Role of Choline in Ocular Diseases
Abstract
1. Introduction
2. Choline in Dry Eye Syndrome and Ocular Surface
3. Choline in the Retina
4. Choline in Retinal Vessels
5. Choline in the Optic Nerve
6. Choline in the Lens
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| LDL | Low-density lipoprotein |
References
- Sanders, L.M.; Zeisel, S.H. Choline. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical Role During Fetal Development and Dietary Requirements in Adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- Pomfret, E.A.; Dacosta, K.-A.; Schurman, L.L.; Zeisel, S.H. Measurement of choline and choline metabolite concentrations using high-pressure liquid chromatography and gas chromatography-mass spectrometry. Anal. Biochem. 1989, 180, 85–90. [Google Scholar] [CrossRef]
- Shah, S.; Jani, H. Prevalence and associated factors of dry eye: Our experience in patients above 40 years of age at a Tertiary Care Center. Oman J. Ophthalmol. 2015, 8, 151–156. [Google Scholar] [CrossRef]
- Bulat, N.; Cuşnir, V.V.; Procopciuc, V.; Cușnir, V.; Cuşnir, N.V. Diagnosing the Dry Eye Syndrome in modern society and among patients with glaucoma: A prospective study. Romanian J. Ophthalmol. 2020, 64, 35–42. [Google Scholar] [CrossRef]
- Bae, S.H.; Shin, Y.J.; Kim, H.K.; Hyon, J.Y.; Wee, W.R.; Park, S.G. Vitamin D Supplementation for Patients with Dry Eye Syndrome Refractory to Conventional Treatment. Sci. Rep. 2016, 6, 33083. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Shimazaki, J. Definition and Diagnostic Criteria of Dry Eye Disease: Historical Overview and Future Directions. Investig. Opthalmol. Vis. Sci. 2018, 59, DES7–DES12. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Gao, J.; Siemasko, K.F.; Beuerman, R.W.; Pflugfelder, S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004, 78, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Koopman, F.A.; Stoof, S.P.; Straub, R.H.; Van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. Restoring the Balance of the Autonomic Nervous System as an Innovative Approach to the Treatment of Rheumatoid Arthritis. Mol. Med. 2011, 17, 937–948. [Google Scholar] [CrossRef]
- Mauduit, P.; Jammes, H.; Rossignol, B. M3 muscarinic acetylcholine receptor coupling to PLC in rat exorbital lacrimal acinar cells. Am. J. Physiol. Physiol. 1993, 264, C1550–C1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Tan, D.T.H.; Beuerman, R.W. Expression and Function of Muscarinic Receptor Subtypes on Human Cornea and Conjunctiva. Investig. Opthalmol. Vis. Sci. 2007, 48, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.R.; Sullivan, D.A. Neurotransmitter Influence on Human Meibomian Gland Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2011, 52, 8543–8548. [Google Scholar] [CrossRef]
- Estrada-Cortés, E.; Negrón-Peréz, V.; Tríbulo, P.; Zenobi, M.; Staples, C.; Hansen, P. Effects of choline on the phenotype of the cultured bovine preimplantation embryo. J. Dairy Sci. 2020, 103, 10784–10796. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Opthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Gao, J.; Schwalb, T.A.; Ngo, M.; Tieu, D.D.; Chan, C.C.; Reis, B.L.; Whitcup, S.M.; Thompson, D.; Smith, J.A. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2609–2614. [Google Scholar]
- Čejková, J.; Ardan, T.; Šimonová, Z.; Čejka, Č.; Malec, J.; Jirsová, K.; Filipec, M.; Dotřelová, D.; Brůnová, B. Nitric oxide synthase induction and cytotoxic nitrogen-related oxidant formation in conjunctival epithelium of dry eye (Sjögren‘s syndrome). Nitric Oxide 2007, 17, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef]
