Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Structure, Source, Metabolism, and Bioavailability of Curcumin
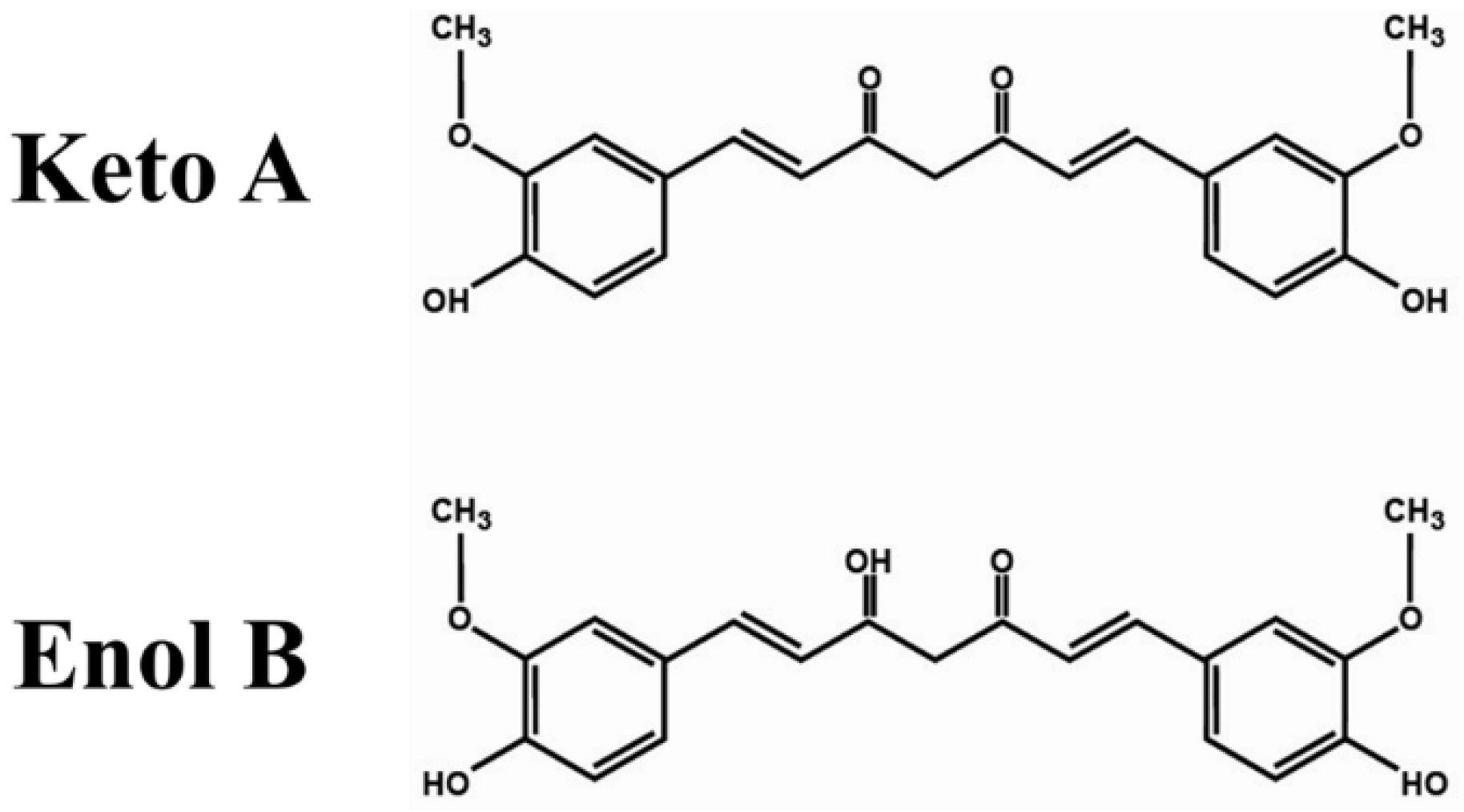
3.1. Chemical Structure
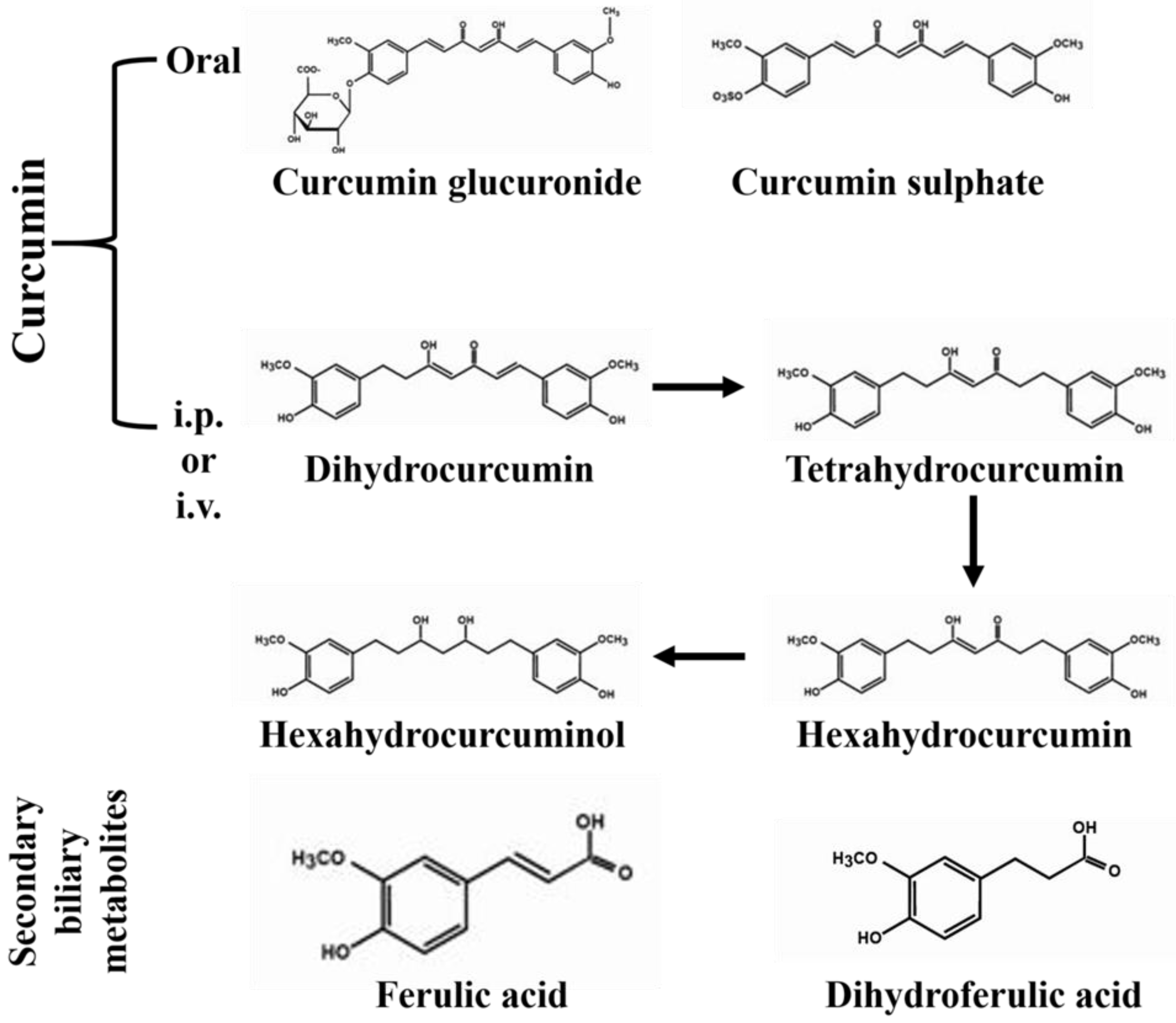
3.2. Source and Metabolism
3.3. Bioavailability
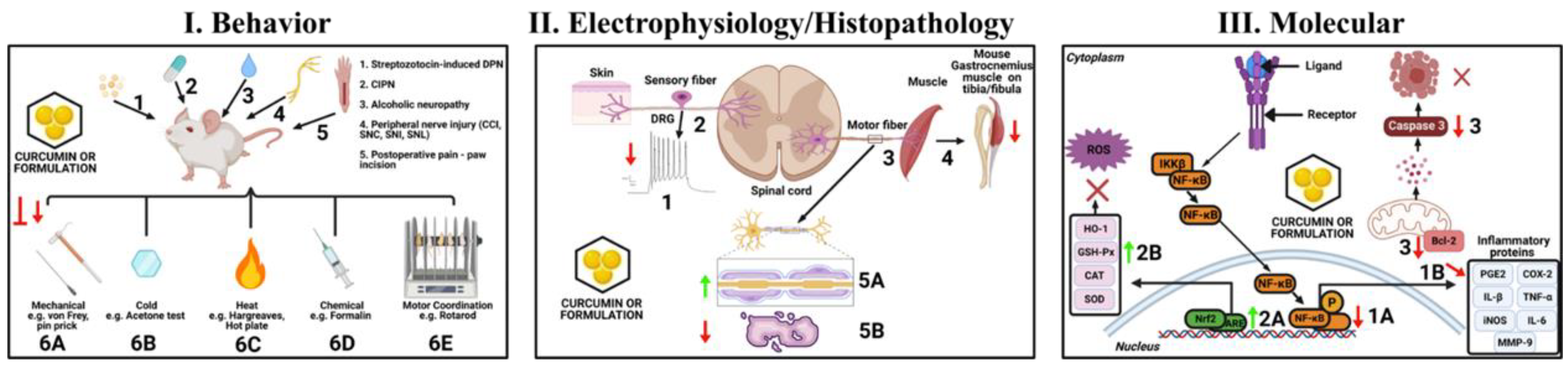
4. Curcumin and Neuropathic Pain—Preclinical Studies
4.1. Alcoholic Neuropathy
4.2. Chemotherapy-Induced Peripheral Neuropathy (CIPN)
4.3. Diabetic Painful Neuropathy (DPN)
4.4. Sciatic Nerve Chronic Constriction Injury (CCI)
4.5. Other Peripheral Neuropathic Pain Models
4.5.1. Sciatic Nerve Crush (SNC) Injury
4.5.2. Spared Nerve Injury (SNI)
4.5.3. Spinal Nerve Ligation (SNL)
5. Curcumin and Postoperative Pain and Preemptive Analgesia—Preclinical Studies
5.1. Postoperative Pain
5.2. Preemptive Analgesia
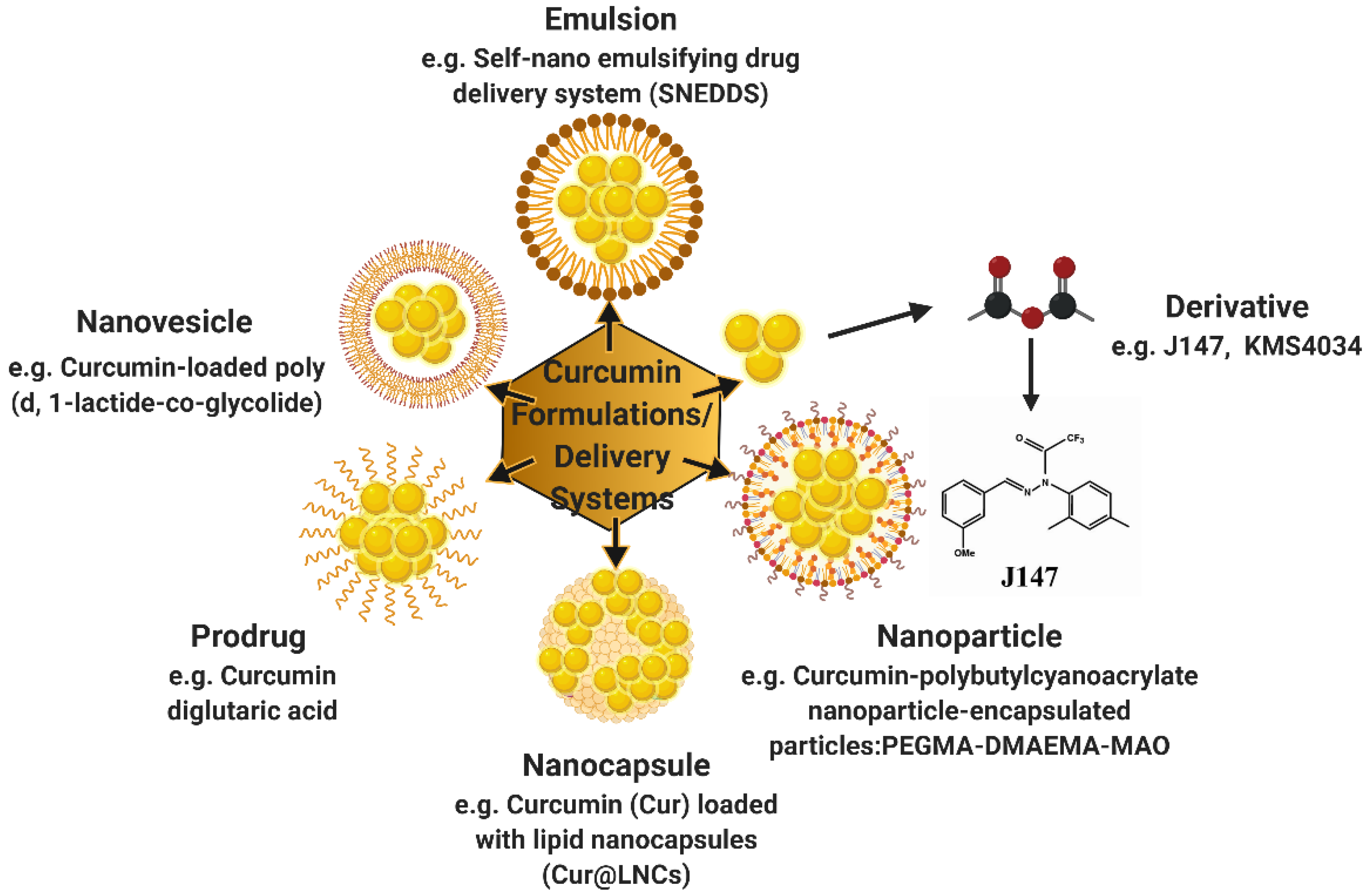
6. Curcumin Formulations and Neuropathic Pain—Preclinical Studies
7. Curcumin and Its Formulations on Neuropathic Pain or Postoperative Pain—Clinical Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpaa, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Smith, B.H.; Torrance, N. Epidemiology of neuropathic pain and its impact on quality of life. Curr. Pain Headache Rep. 2012, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic pain: Central vs. Peripheral mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Ramsay, M.A. Acute postoperative pain management. Proc. Bayl. Univ. Med. Cent. 2000, 13, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Koppert, W.; Schmelz, M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 65–83. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11 (Suppl. 2), S105–S120. [Google Scholar] [CrossRef]
- Barletta, J.F.; Asgeirsson, T.; Senagore, A.J. Influence of intravenous opioid dose on postoperative ileus. Ann. Pharmacother. 2011, 45, 916–923. [Google Scholar] [CrossRef]
- Goettsch, W.G.; Sukel, M.P.P.; van der Peet, D.L.; van Riemsdijk, M.M.; Herings, R.M.C. In-hospital use of opioids increases rate of coded postoperative paralytic ileus. Pharmacoepidemiol. Drug Saf. 2007, 16, 668–674. [Google Scholar] [CrossRef]
- Quintans, J.S.; Antoniolli, A.R.; Almeida, J.R.; Santana-Filho, V.J.; Quintans-Junior, L.J. Natural products evaluated in neuropathic pain models—A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 114, 442–450. [Google Scholar] [CrossRef]
- Basu, P.; Basu, A. In vitro and in vivo effects of flavonoids on peripheral neuropathic pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Kim, Y.T. Food-derived natural compounds for pain relief in neuropathic pain. BioMed Res. Int. 2016, 2016, 7917528. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Hosseinzadeh, H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran. J. Basic Med. Sci. 2018, 21, 347–358. [Google Scholar]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New pharmacological approaches using polyphenols on the physiopathology of neuropathic pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, K. Selected natural phenolic compounds—Potential treatment for peripheral neuropathy? Ceska Slov. Farm. 2014, 63, 55–70. [Google Scholar] [PubMed]
- Alghamdi, S. Antinociceptive effect of the citrus flavonoid eriocitrinon postoperative pain conditions. J. Pain Res. 2020, 13, 805–815. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, J.H. Herbal medicine for the management of postoperative pain: A protocol for the systematic review of randomized controlled trials. Medicine 2019, 98, e14016. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Cheppudira, B.; Fowler, M.; McGhee, L.; Greer, A.; Mares, A.; Petz, L.; Devore, D.; Loyd, D.R.; Clifford, J.L. Curcumin: A novel therapeutic for burn pain and wound healing. Expert Opin. Investig. Drugs 2013, 22, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Eke-Okoro, U.J.; Raffa, R.B.; Pergolizzi, J.V., Jr.; Breve, F.; Taylor, R., Jr.; NEMA Research Group. Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia. J. Clin. Pharm. 2018, 43, 460–466. [Google Scholar] [CrossRef]
- Caillaud, M.; Aung Myo, Y.P.; McKiver, B.D.; Osinska Warncke, U.; Thompson, D.; Mann, J.; Del Fabbro, E.; Desmouliere, A.; Billet, F.; Damaj, M.I. Key developments in the potential of curcumin for the treatment of peripheral neuropathies. Antioxidants 2020, 9, 950. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, H.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, M.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing boswellia serrata, curcuma longa, and vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, e12842. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, D.J.; Marquez, A.; Calcutt, N.A.; Schubert, D. A novel curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology 2018, 129, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Rao, J.; Zou, L.; Zhao, S.; Yi, Z.; Wu, B.; Li, L.; Yuan, H.; Shi, L.; Zhang, C.; et al. Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the p2y12 receptor in the dorsal root ganglia. Front. Neurosci. 2017, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, S.; Chen, H. A novel fabrication of dose-dependent injectable curcumin biocomposite hydrogel system anesthetic delivery method for care and management of musculoskeletal pain. Dose Response 2020, 18, 1559325820929555. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.P.; Negi, G.; Kumar, A.; Pawar, Y.B.; Munjal, B.; Bansal, A.K.; Sharma, S.S. Snedds curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: An insight into its mechanism for neuroprotection. Nanomedicine 2013, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, T.J.; Choi, J.M.; Seo, K.S.; Kim, H.J.; Yoon, T.G.; Lee, Y.S.; Han, H.; Chung, H.J.; Oh, Y.; et al. Antinociceptive curcuminoid, kms4034, effects on inflammatory and neuropathic pain likely via modulating trpv1 in mice. Br. J. Anaesth. 2013, 111, 667–672. [Google Scholar] [CrossRef]
