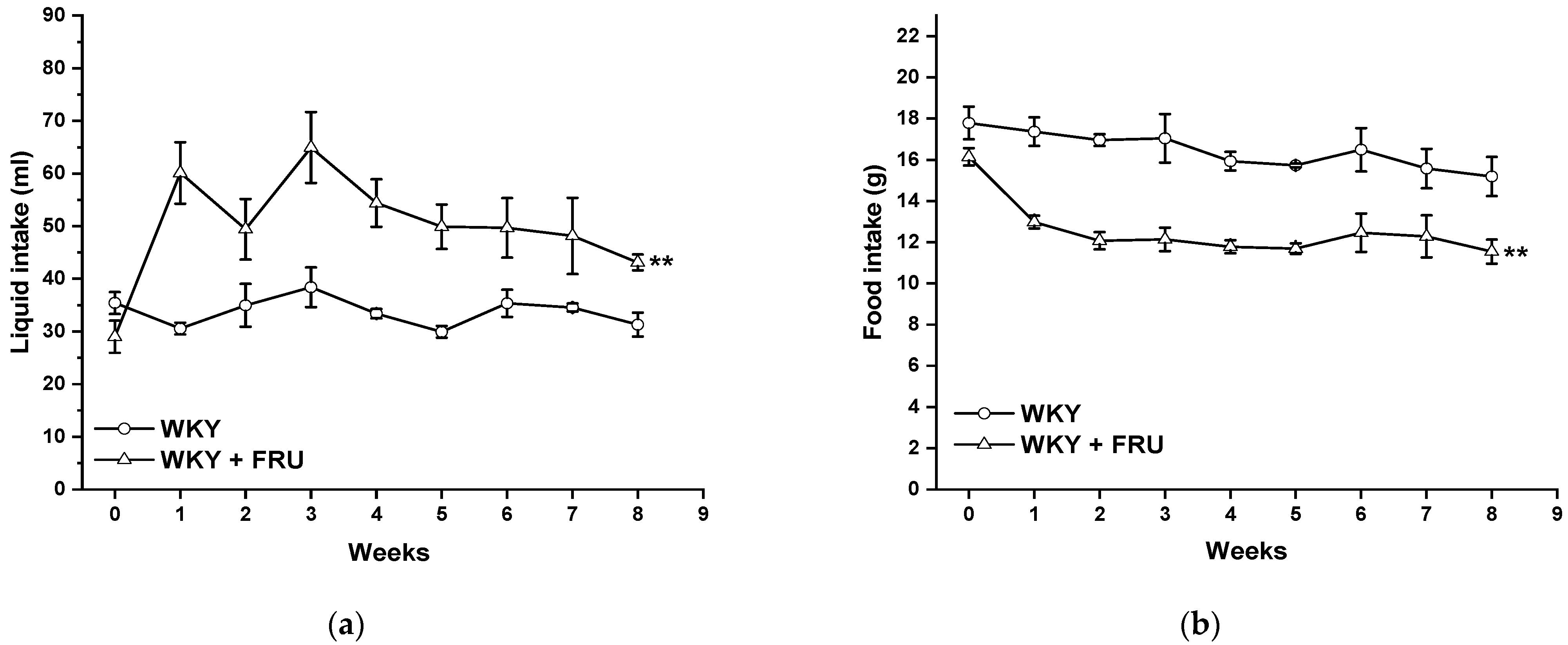
2.1. Blood Pressure, Cardiac Hypertrophy, Weight Gain, and Fluid and Food Intake
2.2. Plasma Parameters
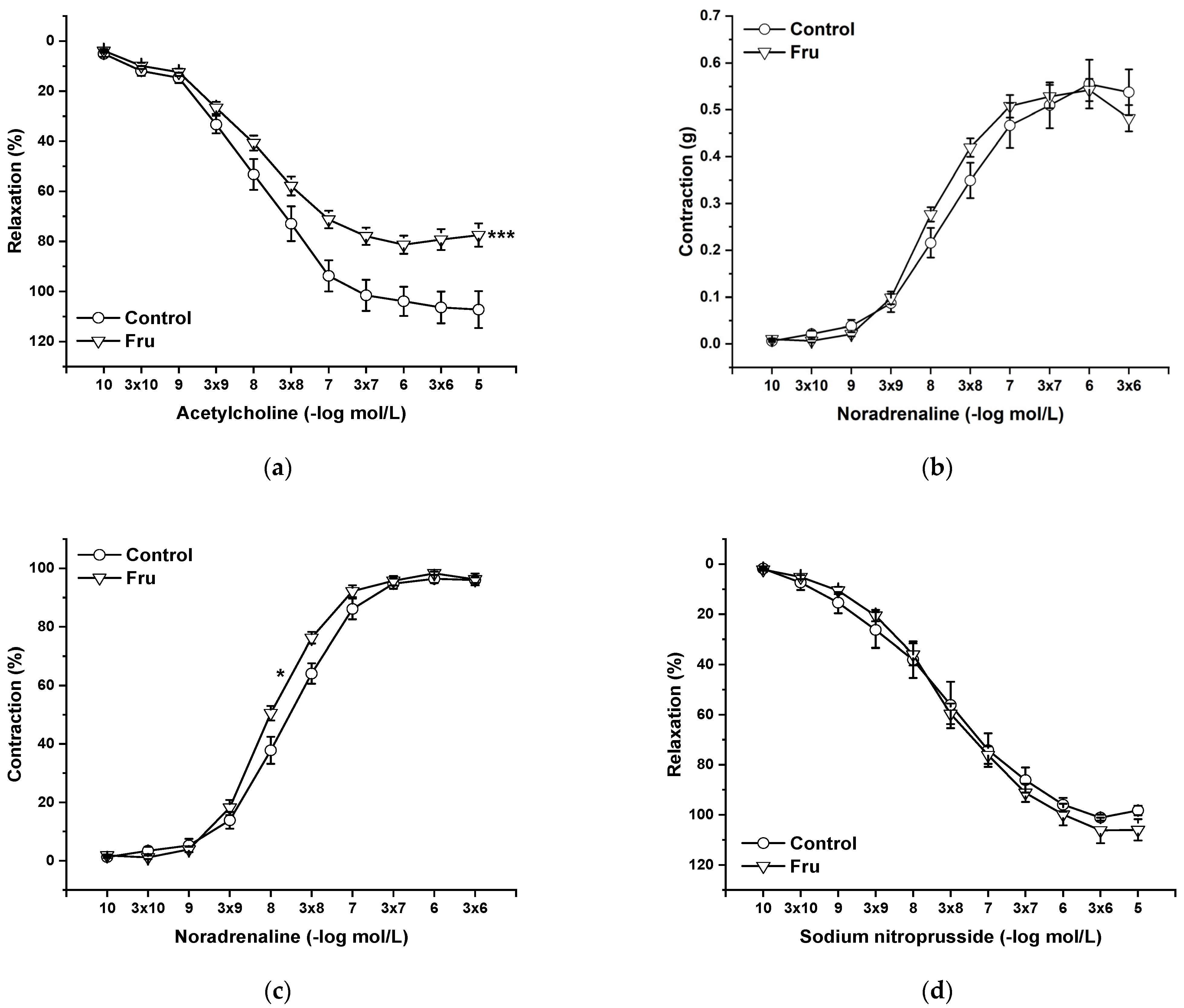
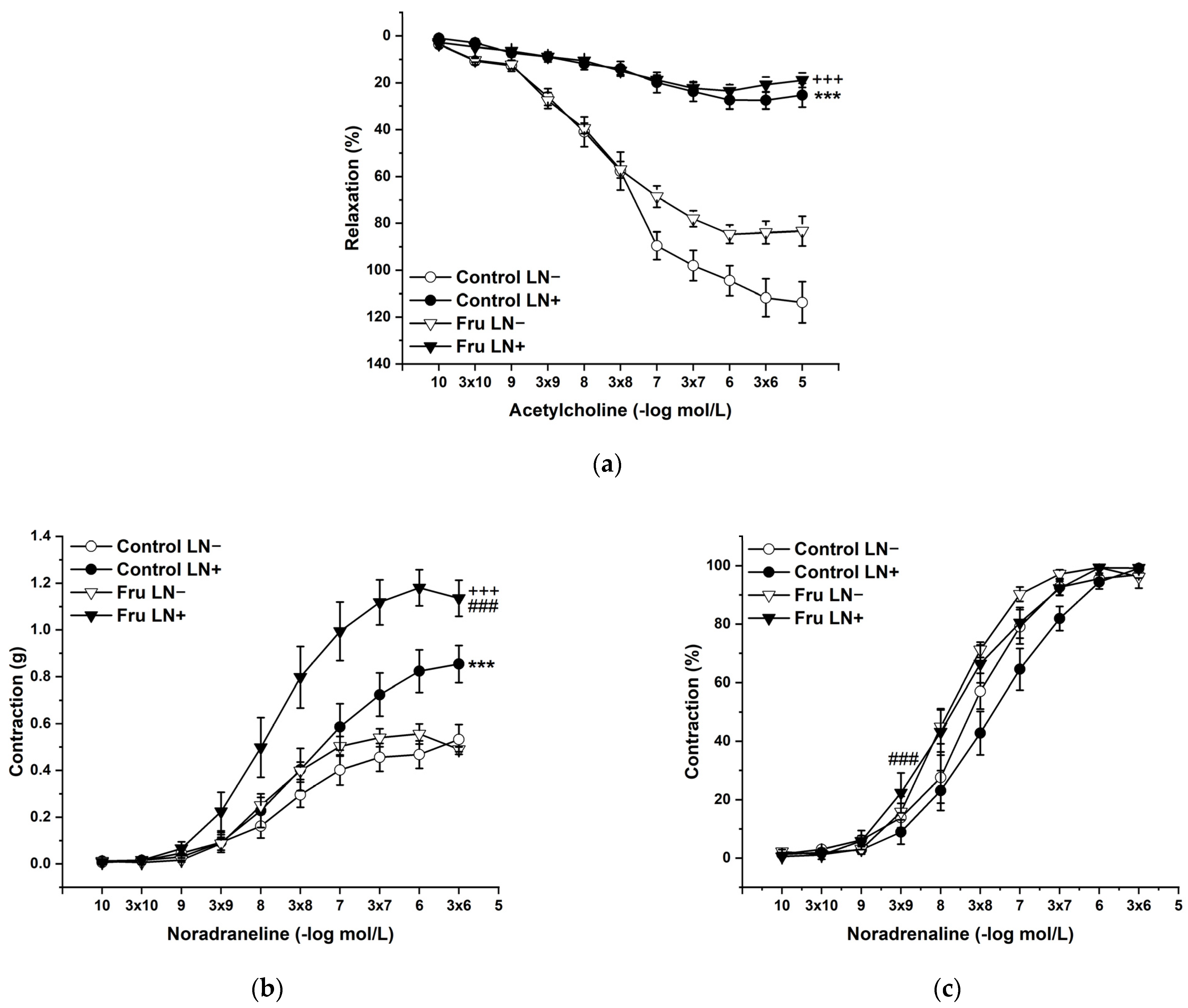
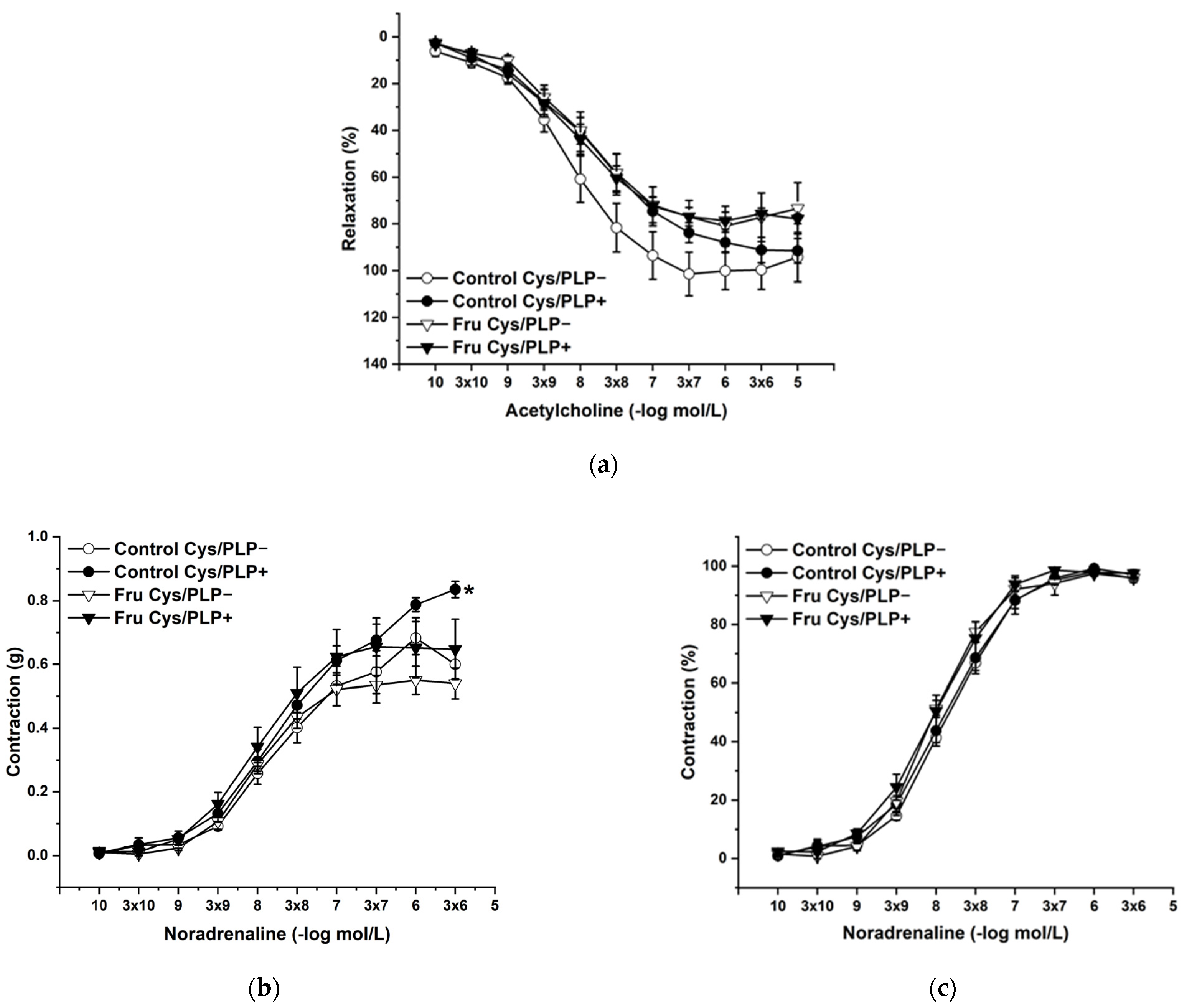
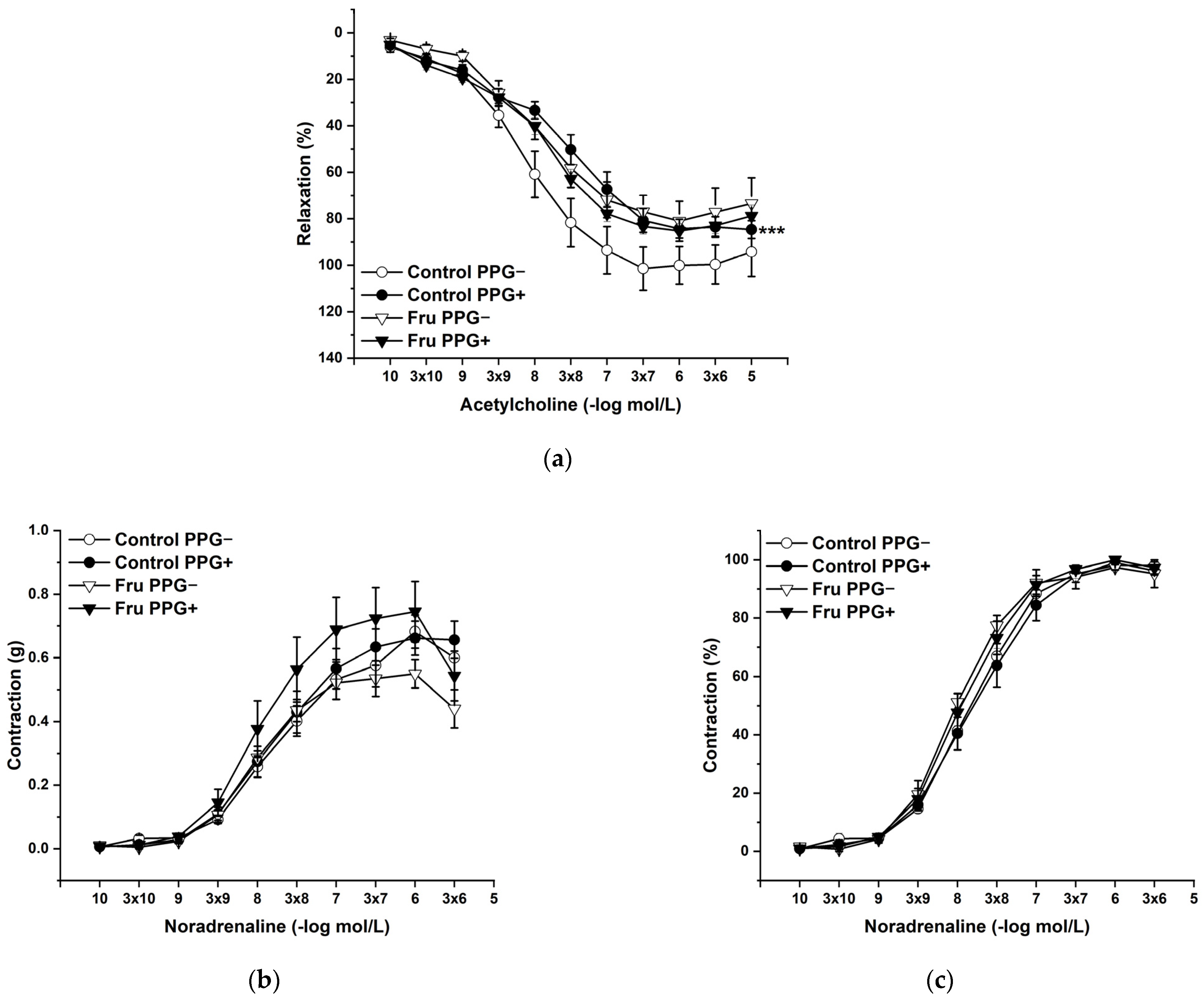
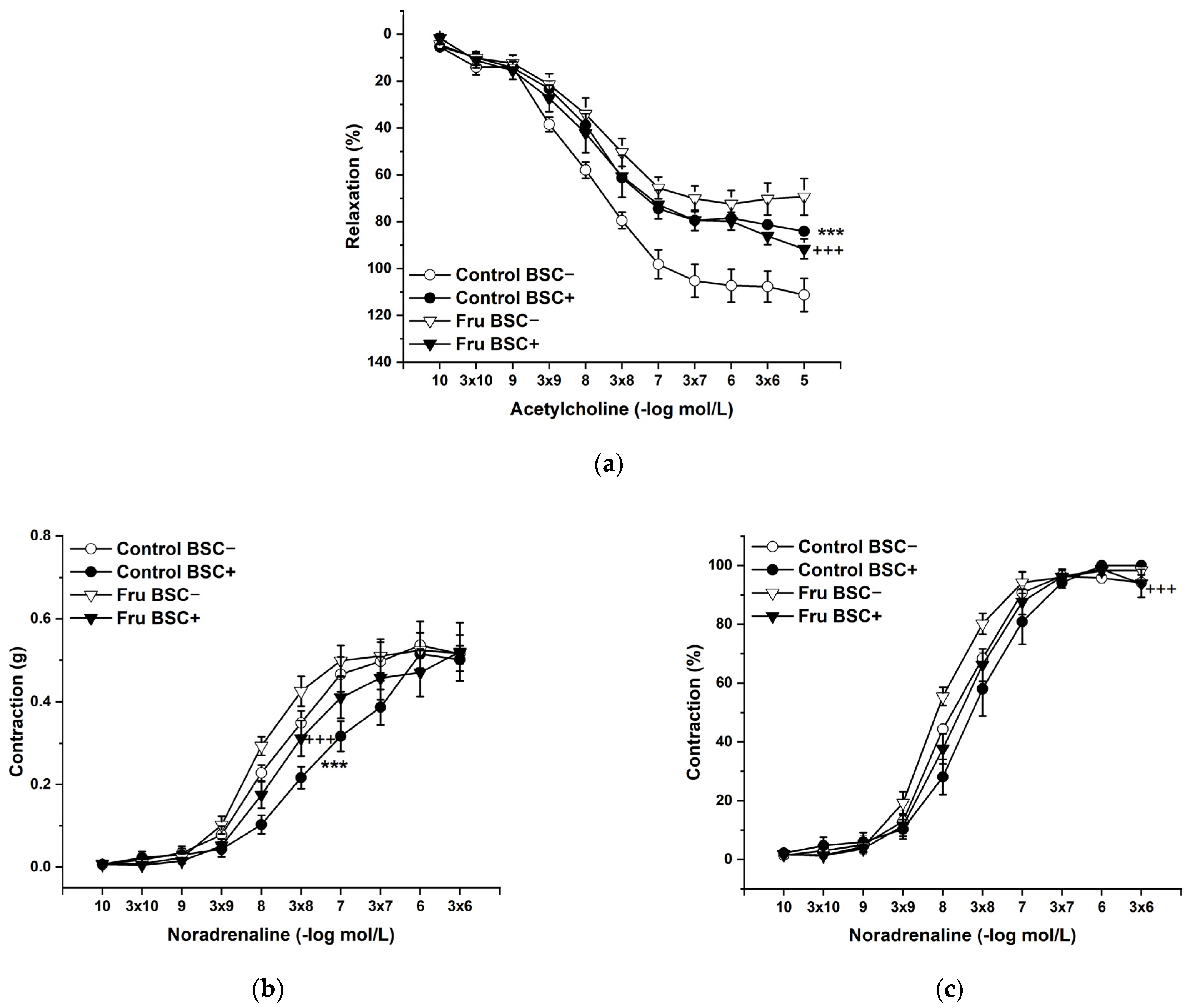
2.3. Vasoactive Responses
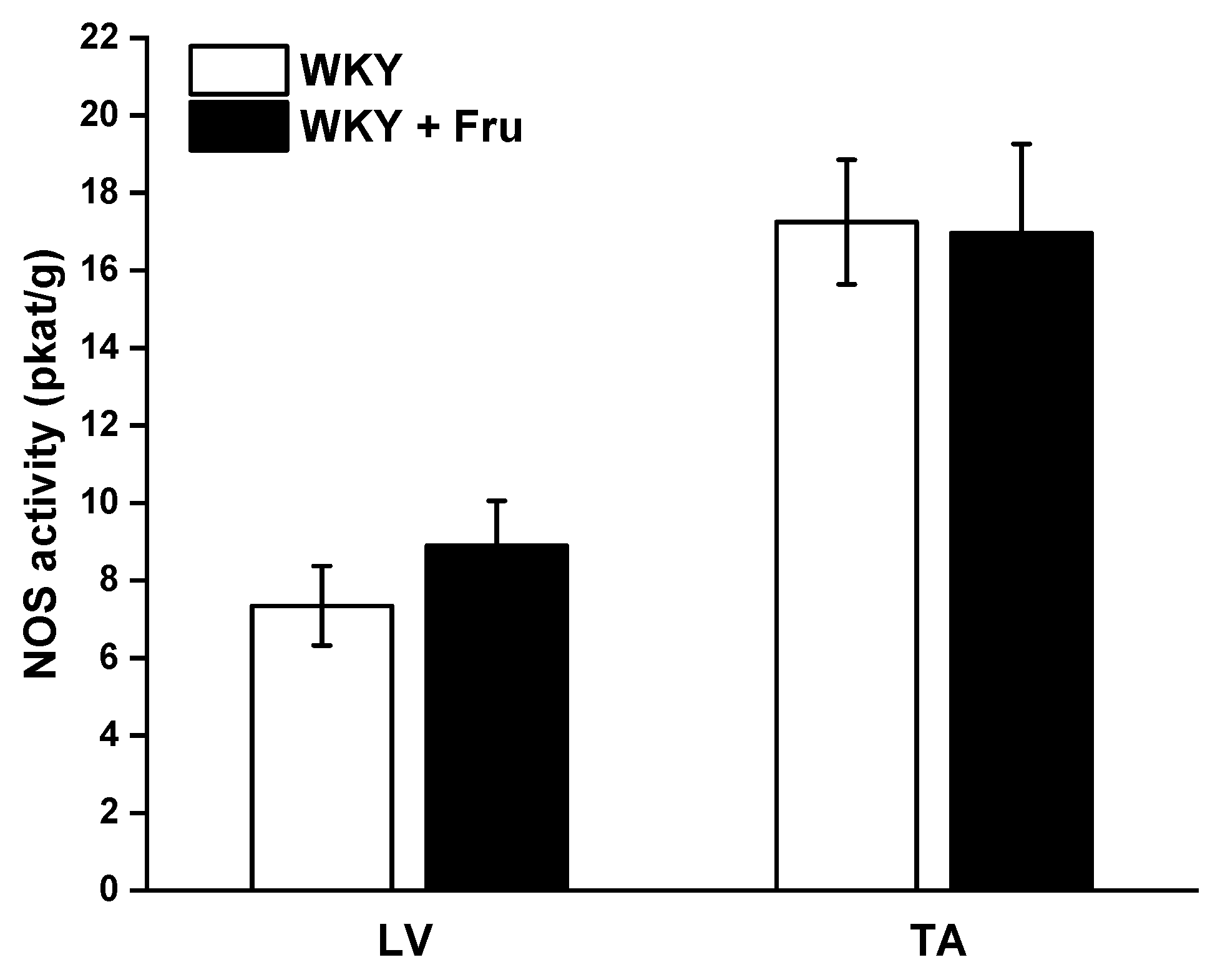
2.4. Total Activity of NO Synthase
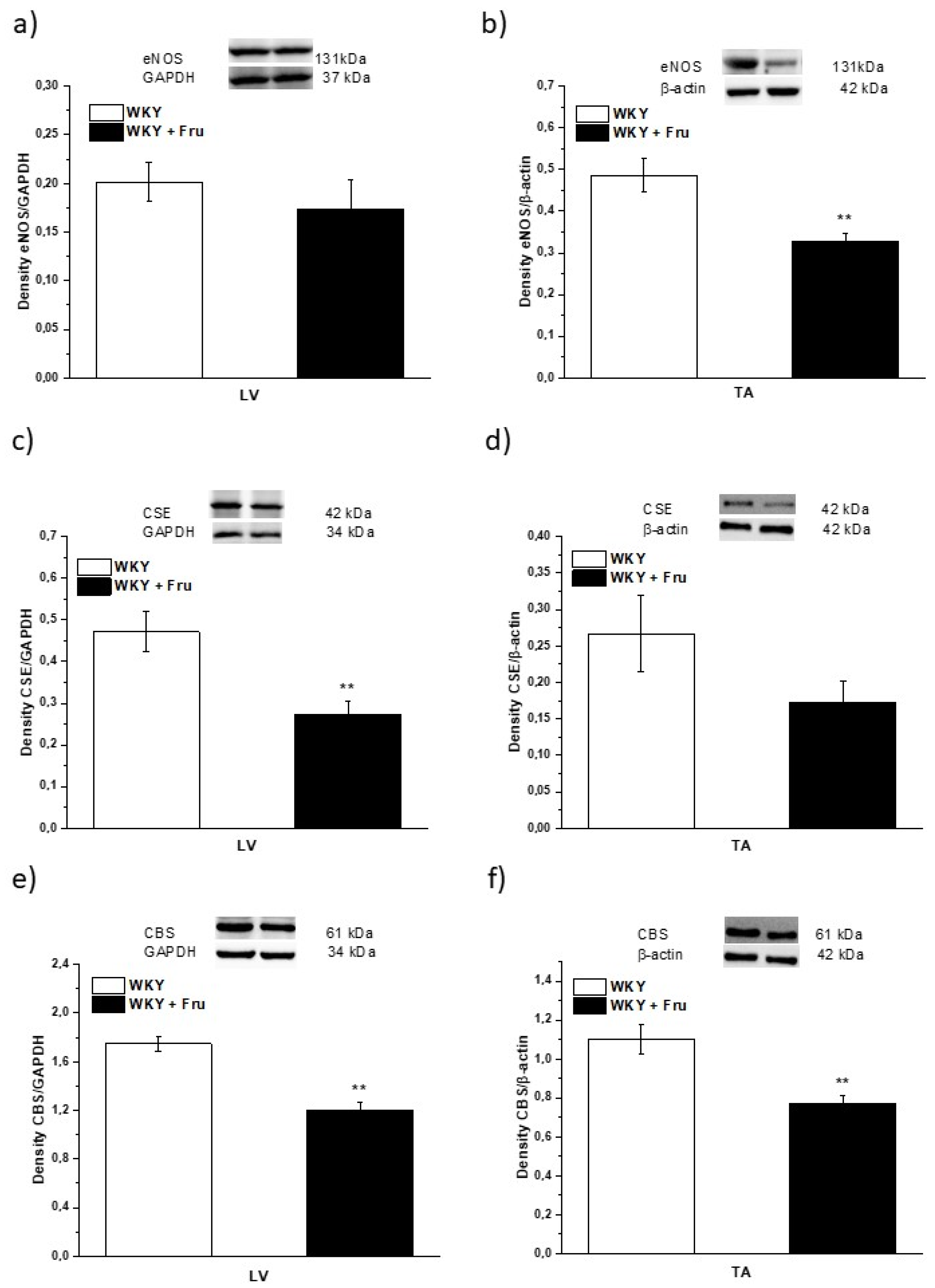
2.5. Expression of Enzymes That Produce NO and H2S
3. Discussion
4. Materials and Methods
4.1. Guide for the Use and Care of Laboratory Animals
4.2. Basic Cardiovascular and Biometric Parameters
4.3. Collection of Plasma Samples
4.4. Functional Study
Experimental Protocol
4.5. Measurement of the Total NO Synthase Activity
4.6. Expression of Enzymes Producing NO and H2S
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaligram, S.; Sanguesa, G.; Akther, F.; Alegret, M.; Laguna, J.C.; Rahimian, R. Differential effects of high consumption of fructose or glucose on mesenteric arterial function in female rats. J. Nutr. Biochem. 2018, 57, 136–144. [Google Scholar] [CrossRef]