- Mehta, A.K.; Singh, B.P.; Arora, N.; Gaur, S.N. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 2010, 215, 527–534. [Google Scholar] [CrossRef]
- Hoover, D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017, 179, 1–16. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nat. Cell Biol. 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, X.; Huang, Z.; Yan, Z.; Li, P.; Duan, R.; Feng, H.; Jiang, J.; Peng, H.; Liu, W. Protective effects of choline against hypoxia-induced injuries of vessels and endothelial cells. Exp. Ther. Med. 2017, 13, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L. The Addition of Choline to Parenteral Nutrition. Gastroenterology 2009, 137, S119–S128. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.R.; Dartt, D.A. Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 2013, 117, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Jiménez, R.; Navas, A.; López-Lizárraga, E.P.; De Ribot, F.M.; Peña, A.; Graue-Hernández, E.O.; Garfias, Y. Ocular Surface as Barrier of Innate Immunity. Open Ophthalmol. J. 2015, 9, 49–55. [Google Scholar] [CrossRef]
- Gasteiger, G.; D’Osualdo, A.; Schubert, D.A.; Weber, A.; Bruscia, E.M.; Hartl, D. Cellular Innate Immunity: An Old Game with New Players. J. Innate Immun. 2017, 9, 111–125. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.-U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef]
- Kassai, M.; Teopipithaporn, R.; Grant, K.B. Hydrolysis of phosphatidylcholine by cerium(IV) releases significant amounts of choline and inorganic phosphate at lysosomal pH. J. Inorg. Biochem. 2011, 105, 215–223. [Google Scholar] [CrossRef]
- Garcia, M.; Mamedova, L.K.; Barton, B.; Bradford, B.J. Choline Regulates the Function of Bovine Immune Cells and Alters the mRNA Abundance of Enzymes and Receptors Involved in Its Metabolism in vitro. Front. Immunol. 2018, 9, 2448. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Zhong, Z.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cell Metab. 2019, 29, 1350–1362.e7. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Coira, I.; Barros-Zulaica, N.; Rodrigo-Angulo, M.; Núñez, Á. Modulation of Specific Sensory Cortical Areas by Segregated Basal Forebrain Cholinergic Neurons Demonstrated by Neuronal Tracing and Optogenetic Stimulation in Mice. Front. Neural Circuits 2016, 10, 28. [Google Scholar] [CrossRef]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Rowley, T.J.; McKinstry, A.; Greenidge, E.; Smith, W.; Flood, P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br. J. Anaesth. 2010, 105, 201–207. [Google Scholar] [CrossRef]
- Kusuda, R.; Carreira, E.U.; Ulloa, L.; Cunha, F.Q.; Kanashiro, A.; Cunha, T.M. Choline attenuates inflammatory hyperalgesia activating nitric oxide/cGMP/ATP-sensitive potassium channels pathway. Brain Res. 2020, 1727, 146567. [Google Scholar] [CrossRef]
- Choi, J.J.; Hwang, J.S.; Shin, Y.J. Effect of Oral Choline Alfoscerate on Patients with Keratoconjunctivitis Sicca. Nutrients 2020, 12, 1526. [Google Scholar] [CrossRef]