- Limcharoen, T.; Dasuni Wasana, P.W.; Hasriadi; Muangnoi, C.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Curcumin diglutaric acid, a prodrug of curcumin reduces pain hypersensitivity in chronic constriction injury of sciatic nerve induced-neuropathy in mice. Pharmaceuticals 2020, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Cao, L.; Zhang, R.; Bai, F.; Wei, P. A curcumin derivative j147 ameliorates diabetic peripheral neuropathy in streptozotocin (stz)-induced dpn rat models through negative regulation ampk on trpa1. Acta Cirúrgica Bras. 2018, 33, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, S.; Ranjan, A.P.; Di Giannuario, A.; Mukerjee, A.; Marzoli, F.; Di Giovannandrea, R.; Vishwanatha, J.K. Curcumin-loaded poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and bdnf release in spinal cord after acute administration in mice. Colloids Surf. B Biointerface 2017, 158, 379–386. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Na, H.-K.; Kim, S.-H.; Surh, Y.-J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008, 46, 1279–1287. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.K.; Roy, S.; Bagchi, B. Hydrophobic hydration driven self-assembly of curcumin in water: Similarities to nucleation and growth under large metastability, and an analysis of water dynamics at heterogeneous surfaces. J. Chem. Phys. 2014, 141, 18C501. [Google Scholar] [CrossRef] [PubMed]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. Dft and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Ravindran, P.; Babu, K.N.; Sivaraman, K. Turmeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Rao, L.J.M.; Sakariah, K. Chemistry and biological activities of c. Longa. Trends Food Sci. Technol. 2005, 16, 533–548. [Google Scholar] [CrossRef]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.-H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharm. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Pan, M.-H.; Huang, T.-M.; Lin, J.-K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Ireson, C.; Orr, S.; Jones, D.J.L.; Verschoyle, R.; Lim, C.-K.; Luo, J.-L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from curcuma longa by lc–ms/ms. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Douglass, B.J.; Clouatre, D.L. Beyond yellow curry: Assessing commercial curcumin absorption technologies. J. Am. Coll. Nutr. 2015, 34, 347–358. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ji, J.; Bucci, L.R.; Preuss, H.G. A comparative pharmacokinetic assessment of a novel highly bioavailable curcumin formulation with 95% curcumin: A randomized, double-blind, crossover study. J. Am. Coll. Nutr. 2018, 37, 51–59. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase i clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Schiborr, C.; Eckert, G.P.; Rimbach, G.; Frank, J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2010, 397, 1917–1925. [Google Scholar] [CrossRef]
- Chang, M.-T.; Tsai, T.-R.; Lee, C.-Y.; Wei, Y.-S.; Chen, Y.-J.; Chen, C.-R.; Tzen, J.T.C. Elevating bioavailability of curcumin via encapsulation with a novel formulation of artificial oil bodies. J. Agric. Food Chem. 2013, 61, 9666–9671. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase i clinical trial of oral curcumin. Biomark. Syst. Act. Compliance 2004, 10, 6847–6854. [Google Scholar]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Mahale, J.; Singh, R.; Howells, L.M.; Britton, R.G.; Khan, S.M.; Brown, K. Detection of plasma curcuminoids from dietary intake of turmeric-containing food in human volunteers. Mol. Nutr. Food Res. 2018, 62, 1800267. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase i/ii study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yerdelen, D.; Koc, F.; Uysal, H. Strength-duration properties of sensory and motor axons in alcoholic polyneuropathy. Neurol. Res. 2008, 30, 746–750. [Google Scholar] [CrossRef]
- Koike, H.; Iijima, M.; Sugiura, M.; Mori, K.; Hattori, N.; Ito, H.; Hirayama, M.; Sobue, G. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann. Neurol. 2003, 54, 19–29. [Google Scholar] [CrossRef]
- Koike, H.; Sobue, G. Alcoholic neuropathy. Curr. Opin. Neurol. 2006, 19, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.P.; Pelham, R.W.; Rasool, C.G.; Chatterjee, A.; Lash, R.W.; Brown, L.; Munsat, T.L.; Bradley, W.G. Animal models of alcoholic neuropathy: Morphologic, electrophysiologic, and biochemical findings. Muscle Nerve 1979, 2, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Monforte, R.; Estruch, R.; Valls-Sole, J.; Nicolas, J.; Villalta, J.; Urbano-Marquez, A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch. Neurol. 1995, 52, 45–51. [Google Scholar] [CrossRef]
- Palliyath, S.; Schwartz, B.D. Peripheral nerve functions improve in chronic alcoholic patients on abstinence. J. Stud. Alcohol 1993, 54, 684–686. [Google Scholar] [CrossRef]
- McDonough, K.H. Antioxidant nutrients and alcohol. Toxicology 2003, 189, 89–97. [Google Scholar] [CrossRef]
- Montoliu, C.; Valles, S.; Renau-Piqueras, J.; Guerri, C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: Effect of chronic alcohol consumption. J. Neurochem. 1994, 63, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Raygude, K.S.; Ghosh, P.; Ghule, A.E.; Bodhankar, S.L. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci. Lett. 2012, 511, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Yoon, M.H.; Choi, J.I.; Kim, W.M.; Lee, H.G.; Kim, Y.O. Effect of sildenafil on neuropathic pain and hemodynamics in rats. Yonsei Med. J. 2010, 51, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Jia, L.; Lu, X.; Lu, M.; Zhang, L.; Zhang, Y.; Zhang, R.; Zhang, Z.G. Sildenafil ameliorates long term peripheral neuropathy in type ii diabetic mice. PLoS ONE 2015, 10, e0118134. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, A.; Kumar, B.; Singh, S.K.; Bhatia, A.; Gulati, M.; Prakash, T.; Bawa, P.; Malik, A.H. Protective effect of co-administration of curcumin and sildenafil in alcohol induced neuropathy in rats. Eur. J. Pharmacol. 2017, 805, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yardim, A.; Kandemir, F.M.; Comakli, S.; Ozdemir, S.; Caglayan, C.; Kucukler, S.; Celik, H. Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem. Res. 2020, 46, 379–395. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of nf-κb and mitigating inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Agthong, S.; Kaewsema, A.; Charoensub, T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp. Neurobiol. 2015, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Prasanth, K.G.; Balaji, B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm. Biol. 2015, 53, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Al Moundhri, M.S.; Al-Salam, S.; Al Mahrouqee, A.; Beegam, S.; Ali, B.H. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: Some behavioral, biochemical, and histopathological studies. J. Med. Toxicol. 2013, 9, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, E.S.H.; Gomaa, A.M.S. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: Role of angiotensin converting enzyme 1. Appl. Physiol. Nutr. Metab. 2015, 40, 1061–1067. [Google Scholar] [CrossRef]