- Sangüesa, G.; Shaligram, S.; Akthur, F.; Roglans, N.; Laguna, J.C.; Rahimian, R.; Alegret, M. Type of supplemented simple sugar, not merely calorie intake, determines adverse effects on metabolism and aortic function in female rats. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H289–H304. [Google Scholar] [CrossRef]
- Miranda, C.A.; Schönholzer, T.E.; Klöppel, E.; Sinzato, Y.K.; Volpato, G.T.; Damasceno, D.C.; Campos, K.E. Repercussions of low fructose-drinking water in male rats. An. Acad. Bras. Ciências 2019, 91, e20170705. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Invest. 2018, 28, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díazcouder, A.; Romero-Nava, R.; Carbó, R.; Sánchez-Lozada, L.G.; Sánchez-Muñoz, F. High Fructose Intake and Adipogenesis. Int. J. Mol. Sci. 2019, 2, 2787. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.M.; Suleimani, Y.M.A.; Ashique, M.; Manoj, P.; Ali, B.H. Effect of infliximab and tocilizumab on fructose-induced hyperinsulinemia and hypertension in rats. Biomed. Pharmacother 2018, 105, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Zemancikova, A.; Torok, J. Effect of perivascular adipose tissue on arterial adrenergic contractions in normotensive and hypertensive rats with high fructose intake. Physiol. Res. 2017, 66, S537–S544. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Samsonov, A.P.; Gheibi, S.; Vazquez, A.B.; Kashfi, K. Regulation of carbohydrate metabolism by nitric oxide and hydrogen sulfide: Implications in diabetes. Biochem. Pharmacol. 2020, 176, 113819. [Google Scholar] [CrossRef]
- Pan, Y.; Kong, L.D. High fructose diet-induced metabolic syndrome: Pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol. Res. 2018, 130, 438–450. [Google Scholar] [CrossRef]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Briones, L.; Andrews, M.; Pizarro, F.; Arredondo-Olguin, M. Expression of genes associated with inflammation and iron metabolism in 3T3-L1 cells induced with macrophages-conditioned medium, glucose and iron. Biometals 2018, 31, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Frese, M.; Romero, J.; Cunningham, J.H.; Mills, K.E. Chronic fructose substitution for glucose or sucrose in food or beverages has little effect on fasting blood glucose, insulin, or triglycerides: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, E.; Kim, M.; Kim, I. Acute Exposure to Fructose Impairs Endothelium-Dependent Relaxation via Oxidative Stress in Isolated Rat Aortic Rings. Vasc. Res. 2020, 57, 213–222. [Google Scholar] [CrossRef]
- Sousa, G.J.; Oliveira, P.W.C.; Nogueira, B.V.; Melo, A.F., Jr.; Faria, T.O.; Meira, E.F.; Mill, J.G.; Bissoli, N.S.; Baldo, M.P. Fructose intake exacerbates the contractile response elicited by norepinephrine in mesenteric vascular bed of rats via increased endothelial prostanoids. J. Nutr. Biochem. 2017, 48, 21–28. [Google Scholar] [CrossRef]
- Rehakova, R.; Klimentova, J.; Cebova, M.; Barta, A.; Matuskova, Z.; Labas, P.; Pechanova, O. Effect of deuterium-depleted water on selected cardiometabolic parameters in fructose-treated rats. Physiol. Res. 2016, 65, S401–S407. [Google Scholar] [CrossRef] [PubMed]
- White, J.S. Challenging the fructose hypothesis: New perspectives on fructose consumption and metabolism. Adv. Nutr. 2013, 4, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Buchwalow, I.B.; Cacanyiova, S.; Neumann, J.; Samoilova, V.E.; Boecker, W.; Kristek, F. The role of arterial smooth muscle in vasorelaxation. Biochem. Biophys Res. Commun. 2008, 377, 504–507. [Google Scholar] [CrossRef]