- Scapicchio, P.L. Revisiting choline alphoscerate profile: A new, perspective, role in dementia? Int. J. Neurosci. 2013, 123, 444–449. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Li, J.; Wang, Y.; Chen, Q.; Hou, C.; Garrett, Q. Efficacy of Osmoprotectants on Prevention and Treatment of Murine Dry Eye. Investig. Opthalmol. Vis. Sci. 2013, 54, 6287–6297. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Su, Z.; Deng, R.; Lin, J.; Li, D.-Q.; Pflugfelder, S.C. Effects ofl-Carnitine, Erythritol and Betaine on Pro-inflammatory Markers in Primary Human Corneal Epithelial Cells Exposed to Hyperosmotic Stress. Curr. Eye Res. 2015, 40, 657–667. [Google Scholar] [CrossRef]
- Butovich, I.A. Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 2017, 163, 2–16. [Google Scholar] [CrossRef]
- Hwang, H.S.; Parfitt, G.J.; Brown, D.J.; Jester, J.V. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp. Eye Res. 2017, 163, 37–45. [Google Scholar] [CrossRef] [PubMed]
- McCulley, J.P.; Shine, W.E. Meibomian Gland Function and the Tear Lipid Layer. Ocul. Surf. 2003, 1, 97–106. [Google Scholar] [CrossRef]
- Trinh, H.K.T.; Kim, S.-C.; Cho, K.; Ban, G.-Y.; Yoo, H.-J.; Cho, J.-Y.; Park, H.-S.; Kim, S.-H. Exploration of the Sphingolipid Metabolite, Sphingosine-1-phosphate and Sphingosine, as Novel Biomarkers for Aspirin-exacerbated Respiratory Disease. Sci. Rep. 2016, 6, 36599. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, V.; Tan, J.; Nguyen, J.; Lee, J.; Allegood, J.; Galor, A.; Mandal, N. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul. Surf. 2019, 17, 318–326. [Google Scholar] [CrossRef]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef]
- Robciuc, A.; Hyötyläinen, T.; Jauhiainen, M.; Holopainen, J.M. Hyperosmolarity-induced lipid droplet formation depends on ceramide production by neutral sphingomyelinase 2. J. Lipid Res. 2012, 53, 2286–2295. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; 2010; pp. 1–23. [Google Scholar] [CrossRef]
- Taha, T.A.; Argraves, K.M.; Obeid, L.M. Sphingosine-1-phosphate receptors: Receptor specificity versus functional redundancy. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2004, 1682, 48–55. [Google Scholar] [CrossRef]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.P.; De Palma, R.M.; Oaks, J.J.; Oleinik, N.V.; Peterson, Y.K.; Stahelin, R.V.; Skordalakes, E.; Ponnusamy, S.; Garrett-Mayer, E.; Smith, C.D.; et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 2015, 8, ra58. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xu, G.-T.; Zhang, J.; Zhang, J.; Zhang, Y.; Ye, W. FTY720 ameliorates Dry Eye Disease in NOD mice: Involvement of leukocytes inhibition and goblet cells regeneration in ocular surface tissue. Exp. Eye Res. 2015, 138, 145–152. [Google Scholar] [CrossRef]
- Gnädinger, M.; Heimann, R.; Markstein, R. Choline acetyltransferase in corneal epithelium. Exp. Eye Res. 1973, 15, 395–399. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Galitovskiy, V.; Shchepotin, I.B.; Jester, J.V.; Grando, S.A. The Acetylcholine Signaling Network of Corneal Epithelium and Its Role in Regulation of Random and Directional Migration of Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2014, 55, 6921–6933. [Google Scholar] [CrossRef][Green Version]
- Wilson, W.S.; Mckean, C.E. Regional distribution of acetylcholine and associated enzymes and their regeneration in corneal epithelium. Exp. Eye Res. 1986, 43, 235–242. [Google Scholar] [CrossRef]
- Cinar, E.; Yuce, B.; Aslan, F.; Erbakan, G. Neuroprotective Effect of Citicoline Eye Drops on Corneal Sensitivity After LASIK. J. Refract. Surg. 2019, 35, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, K.B.; Plewa, S.; Długaszewska, J.; Froelich, A.; Muszalska-Kolos, I. Design and evaluation of pharmaceutical availability, stability and quality of modified viscosity eye drops with choline salicylate. Eur. J. Pharm. Sci. 2021, 159, 105725. [Google Scholar] [CrossRef] [PubMed]