- Meng, B.; Shen, L.L.; Shi, X.T.; Gong, Y.S.; Fan, X.F.; Li, J.; Cao, H. Effects of curcumin on ttx-r sodium currents of dorsal root ganglion neurons in type 2 diabetic rats with diabetic neuropathic pain. Neurosci. Lett. 2015, 605, 59–64. [Google Scholar] [CrossRef]
- Banafshe, H.R.; Hamidi, G.A.; Noureddini, M.; Mirhashemi, S.M.; Mokhtari, R.; Shoferpour, M. Effect of curcumin on diabetic peripheral neuropathic pain: Possible involvement of opioid system. Eur. J. Pharmacol. 2014, 723, 202–206. [Google Scholar] [CrossRef]
- Zhao, W.C.; Zhang, B.; Liao, M.J.; Zhang, W.X.; He, W.Y.; Wang, H.B.; Yang, C.X. Curcumin ameliorated diabetic neuropathy partially by inhibition of nadph oxidase mediating oxidative stress in the spinal cord. Neurosci. Lett. 2014, 560, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, D.B.; Liu, H.Y.; Hou, W.G.; Dong, Y.S. Curcumin attenuates diabetic neuropathic pain by downregulating tnf-alpha in a rat model. Int. J. Med. Sci. 2013, 10, 377–381. [Google Scholar] [CrossRef]
- Acar, A.; Akil, E.; Alp, H.; Evliyaoglu, O.; Kibrisli, E.; Inal, A.; Unan, F.; Tasdemir, N. Oxidative damage is ameliorated by curcumin treatment in brain and sciatic nerve of diabetic rats. Int. J. Neurosci. 2012, 122, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, S.K.; Agrewala, J.N.; Chopra, K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur. J. Pharmacol. 2006, 536, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.N.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Maklad, Y.A.; Ahmed, A.A.; Kenawy, S.A. Protective effects of combined therapy of gliclazide with curcumin in experimental diabetic neuropathy in rats. Behav. Pharmacol. 2012, 23, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chopra, K.; Kulkarni, S.K. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: Participation of nitric oxide and tnf-alpha. Phytother. Res. 2007, 21, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.J.; Deangelis, L.M. Therapy of chemotherapy-induced peripheral neuropathy. Br. J. Haematol. 2009, 145, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, C.C.; Koeppen, S.; Heimans, J.J.; Huijgens, P.C.; Scheulen, M.E.; Strumberg, D.; Kiburg, B.; Postma, T.J. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology 2005, 64, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gutierrez, G.; Sereno, M.; Miralles, A.; Casado-Saenz, E.; Gutierrez-Rivas, E. Chemotherapy-induced peripheral neuropathy: Clinical features, diagnosis, prevention and treatment strategies. Clin. Transl. Oncol. 2010, 12, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; Flatters, S.J.L. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain 2017, 158, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.S.; Li, Y.X.; Zhang, M.T.; Du, J.; Ma, P.S.; Yao, W.X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.Q. Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Khasabov, S.G.; Olson, J.K.; Uhelski, M.L.; Kim, A.H.; Albino-Ramirez, A.M.; Wagner, C.L.; Seybold, V.S.; Simone, D.A. Pioglitazone, a ppargamma agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain 2019, 160, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Doyle, T.; Salvemini, D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014, 10, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Toyama, S.; Shimoyama, N.; Szeto, H.H.; Schiller, P.W.; Shimoyama, M. Protective effect of a mitochondria-targeted peptide against the development of chemotherapy-induced peripheral neuropathy in mice. ACS Chem. Neurosci. 2018, 9, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Doyle, T.; Bryant, L.; Esposito, E.; Cuzzocrea, S.; Ryerse, J.; Bennett, G.J.; Salvemini, D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain 2013, 154, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef]
- Fukuda, Y.; Li, Y.; Segal, R.A. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef]
- Carvalho, L.F.; Silva, A.M.F.; Carvalho, A.A. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin. Exp. Pharmacol. Physiol. 2017, 44, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, A.; Mitsuma, A.; Maeda, O.; Kajiyama, H.; Kiyoi, H.; Kodera, Y.; Nagino, M.; Goto, H.; Ando, Y. Quantitative assessment of chemotherapy-induced peripheral neurotoxicity using a point-of-care nerve conduction device. Cancer Sci. 2016, 107, 1453–1457. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Hua, C.L.; Sun, N.; Brown, J.S. Miami university background an estimated 30.3 million people in the united states, or 9.4% of the national population, have diabetes (cdc, 2017). In Diabetes is Associated with Increased Morbidity; Miami University: Oxford, OH, USA, 2017. [Google Scholar]
- Barrett, A.M.; Lucero, M.A.; Le, T.; Robinson, R.L.; Dworkin, R.H.; Chappell, A.S. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: A review. Pain Med. 2007, 8 (Suppl. 2), S50–S62. [Google Scholar] [CrossRef]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. 1), 8–14. [Google Scholar] [CrossRef]
- Kaur, S.; Pandhi, P.; Dutta, P. Painful diabetic neuropathy: An update. Ann. Neurosci. 2011, 18, 168–175. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dickenson, A.H. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef]
- Harati, Y. Diabetic neuropathies: Unanswered questions. Neurol. Clin. 2007, 25, 303–317. [Google Scholar] [CrossRef]