- Talukder, M.A.H.; Fujiki, T.; Morikawa, K.; Motoishi, M.; Kubota, H.; Morishita, T.; Tsutsui, M.; Takeshita, A.; Shimokawa, H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J. Cardiovasc. Pharmacol. 2004, 44, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Biecker, E.; Neef, M.; Sägesser, H.; Shaw, S.; Koshy, A.; Reichen, J. Nitric oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase 3 knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Dobesová, Z.; Cejka, J.; Kunes, J.; Zicha, J. Vasoactive systems in L-NAME hypertension: The role of inducible nitric oxide synthase. J. Hypertens. 2004, 22, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef]
- Berenyiova, A.; Grman, M.; Misak, A.; Golas, S.; Cuchorova, J.; Cacanyiova, S. The Possible Role of the Nitroso-Sulfide Signaling Pathway in the Vasomotoric Effect of Garlic Juice. Molecules 2020, 25, 590. [Google Scholar] [CrossRef] [PubMed]
- Cacanyiova, S.; Krskova, K.; Zorad, S.; Frimmel, K.; Drobna, M.; Valaskova, Z.; Misak, A.; Golas, S.; Breza, J.; Breza, J., Jr.; et al. Arterial Hypertension and Plasma Glucose Modulate the Vasoactive Effects of Nitroso-Sulfide Coupled Signaling in Human Intrarenal Arteries. Molecules 2020, 25, 2886. [Google Scholar] [CrossRef] [PubMed]
- Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Hüttl, M.; Markova, I.; Berenyiova, A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules 2021, 11, 108. [Google Scholar] [CrossRef]
- Levitt, M.D. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox Signal. 2011, 15, 373–378. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2316–H2323. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.W.; Deng, L.W. Role of H2S Donors in Cancer Biology. Handb. Exp. Pharmacol. 2015, 230, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Liu, T.T.; Guo, L.; Lin, Y.; Nie, X.W.; Bian, J.S. Hydrogen Sulfide: Recent Progression and Perspectives for the Treatment of Diabetic Nephropathy. Molecules 2019, 24, 2857. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Surmiak, M.; Hubalewska-Mazgaj, M.; Kwiecien, S.; Wallace, J.L.; Brzozowski, T. The effect of hydrogen sulfide-releasing naproxen (ATB-346) versus naproxen on formation of stress-induced gastric lesions, the regulation of systemic inflammation, hypoxia and alterations in gastric microcirculation. J. Physiol. Pharmacol. 2017, 68, 749–756. [Google Scholar] [PubMed]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar] [PubMed]
- Cacanyiova, S.; Berenyiova, A.; Kristek, F.; Drobna, M.; Ondrias, K.; Grman, M. The adaptive role of nitric oxide and hydrogen sulphide in vasoactive responses of thoracic aorta is triggered already in young spontaneously hypertensive rats. J. Physiol. Pharmacol. 2016, 67, 501–512. [Google Scholar] [PubMed]
- Berenyiova, A.; Drobna, M.; Cebova, M.; Kristek, F.; Cacanyiova, S. Changes in the vasoactive effects of nitric oxide, hydrogen sulfide and the structure of the rat thoracic aorta: The role of age and essential hypertension. J. Physiol. Pharmacol. 2018, 69, 10–26. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Bernatova, I.; Pelouch, V.; Simko, F. Protein remodelling of the heart in NO-deficient hypertension: The effect of captopril. J. Mol. Cell Cardiol. 1997, 29, 3365–3374. [Google Scholar] [CrossRef]

| n | WG (g) | HW/BW (mg/g) | HW/TL (mg/mm) | RW/TL (mg/mm) | |
|---|---|---|---|---|---|
| WKY | 6 | 78.7 ± 7.91 | 3.37 ± 0.1 | 29.7 ± 2.2 | 96.5 ± 16.9 |
| WKY + FRU | 6 | 80.5 ± 8.7 | 3.49 ± 0.1 | 31.4 ± 1.1 | 82.8 ± 14.8 |
| TAG mmol/L | HDL mg/dL | ALT U/L | y-GT U/L | CYS mg/L | CRE µmol/L | GLU mmol/L | |
|---|---|---|---|---|---|---|---|