- Charkoftaki, G.; Jester, J.V.; Thompson, D.C.; Vasiliou, V. Nitrogen mustard-induced corneal injury involves the sphingomyelin-ceramide pathway. Ocul. Surf. 2018, 16, 154–162. [Google Scholar] [CrossRef]
- Magny, R.; Auzeil, N.; Olivier, E.; Kessal, K.; Regazzetti, A.; Dutot, M.; Mélik-Parsadaniantz, S.; Rat, P.; Baudouin, C.; Laprévote, O.; et al. Lipidomic analysis of human corneal epithelial cells exposed to ocular irritants highlights the role of phospholipid and sphingolipid metabolisms in detergent toxicity mechanisms. Biochimie 2020, 178, 148–157. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 1–19. [Google Scholar] [CrossRef]
- Singh, R.; Cuzzani, O.; Binette, F.; Sternberg, H.; West, M.D.; Nasonkin, I.O. Pluripotent Stem Cells for Retinal Tissue Engineering: Current Status and Future Prospects. Stem Cell Rev. Rep. 2018, 14, 463–483. [Google Scholar] [CrossRef]
- Adki, K.M. Potential Biomarkers in Diabetic Retinopathy. Curr. Diabetes Rev. 2020, 16, 971–983. [Google Scholar] [CrossRef]
- Wang, Y.; Surzenko, N.; Friday, W.B.; Zeisel, S.H. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J. 2016, 30, 1566–1578. [Google Scholar] [CrossRef]
- Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 2010, 93, 61–84. [Google Scholar] [PubMed]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Gonzalez, I.; Friday, W.B.; Munson, C.A.; Bachleda, A.; Weiss, E.R.; Alam, N.M.; Sha, W.; Zeisel, S.H.; Surzenko, N. Low availability of choline in utero disrupts development and function of the retina. FASEB J. 2019, 33, 9194–9209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, W.-Q.; Hoshino, A.; Huang, J.; Rieke, F.; Reh, T.A.; Wong, R.O. Development of ON and OFF cholinergic amacrine cells in the human fetal retina. J. Comp. Neurol. 2019, 527, 174–186. [Google Scholar] [CrossRef]
- Arenzana, F.J.; Clemente, D.; Sánchez-González, R.; Porteros, Á.; Aijón, J.; Arévalo, R. Development of the cholinergic system in the brain and retina of the zebrafish. Brain Res. Bull. 2005, 66, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, C.; Vielma, A.H.; Palacios, A.G.; Schmachtenberg, O. Acetylcholine induces GABA release onto rod bipolar cells through heteromeric nicotinic receptors expressed in A17 amacrine cells. Front. Cell. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, O.; Tooyama, I.; Aimi, Y.; Bellier, J.-P.; Hisano, T.; Matsuo, A.; Park, M.; Kimura, H. Demonstration of Cholinergic Ganglion Cells in Rat Retina: Expression of an Alternative Splice Variant of Choline Acetyltransferase. J. Neurosci. 2003, 23, 2872–2881. [Google Scholar] [CrossRef]
- Hutchins, J.B.; Hollyfield, J.G. Acetylcholinesterase in the human retina. Brain Res. 1987, 400, 300–311. [Google Scholar] [CrossRef]
- Nichols, C.W.; Koelle, G.B. Comparison of the localization of acetylcholinesterase and non-specific cholinesterase activities in mammalian and avian retians. J. Comp. Neurol. 1968, 133, 1–15. [Google Scholar] [CrossRef]
- Layer, P.G.; Klaczinski, J.; Salfelder, A.; Sperling, L.E.; Thangaraj, G.; Tuschl, C.; Vogel-Höpker, A. Cholinesterases in development: AChE as a firewall to inhibit cell proliferation and support differentiation. Chem. Interact. 2013, 203, 269–276. [Google Scholar] [CrossRef]