- Ramos, K.M.; Jiang, Y.; Svensson, C.I.; Calcutt, N.A. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes 2007, 56, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The renin-angiotensin system in the pathophysiology of type 2 diabetes. Obes. Facts 2012, 5, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Carrasco, J.L.; Zambrano, S.; Blanca, A.J.; Mate, A.; Vazquez, C.M. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of nf-kb. J. Inflamm. 2010, 7, 21. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The nrf2/keap1/are pathway and oxidative stress as a therapeutic target in type ii diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef]
- Uzar, E.; Alp, H.; Cevik, M.U.; Firat, U.; Evliyaoglu, O.; Tufek, A.; Altun, Y. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol. Sci. 2012, 33, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable abts radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Rauter, I.; Krauth, M.T.; Flicker, S.; Gieras, A.; Westritschnig, K.; Vrtala, S.; Balic, N.; Spitzauer, S.; Huss-Marp, J.; Brockow, K.; et al. Allergen cleavage by effector cell-derived proteases regulates allergic inflammation. FASEB J. 2006, 20, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, B.; Saluja, A.K.; Bhatia, M.; Frossard, J.L.; Lee, H.S.; Bhagat, L.; Steer, M.L. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology 1998, 115, 1238–1247. [Google Scholar] [CrossRef]
- Lambeth, J.D. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Cao, H.; Zheng, J.W.; Li, J.J.; Meng, B.; Li, J.; Ge, R.S. Effects of curcumin on pain threshold and on the expression of nuclear factor kappa b and cx3c receptor 1 after sciatic nerve chronic constrictive injury in rats. Chin. J. Integr. Med. 2014, 20, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.X.; Hong, C.; Jun, L.; Renshan, G.; Qinquan, L. Curcumin attenuates mechanical and thermal hyperalgesia in chronic constrictive injury model of neuropathic pain. Pain 2014, 3, 59–69. [Google Scholar] [CrossRef]
- Yu, X.; Cao, H. Curcumin attenuates the expression of nmdar-nr1 in chronic constructive injury model of neuropathic pain. Int. J. Pharmacol. Res. 2015, 5, 35. [Google Scholar]
- Zhao, X.; Xu, Y.; Zhao, Q.; Chen, C.R.; Liu, A.M.; Huang, Z.L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuropharmacology 2012, 62, 843–854. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Chang, R.; Yang, D.; Song, Z.; Guo, Q.; Huang, C. Curcumin alleviates neuropathic pain by inhibiting p300/cbp histone acetyltransferase activity-regulated expression of bdnf and cox-2 in a rat model. PLoS ONE 2014, 9, e91303. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, C.-E.; Jung, D.; Kwak, K.; Park, S.; Lim, D.; Kim, S.; Baek, W. Curcumin could prevent the development of chronic neuropathic pain in rats with peripheral nerve injury. Curr. Ther. Res. Clin. Exp. 2013, 74, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moini Zanjani, T.; Ameli, H.; Labibi, F.; Sedaghat, K.; Sabetkasaei, M. The attenuation of pain behavior and serum cox-2 concentration by curcumin in a rat model of neuropathic pain. Korean J. Pain 2014, 27, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Rajakariar, R.; Yaqoob, M.; Gilroy, D. Cox-2 in inflammation and resolution. Mol. Interv. 2006, 6, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; He, M.-l.; Bohlin, L. Is cox-2 a perpetrator or a protector? Selective cox-2 inhibitors remain controversial. Acta Pharmacol. Sin. 2005, 26, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Brune, K. Cyclooxygenase-2—10 years later. J. Pharmacol. Exp. Ther. 2002, 300, 367–375. [Google Scholar] [CrossRef]
- Dover, A.R.; Hadoke, P.W.; Macdonald, L.J.; Miller, E.; Newby, D.E.; Walker, B.R. Intravascular glucocorticoid metabolism during inflammation and injury in mice. Endocrinology 2007, 148, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lim, G.; Zeng, Q.; Sung, B.; Yang, L.; Mao, J. Central glucocorticoid receptors modulate the expression and function of spinal nmda receptors after peripheral nerve injury. J. Neurosci. 2005, 25, 488–495. [Google Scholar] [CrossRef]
- Tian, F.; Hu, X.Z.; Wu, X.; Jiang, H.; Pan, H.; Marini, A.M.; Lipsky, R.H. Dynamic chromatin remodeling events in hippocampal neurons are associated with nmda receptor-mediated activation of bdnf gene promoter 1. J. Neurochem. 2009, 109, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, D.; Kocman, A.E.; Yildirim, E.; Ozatik, O.; Aydin, S.; Kose, A. Comparison of the effects of curcumin, tramadol and surgical treatments on neuropathic pain induced by chronic constriction injury in rats. Turk. Neurosurg. 2018, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.P.; Chen, L.; Wang, W.; Wu, D.; Wang, L.Y.; Zhang, T.; Zhang, H.; Xu, L.X.; Li, Y.Q. Combination of tramadol with minocycline exerted synergistic effects on a rat model of nerve injury-induced neuropathic pain. Neurosignals 2013, 21, 184–196. [Google Scholar] [CrossRef]
- Sun, W.; Sun, C.; Lin, H.; Zhao, H.; Wang, J.; Ma, H.; Chen, B.; Xiao, Z.; Dai, J. The effect of collagen-binding ngf-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials 2009, 30, 4649–4656. [Google Scholar] [CrossRef]
- Yin, Z.S.; Zhang, H.; Bo, W.; Gao, W. Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. Am. J. Neuroradiol. 2010, 31, 509–515. [Google Scholar] [CrossRef]
- Sun, W.; Sun, C.; Zhao, H.; Lin, H.; Han, Q.; Wang, J.; Ma, H.; Chen, B.; Xiao, Z.; Dai, J. Improvement of sciatic nerve regeneration using laminin-binding human ngf-beta. PLoS ONE 2009, 4, e6180. [Google Scholar] [CrossRef]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.-A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.-M.; et al. Local low dose curcumin treatment improves functional recovery and remyelination in a rat model of sciatic nerve crush through inhibition of oxidative stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Moharrami Kasmaie, F.; Jahromi, Z.; Gazor, R.; Zaminy, A. Comparison of melatonin and curcumin effect at the light and dark periods on regeneration of sciatic nerve crush injury in rats. EXCLI J. 2019, 18, 653–665. [Google Scholar] [PubMed]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Omidi, A.; Karbalay-Doust, S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol. Res. Pract. 2011, 207, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Yuce, S. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann. Plast. Surg. 2015, 74, 684–692. [Google Scholar] [CrossRef]