| WKY | 1.05 ± 0.35 | 62.18 ± 6.07 | 18.72 ± 2.25 | 0.17 ± 0.08 | 1.98 ± 0.16 | 44.2 ± 4.04 | 9.28 ± 1.12 |
| WKY + FRU | 1.18 ± 0.21 | 68.83 ± 4.59 | 31.95 ± 4.75 * | 0.62 ± 0.16 * | 2.15 ± 0.02 | 54.17 ± 3.1 | 10.63 ± 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenyiova, A.; Golas, S.; Drobna, M.; Cebova, M.; Cacanyiova, S. Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta. Int. J. Mol. Sci. 2021, 22, 4749. https://doi.org/10.3390/ijms22094749
Berenyiova A, Golas S, Drobna M, Cebova M, Cacanyiova S. Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta. International Journal of Molecular Sciences. 2021; 22(9):4749. https://doi.org/10.3390/ijms22094749
Chicago/Turabian StyleBerenyiova, Andrea, Samuel Golas, Magdalena Drobna, Martina Cebova, and Sona Cacanyiova. 2021. "Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta" International Journal of Molecular Sciences 22, no. 9: 4749. https://doi.org/10.3390/ijms22094749
APA StyleBerenyiova, A., Golas, S., Drobna, M., Cebova, M., & Cacanyiova, S. (2021). Fructose Intake Impairs the Synergistic Vasomotor Manifestation of Nitric Oxide and Hydrogen Sulfide in Rat Aorta. International Journal of Molecular Sciences, 22(9), 4749. https://doi.org/10.3390/ijms22094749