- Granja, M.G.; Braga, L.E.G.; Carpi-Santos, R.; De Araujo-Martins, L.; Nunes-Tavares, N.; Calaza, K.C.; Dos Santos, A.A.; Giestal-De-Araujo, E. IL-4 Induces Cholinergic Differentiation of Retinal Cells In Vitro. Cell. Mol. Neurobiol. 2015, 35, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Shibasaki, K.; Uchigashima, M.; Koizumi, A.; Kurachi, M.; Moriwaki, Y.; Misawa, H.; Kawashima, K.; Watanabe, M.; Kishi, S.; et al. Localization of Acetylcholine-Related Molecules in the Retina: Implication of the Communication from Photoreceptor to Retinal Pigment Epithelium. PLoS ONE 2012, 7, e42841. [Google Scholar] [CrossRef]
- Pu, G.A.; Anderson, R.E. Alteration of retinal choline metabolism in an experimental model for photoreceptor cell degeneration. Investig. Ophthalmol. Vis. Sci. 1983, 24, 288–293. [Google Scholar]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Combination of Neuroprotective and Regenerative Agents for AGE-Induced Retinal Degeneration: In Vitro Study. BioMed Res. Int. 2017, 2017, 8604723. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Fujimoto, N.; Adachi-Usami, E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. NeuroReport 2002, 13, 2109–2111. [Google Scholar] [CrossRef]
- Nashine, S.; Kenney, M.C. Role of Citicoline in an in vitro AMD model. Aging 2020, 12, 9031–9040. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Simón, M.V.; Spalm, F.H.P.; Vera, M.S.; Rotstein, N.P. Sphingolipids as Emerging Mediators in Retina Degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tran, J.-T.A.; Brush, R.S.; Saadi, A.; Rahman, A.K.; Yu, M.; Yasumura, D.; Matthes, M.T.; Ahern, K.; Yang, H.; et al. Ceramide Signaling in Retinal Degeneration. Adv. Exp. Med. Biol. 2012, 723, 553–558. [Google Scholar] [CrossRef]
- German, O.L.; Miranda, G.E.; Abrahan, C.E.; Rotstein, N.P. Ceramide is a Mediator of Apoptosis in Retina Photoreceptors. Investig. Opthalmol. Vis. Sci. 2006, 47, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Shah, H.R.; Rosner, B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: Epigenetic implications. Ophthalmology 2011, 118, 1386–1394. [Google Scholar] [CrossRef]
- Lennikov, A.; Mukwaya, A.; Fan, L.; Saddala, M.S.; De Falco, S.; Huang, H. Synergistic interactions of PlGF and VEGF contribute to blood-retinal barrier breakdown through canonical NFκB activation. Exp. Cell Res. 2020, 397, 112347. [Google Scholar] [CrossRef]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Pericyte-Endothelial Interactions in the Retinal Microvasculature. Int. J. Mol. Sci. 2020, 21, 7413. [Google Scholar] [CrossRef]
- Ossani, G.P.; Pelayes, D.; Diaz, M.L.; Lago, N.R.; Fariña, S.L.; Monserrat, A.J.; Zarate, J.O. Ocular lesions and experimental choline deficiency. Medicina 2006, 66, 415–420. [Google Scholar]
- Jin, X.; Wang, R.-H.; Wang, H.; Long, C.-L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Bi, X.; Xu, M.; Yu, X.; Xue, R.; He, X.; Zang, W. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 42553. [Google Scholar] [CrossRef]
- Roe, A.J.; Zhang, S.; Bhadelia, R.A.; Johnson, E.J.; Lichtenstein, A.H.; Rogers, G.T.; Rosenberg, I.H.; Smith, C.E.; Zeisel, S.H.; Scott, T.M. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am. J. Clin. Nutr. 2017, 105, 1283–1290. [Google Scholar] [CrossRef]
- Nentwich, M.M. Diabetic retinopathy—ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef]