- Maurício, A.C. Peripheral Nerve Regeneration: From Surgery to New Therapeutic Approaches Including Biomaterials and Cell-Based Therapies Development; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Rateb, E.E.; Amin, S.N.; El-Tablawy, N.; Rashed, L.A.; El-Attar, S. Effect of melatonin supplemented at the light or dark period on recovery of sciatic nerve injury in rats. EXCLI J. 2017, 16, 138–150. [Google Scholar]
- Gupta, J.; Qureshi, S. Potential benefits of methylcobalamin: A review. Austin J. Pharmacol. Ther. 2015, 3, 1076–1080. [Google Scholar]
- Sang, Q.; Sun, D.; Chen, Z.; Zhao, W. Ngf and pi3k/akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed. Pharm. 2018, 103, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Zhang, M.-T.; Mao-Ying, Q.-L.; Hu, L.-Y.; Wu, G.-C.; Mi, W.-L.; Wang, Y.-Q. Curcumin ameliorates neuropathic pain by down-regulating spinal il-1β via suppressing astroglial nalp1 inflammasome and jak2-stat3 signalling. Sci. Rep. 2016, 6, 28956. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Quintos, T.; Salgado-Moreno, G.; Pérez-Ramos, J.; Coen, A.; Godínez-Chaparro, B. Anti-allodynic effect induced by curcumin in neuropathic rat is mediated through the no-cyclic-GMP-ATP sensitive K+ channels pathway. BMC Complement. Med. Ther. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.D.; Jung, H.C.; Cheong, Y.K. The effect of intrathecal curcumin on mechanical allodynia in rats after l5 spinal nerve ligation. Korean J. Anesthesiol. 2014, 67, S122–S123. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Shields, S.D.; Eckert, W.A., 3rd; Basbaum, A.I. Spared nerve injury model of neuropathic pain in the mouse: A behavioral and anatomic analysis. J. Pain 2003, 4, 465–470. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S. Targeting interleukin-1β for pain. CNS Neurol. Disord. Drug Targets 2011, 10, 571–575. [Google Scholar] [CrossRef]
- Kiso, T.; Watabiki, T.; Tsukamoto, M.; Okabe, M.; Kagami, M.; Nishimura, K.; Aoki, T.; Matsuoka, N. Pharmacological characterization and gene expression profiling of an l5/l6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience 2008, 153, 492–500. [Google Scholar] [CrossRef]
- Woolf, C.J. Overcoming obstacles to developing new analgesics. Nat. Med. 2010, 16, 1241–1247. [Google Scholar] [CrossRef]
- Apfelbaum, J.; Chen, C.; Mehta, S.; Gan, T. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Phillips, C.; Gelesko, S.; Proffit, W.R.; White, R.P., Jr. Recovery after third-molar surgery: The effects of age and sex. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 700.e1–700.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Y.; Yun, X.; Ou, Y.; Zhang, W.; Li, J.X. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci. Rep. 2014, 4, 4932. [Google Scholar] [CrossRef] [PubMed]
- Sahbaie, P.; Sun, Y.; Liang, D.Y.; Shi, X.Y.; Clark, J.D. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth. Analg. 2014, 118, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Lantero, A.; Tramullas, M.; Diaz, A.; Hurle, M.A. Transforming growth factor-beta in normal nociceptive processing and pathological pain models. Mol. Neurobiol. 2012, 45, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Shin, J.; Yoon, J.; Yin, M.; Yoon, M. Differential expression of spinal γ-aminobutyric acid and opioid receptors modulates the analgesic effects of intrathecal curcumin on postoperative/inflammatory pain in rats. Anesth. Pain Med. 2018, 13, 82–92. [Google Scholar] [CrossRef]
- Ma, P.; Tumin, D.; Cismowski, M.; Tobias, J.D.; Gomez, D.; McConnell, P.; Naguib, A.; Yates, A.R.; Winch, P. Effects of preoperative curcumin on the inflammatory response during mechanical circulatory support: A porcine model. Cardiol. Res. 2018, 9, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Bolboaca, S.D.; Stanescu, I.C.; Sfrangeu, C.A.; Bulboaca, A.C. Preemptive analgesic and antioxidative effect of curcumin for experimental migraine. BioMed Res. Int. 2017, 2017, 4754701. [Google Scholar] [CrossRef]
- Nurullahoglu, K.E.; Okudan, N.; Belviranli, M.; Oz, M. The comparison of preemptive analgesic effects of curcumin and diclofenac. Bratisl. Lekárske Listy 2014, 115, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Chong, M.S. Preemptive analgesia—Treating postoperative pain by preventing the establishment of central sensitization. Anesth. Analg. 1993, 77, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Katz, J. Pre-emptive analgesia: Evidence, current status and future directions. Eur. J. Anaesthesiol. Suppl. 1995, 10, 8–13. [Google Scholar] [PubMed]
- Kissin, I. Preemptive analgesia: Problems with assessment of clinical significance. Methods Mol. Biol. 2010, 617, 475–482. [Google Scholar] [PubMed]
- Van der Marel, C.D.; Anderson, B.J.; Romsing, J.; Jacqz-Aigrain, E.; Tibboel, D. Diclofenac and metabolite pharmacokinetics in children. Paediatr. Anaesth. 2004, 14, 443–451. [Google Scholar] [CrossRef]
- Buggy, D.J.; Wall, C.; Carton, E.G. Preoperative or postoperative diclofenac for laparoscopic tubal ligation. Br. J. Anaesth. 1994, 73, 767–770. [Google Scholar] [CrossRef]
- Gillberg, L.E.; Harsten, A.S.; Stahl, L.B. Preoperative diclofenac sodium reduces post-laparoscopy pain. Can. J. Anaesth. 1993, 40 Pt 1, 406–408. [Google Scholar] [CrossRef][Green Version]
- Evers, S.; Afra, J.; Frese, A.; Goadsby, P.J.; Linde, M.; May, A.; Sandor, P.S.; European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine—Revised report of an EFNS task force. Eur. J. Neurol. 2009, 16, 968–981. [Google Scholar] [CrossRef]
- Freitag, F.G. The cycle of migraine: Patients’ quality of life during and between migraine attacks. Clin. Ther. 2007, 29, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.R.; Den Boer, M.O. Pharmacology of antimigraine drugs. J. Neurol. 1991, 238, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A pilot cross-over study to evaluate human oral bioavailability of bcm-95cg (biocurcumax), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Hirano, S.; Katanasaka, Y.; Miyazaki, Y.; Funamoto, M.; Okamura, N.; Hojo, Y.; Suzuki, H.; Doi, O.; Yokoji, T.; et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study. J. Nutr. Sci. Vitaminol. 2015, 61, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Munch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Prior, M.; Dargusch, R.; Roberts, A.; Riek, R.; Eichmann, C.; Chiruta, C.; Akaishi, T.; Abe, K.; Maher, P.; et al. A novel neurotrophic drug for cognitive enhancement and alzheimer’s disease. PLoS ONE 2011, 6, e27865. [Google Scholar] [CrossRef]