- Chen, C.; Shah, C.P. Review of therapeutic advances in diabetic retinopathy. Ther. Adv. Endocrinol. Metab. 2011, 2, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kaneko, Y.; Mori, A.; Saito, M.; Sakamoto, K.; Nakahara, T.; Ishii, K. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocin-treated rats. Vasc. Pharmacol. 2007, 46, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Bianchimano, P.; Corona, C.; Eleftheriou, C.G.; Sagdullaev, B.T. Optogenetic Stimulation of Cholinergic Amacrine Cells Improves Capillary Blood Flow in Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Sniatecki, J.J.; Goloborodko, E.; Steege, A.; Zavaritskaya, O.; Vetter, J.M.; Grus, F.H.; Patzak, A.; Wess, J.; Pfeiffer, N. Identification of the Muscarinic Acetylcholine Receptor Subtype Mediating Cholinergic Vasodilation in Murine Retinal Arterioles. Investig. Opthalmol. Vis. Sci. 2011, 52, 7479–7484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lim, H.-H.; Lee, S.-H.; Shin, M.-S.; Kim, C.-J.; Yang, H.J. Betaine inhibits vascularization via suppression of Akt in the retinas of streptozotocin-induced hyperglycemic rats. Mol. Med. Rep. 2015, 12, 1639–1644. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Giovannucci, E.L.; Hankinson, S.E.; Zeisel, S.H.; Dougherty, L.W.; Willett, W.C.; Rimm, E.B. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 2007, 86, 1073–1081. [Google Scholar] [CrossRef]
- Park, S.W.; Jun, H.O.; Kwon, E.; Yun, J.-W.; Kim, J.H.; Park, Y.-J.; Kang, B.-C.; Kim, J.H. Antiangiogenic effect of betaine on pathologic retinal neovascularization via suppression of reactive oxygen species mediated vascular endothelial growth factor signaling. Vasc. Pharmacol. 2017, 90, 19–26. [Google Scholar] [CrossRef]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Cinar, E.; Yuce, B.; Aslan, F.; Erbakan, G. Effect of neuroprotective citicoline eye drops on macular microcirculation. Int. Ophthalmol. 2020, 40, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Terelak-Borys, B.; Skonieczna, K.; Grabska-Liberek, I. Ocular ischemic syndrome—A systematic review. Med. Sci. Monit. 2012, 18, RA138–RA144. [Google Scholar] [CrossRef] [PubMed]
- Kamilov, K.M.; Kasimova, M.S.; Makhkamova, D.K. Analysis of choline alfoscerate effectiveness in chronic ocular ischemic syndrome. Vestn. Oftalmol. 2016, 132, 73. [Google Scholar] [CrossRef] [PubMed]
- Argraves, K.M.; Wilkerson, B.A.; Argraves, W.S.; Fleming, P.A.; Obeid, L.M.; Drake, C.J. Sphingosine-1-phosphate Signaling Promotes Critical Migratory Events in Vasculogenesis. J. Biol. Chem. 2004, 279, 50580–50590. [Google Scholar] [CrossRef]
- Lee, M.-J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’Afi, R.I.; Hla, T. Vascular Endothelial Cell Adherens Junction Assembly and Morphogenesis Induced by Sphingosine-1-Phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Maines, L.W.; French, K.J.; Wolpert, E.B.; Antonetti, D.A.; Smith, C.D. Pharmacologic Manipulation of Sphingosine Kinase in Retinal Endothelial Cells: Implications for Angiogenic Ocular Diseases. Investig. Opthalmol. Vis. Sci. 2006, 47, 5022–5031. [Google Scholar] [CrossRef]
- Levitsky, Y.; Hammer, S.S.; Fisher, K.P.; Huang, C.; Gentles, T.L.; Pegouske, D.J.; Xi, C.; Lydic, T.A.; Busik, J.V.; Proshlyakov, D.A. Mitochondrial Ceramide Effects on the Retinal Pigment Epithelium in Diabetes. Int. J. Mol. Sci. 2020, 21, 3830. [Google Scholar] [CrossRef]
- Parsadaniantz, S.M.; Goazigo, A.R.-L.; Sapienza, A.; Habas, C.; Baudouin, C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells 2020, 9, 535. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V.; Levin, L.A. Glaucoma as a Neurodegenerative Disease. J. Neuro-Ophthalmol. 2015, 35, S22–S28. [Google Scholar] [CrossRef]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Siganos, C.S.; Dalianis, G.; Detorakis, E.T. Normal-tension glaucoma: Pathogenesis and genetics (Review). Exp. Ther. Med. 2018, 17, 563–574. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog. Retin. Eye Res. 2019, 72, 100767. [Google Scholar] [CrossRef] [PubMed]
- Virno, M.; Pecori-Giraldi, J.; Liguori, A.; Gregorio, F. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up). Acta Ophthalmol. Scand. 2000, 78, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5′-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, R.; Toczołowski, J.; Solski, J.; Duma, D.; Grieb, P. Citicoline Treatment Increases Retinal Dopamine Content in Rabbits. Ophthalmic Res. 2002, 34, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Schuettauf, F.; Rejdak, R.; Thaler, S.; Bolz, S.; Lehaci, C.; Mankowska, A.; Zarnowski, T.; Junemann, A.; Zagorski, Z.; Zrenner, E.; et al. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp. Eye Res. 2006, 83, 1128–1134. [Google Scholar] [CrossRef]
- Hayreh, S.S. Visual Field Abnormalities in Nonarteritic Anterior Ischemic Optic Neuropathy. Arch. Ophthalmol. 2005, 123, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Barbano, L.; Di Renzo, A.; Coppola, G.; Ziccardi, L. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: A randomized pilot study. PLoS ONE 2019, 14, e0220435. [Google Scholar] [CrossRef]
- Houser, C.R.; Crawford, G.D.; Salvaterra, P.M.; Vaughn, J.E. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: A study of cholinergic neurons and synapses. J. Comp. Neurol. 1985, 234, 17–34. [Google Scholar] [CrossRef]
- Kim, G.-H.; Kim, H.-G.; Jeon, C.-J. Immunocytochemical Localization of Choline Acetyltransferase in the Microbat Visual Cortex. Acta Histochem. ET Cytochem. 2018, 51, 153–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Tzounopoulos, T. Physiological Activation of Cholinergic Inputs Controls Associative Synaptic Plasticity via Modulation of Endocannabinoid Signaling. J. Neurosci. 2011, 31, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Sajedin, A.; Menhaj, M.B.; Vahabie, A.-H.; Panzeri, S.; Esteky, H. Cholinergic Modulation Promotes Attentional Modulation in Primary Visual Cortex- A Modeling Study. Sci. Rep. 2019, 9, 20186. [Google Scholar] [CrossRef] [PubMed]
- Sheynin, Y.; Rosa-Neto, P.; Hess, R.F.; Vaucher, E. Cholinergic Modulation of Binocular Vision. J. Neurosci. 2020, 40, 5208–5213. [Google Scholar] [CrossRef]
- Chamoun, M.; Sergeeva, E.G.; Henrich-Noack, P.; Jia, S.; Grigartzik, L.; Ma, J.; You, Q.; Huppé-Gourgues, F.; Sabel, B.A.; Vaucher, E. Cholinergic Potentiation of Restoration of Visual Function after Optic Nerve Damage in Rats. Neural Plast. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. Lipid conformational order and the etiology of cataract and dry eye. J. Lipid Res. 2021, 62, 100039. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Grami, V.; Marrero, Y.; Tang, D.; Yappert, M.C.; Rasi, V.; Borchman, D. Human Lens Phospholipid Changes with Age and Cataract. Investig. Opthalmol. Vis. Sci. 2005, 46, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Estrada, R.; Yappert, M.C.; Borchman, D. Oxidation-induced changes in human lens epithelial cells. Free Radic. Biol. Med. 2006, 41, 1425–1432. [Google Scholar] [CrossRef]