- Prior, M.; Goldberg, J.; Chiruta, C.; Farrokhi, C.; Kopynets, M.; Roberts, A.J.; Schubert, D. Selecting for neurogenic potential as an alternative for alzheimer’s disease drug discovery. Alzheimers Dement. J. Alzheimers Assoc. 2016, 12, 678–686. [Google Scholar] [CrossRef]
- Calcutt, N.A.; Smith, D.R.; Frizzi, K.; Sabbir, M.G.; Chowdhury, S.K.R.; Mixcoatl-Zecuatl, T.; Saleh, A.; Muttalib, N.; Van der Ploeg, R.; Ochoa, J.; et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J. Clin. Investig. 2017, 127, 608–622. [Google Scholar] [CrossRef]
- Roy Chowdhury, S.K.; Smith, D.R.; Saleh, A.; Schapansky, J.; Marquez, A.; Gomes, S.; Akude, E.; Morrow, D.; Calcutt, N.A.; Fernyhough, P. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain J. Neurol. 2012, 135 Pt 6, 1751–1766. [Google Scholar] [CrossRef]
- Katagiri, A.; Shinoda, M.; Honda, K.; Toyofuku, A.; Sessle, B.J.; Iwata, K. Satellite glial cell p2y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol. Pain 2012, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yamanaka, H.; Noguchi, K. Expression of atp receptors in the rat dorsal root ganglion and spinal cord. Anat. Sci. Int. 2013, 88, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic mechanisms and pain--an update. Eur. J. Pharm. 2013, 716, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Ceruti, S. P2y purinergic receptors: New targets for analgesic and antimigraine drugs. Biochem. Pharm. 2013, 85, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Goloncser, F.; Csolle, C.; Kiraly, K.; Ando, R.D.; Baranyi, M.; Kovanyi, B.; Mate, Z.; Hoffmann, K.; Algaier, I.; et al. Central p2y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol. Dis. 2014, 70, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of meriva(r), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Sterzi, S.; Giordani, L.; Morrone, M.; Lena, E.; Magrone, G.; Scarpini, C.; Milighetti, S.; Pellicciari, L.; Bravi, M.; Panni, I.; et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: A randomized, double-blind, placebo-controlled study. Eur. J. Phys. Rehabil. Med. 2016, 52, 321–330. [Google Scholar]
- Pinsornsak, P.; Niempoog, S. The efficacy of curcuma longa l. Extract as an adjuvant therapy in primary knee osteoarthritis: A randomized control trial. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2012, 95 (Suppl. 1), S51–S58. [Google Scholar]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and efficacy of curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Gharbi, M.; Dierckxsens, Y.; Priem, F.; Marty, M.; Seidel, L.; Albert, A.; Heuse, E.; Bonnet, V.; Castermans, C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.A.; Tripathi, C.D.; Agarwal, B.B.; Saluja, S. Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: A double-blind, randomized placebo-controlled study. Surg. Endosc. 2011, 25, 3805–3810. [Google Scholar] [CrossRef]
- Anil, A.; Gujjari, S.K.; Venkatesh, M.P. Evaluation of a curcumin-containing mucoadhesive film for periodontal postsurgical pain control. J. Indian Soc. Periodontol. 2019, 23, 461–468. [Google Scholar] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complement. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (meriva(r)) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Maulina, T.; Diana, H.; Cahyanto, A.; Amaliya, A. The efficacy of curcumin in managing acute inflammation pain on the post-surgical removal of impacted third molars patients: A randomised controlled trial. J. Oral Rehabil. 2018, 45, 677–683. [Google Scholar] [CrossRef]
- Phoolcharoen, N.; Oranratanaphan, S.; Ariyasriwatana, C.; Worasethsin, P. Efficacy of curcuminoids for reducing postoperative pain after laparoscopic gynecologic surgery: A pilot randomized trial. J. Complement. Integr. Med. 2019, 16. [Google Scholar] [CrossRef]
- Di Pierro, F.; Settembre, R. Safety and efficacy of an add-on therapy with curcumin phytosome and piperine and/or lipoic acid in subjects with a diagnosis of peripheral neuropathy treated with dexibuprofen. J. Pain Res. 2013, 6, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Davies, J.L.; Litchy, W.J.; O’Brien, P.C. Longitudinal assessment of diabetic polyneuropathy using a composite score in the rochester diabetic neuropathy study cohort. Neurology 1997, 49, 229–239. [Google Scholar] [CrossRef]
- Davies, M.; Brophy, S.; Williams, R.; Taylor, A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006, 29, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The eurodiab iddm complications study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharm. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Or, S.; Bozkurt, A. Analgesic effect of aspirin, mefenamic acid and their combination in post-operative oral surgery pain. J. Int. Med. Res. 1988, 16, 167–172. [Google Scholar] [CrossRef]
- Harrison, R.F.; Brennan, M. Comparison of two formulations of lignocaine spray with mefenamic acid in the relief of post-episiotomy pain: A placebo-controlled study. Curr. Med. Res. Opin. 1987, 10, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Michelet, D.; Andreu-Gallien, J.; Bensalah, T.; Hilly, J.; Wood, C.; Nivoche, Y.; Mantz, J.; Dahmani, S. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesth. Analg. 2012, 114, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Hamza, M.; Wu, T.-X.; Dionne, R.A. Upregulation of il-6, il-8 and ccl2 gene expression after acute inflammation: Correlation to clinical pain. Pain 2009, 142, 275–283. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. Il-6-dependent pge2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef] [PubMed]
| Animals | Dose a, Route of Administration | Bioavailability | Reference | ||
|---|---|---|---|---|---|
| Tissue | Concentration | Time Measured after Administration | |||
| Animal Studies | |||||
| NMRI and C57/BL6 mouse | 50 mg/kg, force-fed | Brain | Below detection limit | 30, 60, and 120 min | |
| 100 mg/kg, i.p. | 0.004–0.005 mg/g | 20–40 min | [65] | ||
| C57BL6/J male and female mice | 0.148 mg, i.p. | Brain | 0.000739 ± 0.019 mg/g 2.010 µM | 4 h | [55] |
| Plasma | 0.000127 ± 0.035 mg/mL 0.345 µM | ||||
| 0.148 mg, oral | Brain | 0.000519 ± 0.098a mg/g 1.412 µM | 4 h | ||
| Plasma | Below detection level | ||||
| 0.074 mg, intramuscular | Brain | 0.001162 ± 0.004 mg/g 3.157 µM | 4 h | ||
| Plasma | 0.000238 ± 0.048 mg/mL 0.647 µM | ||||
| ~2.5 mg/day, oral (500 ppm) | Brain | 0.000469 ± 0.220 mg/g 1.276 µM | 4 months | ||
| Plasma | 0.000035 ± 0.014 mg/mL 0.095 µM | ||||
| ~10 mg/day, oral (2000 ppm) | Brain | 0.000525 ± 0.125 mg/g 1.428 µM | 4 months | ||
| Plasma | 0.000171 ± 0.019 mg/mL 0.465 µM | ||||
| Male Sprague-Dawley rats | 500 mg/kg, oral | Plasma | 0.00006 ± 0.01 mg/mL | 41.7 ± 5.4 min | [56] |
| 10 mg/kg, intravenous | Plasma | 0.00036 ± 0.05 mg/mL | Not mentioned | ||
| Male Sprague-Dawley rats | 1000 mg/kg, oral | Plasma | 15 ng/mL | 50 min | [66] |
| Female BALB/c mice | 0.1 g/kg, i.p. | Brain | 0.00041 ± 0.01 mg/g | 1 h | [50] |
| Intestine | 0.11704 ± 6.86 mg/g | ||||
| Kidneys | 0.00751 ± 0.08 mg/g | ||||
| Liver | 0.0269 ± 2.58 mg/g | ||||
| Plasma | 0.0006 ± 0.03 mg/g | ||||
| Spleen | 0.02606 ± 1.06 mg/g | ||||
| Clinical Studies | |||||
| Human | 500–8000 mg/day, oral | Serum | 0.51 ± 0.11 µM 0.63 ± 0.06 µM 1.77 ± 1.87 µM | 1–2 h | [67] |
| 4000 mg 6000 mg 8000 mg | Urine | Undetectable | |||
| Human | 500–12,000 mg, oral 500–8000 mg | Serum | Undetectable | 1, 2, 4 h | [63] |
| 10,000 mg | 30.4 ng/mL 39.5 ng/mL 50.5 ng/mL | 1 h 2 h 4 h | |||
| 12,000 mg | 29.7 ng/mL 57.6 ng/mL 51.2 ng/mL | 1 h 2 h 4 h | |||
| Human | 0.45–3.6 g, oral | Plasma | 11.1 ± 0.6 nmol/L | 1 h | [68] |
| Under 3.6 g | Urine | 0.1–1.3 nmol/L | |||
| Human | 2 or 4 g, oral | Plasma | 7 ng/mL | 24 weeks | [69] |
| Human | 100 mg, oral | Plasma | 3.2 nM | 2 h | [70] |
| Human | 8 g, oral | Plasma | 29 to 91 ng/mL | 3 months | [71] |
| Animals (Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Effects | Reference | ||
|---|---|---|---|---|---|
| Behavioral/Other | Electrophysiological/ Functional | Histopathological/ Biochemical/Molecular | |||
| Alcoholic Neuropathy | |||||
| Male Wistar rats | 35% (v/v) ethanol 10 g/kg, b.i.d (bis in die, i.e., twice daily), oral, 10 weeks + curcumin: 20, 40 and 80 mg/kg, oral, 10 weeks + 35% (v/v) ethanol (10 g/kg), oral, 10 weeks | ↑ Mechanical hyperalgesia threshold (Randall–Selitto paw pressure test) ↓ Mechanical allodynia (von Frey hair test) Thermal hyperalgesia (Tail immersion test) | X Reduction in MNCV | ↓ MDA, neural nitrite, and total calcium content ↓ TNF-α and IL-1β and DNA fragmentation in sciatic nerve | [80] |
| Combination Study | |||||
| Wistar Albino rats of either sex | Curcumin per se: 60 mg/kg, i.p. 10 weeks 35% (v/v) ethanol (10 g/kg, twice daily, oral, 10 weeks) + curcumin (30 and 60 mg/kg, oral, 10 weeks) Sildenafil per se: 10 mg/kg, i.p. 10 weeks 35% (v/v) ethanol (10 g/kg, twice daily, oral, 10 weeks) + sildenafil (5 and 10 mg/kg, oral, 10 weeks) 35% (v/v) ethanol (10 g/kg, twice daily, oral, 10 weeks) + curcumin (30 mg/kg, oral, 10 weeks) + sildenafil (5 mg/kg, oral, 10 weeks) | Combination ✓ Improved motor coordination (rotarod test) ↓ Thermal hyperalgesia (hotplate test) and paw heat allodynia (hotplate test), mechanical hyperalgesia (pin prick test), cold allodynia (acetone test), tail cold-hyperalgesia (tail immersion test) | Not tested | ↓ MDA level ✓ GSH level ↓ Ethanol-induced fiber derangement, nerve fiber swelling, Schwann cells activation ✓ Nerve fibers | [83] |
| Chemotherapy-Induced Peripheral Neuropathy (CIPN) | |||||
| Male Sprague-Dawley rats | Paclitaxel (2 mg/kg, i.p., 5 consecutive days) Curcumin (200 mg/kg/day, oral, 10 consecutive days) Paclitaxel (2 mg/kg, i.p., 5 consecutive days) + curcumin (100 or 200 mg/kg/day, oral, 10 consecutive days) | Not tested | Not tested | In spinal cord and sciatic nerve tissues: ↓ NF-κB and GFAP levels ↓ mRNA expressions of TNF-α, IL6, NF-κB, iNOS, Bcl-2 and Bcl-xL ↑ mRNA expression of Nrf2 ↓ mRNA expressions of caspase-3, p53, Apaf-1 ↓ mRNA expressions of autophagy related genes LC3A, LC3B, and Beclin-1 ↓ Immunohistochemical expressions of 8-OHdG, caspase-3 and LC3B ✓ Spinal cord and sciatic nerve histological architecture and integrity with no sciatic nerve damage, including vacuolation, neuronal degeneration, and demyelination | [84] |
| Male Sprague-Dawley rats | Oxaliplatin (4 mg/kg, i.p., twice weekly for 4 weeks) + curcumin (12.5, 25 and 50 mg/kg, oral, 4 weeks) | ↑ Mechanical (von Frey) threshold ↓ Cold allodynia (acetone test) | ↑ MNCV and SNCV | ✓ Injured spinal cord cells ↑ Levels of SOD, GSH-Px, and CAT in spinal cord ↓ MDA level in spinal cord X Protein expression of p-NF-κB/NF-κB in spinal cord ↓ TNF-α, IL-6 and IL-1β levels in spinal cord | [85] |
| Female Wistar rats | Cisplatin (2 mg/kg, i.p., twice a week for 5 weeks) + curcumin (200 mg/kg, oral, once daily for 5 weeks) | ↓ Thermal hypoalgesia (hotplate test) | ✓ Reduction in MNCV | ↑ Myelin thickness in sciatic nerve X Nuclear and nucleolar atrophy, loss of neurons in L4 DRG | [86] |
| Male Swiss Albino mice | Vincristine sulfate (0.1 mg/kg, i.p., once per day for 7 consecutive days) + curcumin (15, 30, 60 mg/kg/day, oral, 14 consecutive days) | ↑ Thermal hyperalgesia (hotplate test) ↓ Thermal allodynia (cold plate test) and mechanical hyperalgesia (pin prick test) O Motor coordination (rotarod test) O Acute phase of nociception (formalin test) ↓ Paw elevation and licking in delayed phase of nociception (formalin test) | ↓ Vincristine-induced rise in SFI | ↓ Calcium level in sciatic nerve ↑ SOD activity in sciatic nerve ↑ Sciatic SOD, CAT, GPx, and GSH ↓ Levels of LPO and NO | [87] |
| Male Wistar rats | Oxaliplatin (4 mg/kg, i.p., twice weekly for 4.5 weeks) + curcumin (10 mg/kg, oral, twice weekly for 4.5 weeks) Cisplatin (2 mg/kg, i.p., twice weekly for 4.5 weeks) + curcumin (10 mg/kg, oral, twice weekly for 4.5 weeks) | O Motor coordination (rotarod test), cold (cold water tail flick test), mechanical (paw pressure test), thermal (tail flick test) nociception | Not tested | ↓ Plasma concentration of neurotensin Co-treatment with oxaliplatin or cisplatin insignificantly ↓ platinum concentration in sciatic nerve ↓ Demyelination | [88] |