- Korenfeld, M.S.; Robertson, S.M.; Stein, J.M.; Evans, D.G.; Rauchman, S.H.; Sall, K.N.; Venkataraman, S.; Chen, B.-L.; Wuttke, M.; Burns, W. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye 2021, 1–10. [Google Scholar] [CrossRef]
- Garner, W.H.; Garner, M.H. Protein Disulfide Levels and Lens Elasticity Modulation: Applications for Presbyopia. Investig. Opthalmol. Vis. Sci. 2016, 57, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Blum, P.S.; Pabst, D.M.; Chakrabarti, I.; Jernigan, H.J., Jr. Effects of Cataractogenesis on the CDP-Choline Pathway: Changes in ATP Concentration and Phosphocholine Synthesis during and after Exposure of Rat Lenses to Sugars in vitro and in vivo. Ophthalmic Res. 2003, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of alpha-crystallin with four major phospholipids of eye lens membranes. Exp. Eye Res. 2021, 202, 108337. [Google Scholar] [CrossRef] [PubMed]
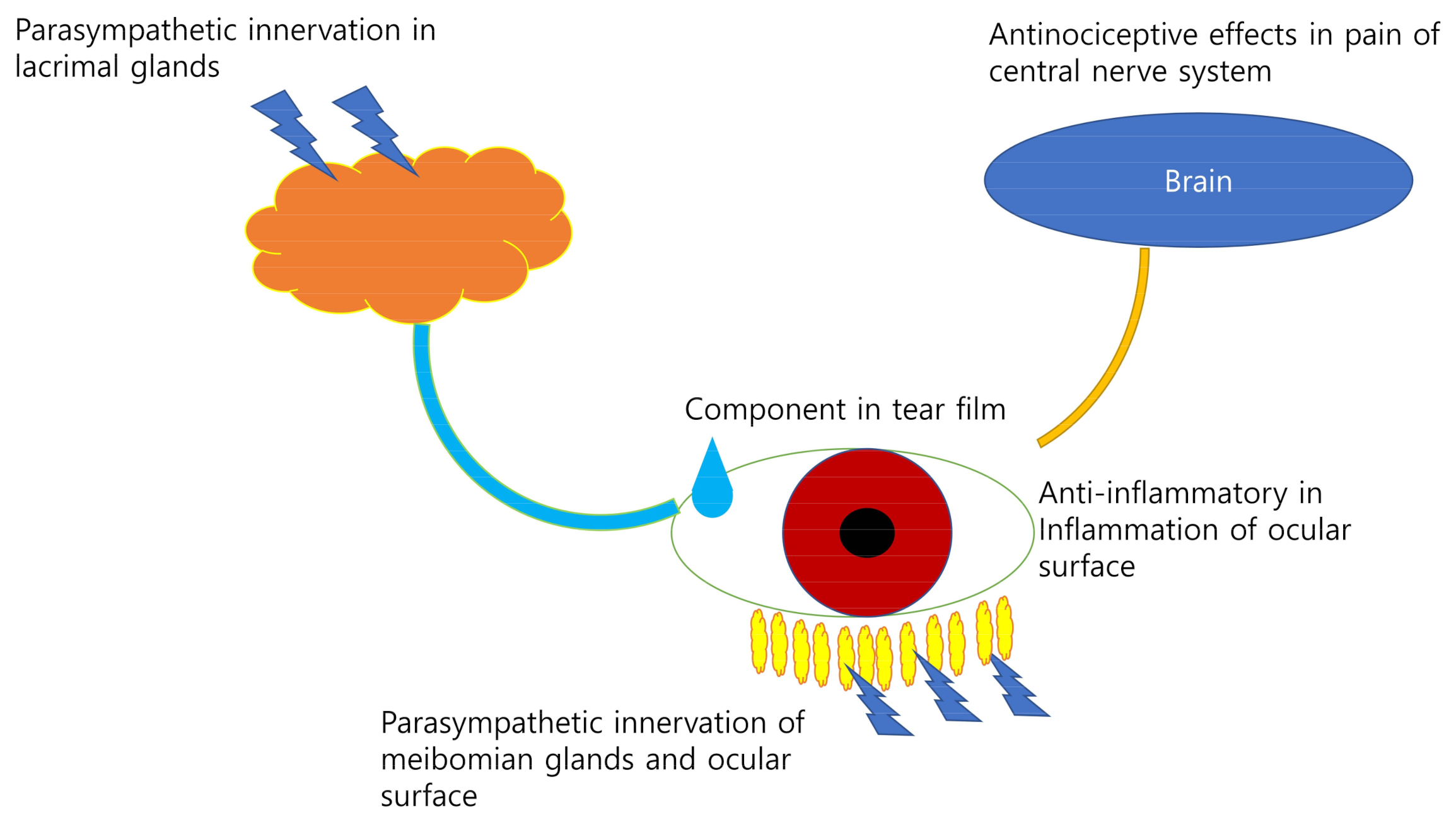
| References | Mode of Action or Mechanism | Organ |
|---|---|---|
| Dartt 2009 [10] | Parasympathetic innervation | Lacrimal glands |
| Naser et al., 2018, Kusuda et al., 2020 [35,37] | Pain signaling pathway | Cholinergic nervous system in brain |
| Zhou et al., 2012, Choi et al., 2020 [7,38] | Components of tear film | Ocular surface |
| Chen et al., 2013, Hua et al., 2015, Robciuc et al., 2012 [40,41,48] | Tear osmolarity and inflammation | Ocular surface |
| McCulley et al., 2003, Paranjpe et al., 2019 [44,46] | Components of meibum | Meibomian glands |
| Cinar et al., 2019 [57] | Wound healing | Ocular surface |
| References | Mode of Action or Mechanism | Disease |
|---|---|---|
| Trujillo-Gonzalez et al., 2019 [67] | Differentiation during retinogenesis | Retinal cytoarchitectural defects |
| Bikbova et al., 2017 [78] | Neuroprotection | Age-related macular degeneration |
| German et al., 2006 [84] | Anti-apoptosis | Age-related macular degeneration |
| Spiegel et al., 2003 [85] | Protection against inflammation, fibrosis and neovascularization | Age-related macular degeneration |
| Nakazawa et al., 2007; Ivanova et al., 2020 [97,98] | Enhancement of blood supply | Diabetic retinopathy |
| Kim et al., 2015; Park et al., 2017 [100,102] | Protection against pathologic neovascularization | Diabetic retinopathy |
| Parisi et al., 2018 [103] | Anti-apoptosis and elevation of retinal dopamine level | Diabetic retinopathy |
| References | Mode of Action or Mechanism | Organ |
|---|---|---|
| Parisi et al., 2018; Gandolfi et al., 2020 [103,117] | Restoration of mitochondrial functions and anti-apoptosis | Retinal ganglion cell and optic nerve |
| Parisi et al., 2019 [121] | Anti-degeneration | Optic nerve |
| Zhao et al., 2011 [124] | Cortical processing | Brain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-S.; Shin, Y.-J. Role of Choline in Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 4733. https://doi.org/10.3390/ijms22094733
Hwang J-S, Shin Y-J. Role of Choline in Ocular Diseases. International Journal of Molecular Sciences. 2021; 22(9):4733. https://doi.org/10.3390/ijms22094733
Chicago/Turabian StyleHwang, Jin-Sun, and Young-Joo Shin. 2021. "Role of Choline in Ocular Diseases" International Journal of Molecular Sciences 22, no. 9: 4733. https://doi.org/10.3390/ijms22094733
APA StyleHwang, J.-S., & Shin, Y.-J. (2021). Role of Choline in Ocular Diseases. International Journal of Molecular Sciences, 22(9), 4733. https://doi.org/10.3390/ijms22094733