| Diabetic Painful Neuropathy (DPN) | |||||
| Male Wistar rats | Streptozotocin (STZ) (100 mg/kg, i.p.) + curcumin (100 mg/kg, oral, 6 weeks) | ↑ Body weight, and kidney weight/body weight ↑ Thermal hyperalgesia (hotplate and tail flick test) and mechanical allodynia (von Frey) | ↓ FBG, TG, total cholesterol, LDL-C, total peroxide, serum creatinine, and BUN ↑ HDL-C ↓ Renal ACE1 ✓ TNF-α and IL-10 in kidneys and sciatic nerves | [89] | |
| Male Sprague-Dawley rats | a Animals with type 2 diabetes with diabetic neuropathic pain + curcumin (100 mg/kg, i.p., 14 days) | ↑ Mechanical withdrawal threshold (von Frey) and thermal withdrawal latency (heat stimulus) | ✓ TTX-R sodium currents INa of small-sized DRG neurons | Not tested | [90] |
| Male Albino Wistar rats | STZ (60 mg/kg, i.p.) + acute or chronic curcumin (50 mg/kg/day, i.p.) Acute treatment: only 30 min prior to pain assessment Chronic treatment: from 7th day till 21st day injected once a day | O Hyperglycemia Chronic treatment X Weight loss Chronic treatment ↑ Thermal hyperalgesia (plantar test) and mechanical allodynia (von Frey) Naloxone pre-treatment ↓ Anti-allodynic effect of chronic curcumin | Not tested | Not tested | [91] |
| Male Sprague-Dawley rats | STZ (60 mg/kg, i.p.) + curcumin (200 mg/kg, intragastric, 14 days) | O Hyperglycemia and increased body weight ↑ Tactile allodynia (von Frey) | Not tested | ✓ Protein expressions of NADPH oxidase subunits gp91phox and p47phox in spinal cord ↓ H2O2 and MDA in spinal cord ↑ SOD in spinal cord | [92] |
| Male Sprague-Dawley rats | STZ (65 mg/kg, i.p.) + curcumin (60 mg/kg, oral, daily from day 3 to day 28) | ↓ Hyperglycemia and body weight ✓ Thermal hyperalgesia (Hargreaves test) and mechanical allodynia (von Frey) | Not tested | ↓ Spinal TNF-α and TNF-α receptor 1 | [93] |
| Female Wistar Albino rats | STZ (50 mg/kg, i.p.) + curcumin (60 mg/kg, oral, 21 days) Non-diabetic rats + curcumin (60 mg/kg, oral, 21 days) | Not tested | Not tested | ↓ MDA, TOS, OSI, and NO in brain and sciatic tissues ↑ TAS in brain and sciatic tissues | [94] |
| Male Albino mice of Laka strain | STZ (200 mg/kg, i.p.) + curcumin (15, 30, and 60 mg/kg, oral,4th 8th week) | ↓ Plasma glucose level ↑ Body weight ↑ Thermal hyperalgesia (tail immersion warm water test and hotplate test) | Not tested | ↓ Serum TNF-α level ↓ Brain nitrite level | [95] |
| Combination Studies | |||||
| Male Sprague-Dawley rats | STZ (45 mg/kg, i.p.) + gliclazide (10 mg/kg, oral, 5 weeks) STZ (45 mg/kg, i.p.) + gliclazide (10 mg/kg, oral, 5 weeks) + gabapentin (30 mg/kg, i.p., 5 weeks) STZ (45 mg/kg, i.p.) + gliclazide (10 mg/kg, oral, 5 weeks) + curcumin (100 mg/kg, oral, 5 weeks) | ↑ Thermal hyperalgesia (hotplate and tail flick test), and mechanical hyperalgesia (tail pinch test) | Not tested | ✓ C-peptide level ↓ Total NO, serum TNF-α and MDA | [96] |
| Male Albino mice of Laka strain | STZ (200 mg/kg, i.p.) + insulin (10 IU/kg, s.c., 8 weeks) STZ (200 mg/kg, i.p.) + curcumin (60 mg/kg, oral, 8th weeks) STZ (200 mg/kg, i.p.) + resveratrol (20 mg/kg, oral, 8th weeks) Insulin (10 IU/ kg, s.c., 8 weeks) + curcumin (60 mg/kg, oral, 8th weeks) Insulin (10 IU/ kg, s.c., 8 weeks) + resveratrol (20 mg/kg, oral, 8th weeks) | Insulin per se ↓ Blood glucose level and ↑ body weight Curcumin or resveratrol per se ↓ Blood glucose level and ↑ body weight Combination treatment O blood glucose level and body weight Combination treatment ↑ thermal hyperalgesia (tail immersion warm water test and hotplate test) threshold | Not tested | Combination treatment ↓ Serum TNF-α level compared to its per se effects Combination treatment ↓ Brain nitrite level compared to its per se effects | [97] |
| Animals (Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Effects | Reference | |
|---|---|---|---|---|
| Behavioral Evaluation/Other Diabetic | Histopathological/ Biochemical/Molecular | |||
| Male Sprague-Dawley rats | CCI + curcumin (100 mg/kg, peritoneal, 14 days) | ✓ Mechanical allodynia (von Frey) and thermal hyperalgesia (Hargreaves test) | X Immunohistochemical and protein expressions of NMDAR-NR1 in spinal cord and DRG | [133] |
| Male Sprague-Dawley rats | CCI + curcumin (100 mg/kg, i.p., 14 days) | ↑ Thermal withdrawal latency (heat stimulus) 7 days after surgery and mechanical withdrawal threshold (von Frey) 10 days after surgery | ↓ NF-κB p65 protein expression in lumbar spinal cord and DRG 7 days after surgery ↓ CX3CR1 positive expression in spinal dorsal horn and DRG 7 days after surgery | [131] |
| Male Sprague-Dawley rats | CCI + curcumin (100 mg/kg, peritoneal, 14 days) | ✓ Thermal hyperalgesia (Hargreaves test) and paw withdrawal mechanical threshold (von Frey) on day 14 | ↓ Serum cortisol concentration X Upregulated expression of 11βHSD1 in spinal dorsal horn and DRG | [132] |
| Male Wistar rats | CCI + curcumin (12.5, 25 and 50 mg/kg, i.p., 7 days) | O Mechanical allodynia (von Frey) High dose ↓ Cold allodynia (acetone test) | High dose ↓ Serum concentration of cyclooxygenase 2 | [137] |
| Male Sprague-Dawley rats | CCI + curcumin (20, 40, or 60 mg/kg, i.p., 14 days) | ↓ Thermal hyperalgesia (Hargreaves test) and mechanical allodynia (von Frey) | ↓ Recruitment of p300/CBP and acetyl-histone H3/acetyl-histone H4 to the promoter of BDNF and Cox-2 genes ↓ mRNA and protein expressions of BDNF and Cox-2 in spinal cord | [135] |
| Male Sprague-Dawley rats | CCI + curcumin (50 mg/kg, oral, 7 days) | ↓ Mechanical allodynia (von Frey) | O Protein expressions of p-ERK, p-JNK, and p-NR1 in DRG | [136] |
| Male C57BL/6J mice | CCI + curcumin (5, 15 or 45 mg/kg, oral, twice per day for 3 weeks) | Chronic treatment ↓Thermal hyperalgesia (hotplate) and mechanical allodynia (von Frey) Depletion of descending noradrenaline (NA) by 6-OHDA X Mechanical allodynia, but not thermal hyperalgesia Depletion of 5-HT by PCPA X thermal hyperalgesia but not mechanical allodynia Antagonists β-AR (propranolol) but not α-AR (phentolamine) X Mechanical allodynia β2-AR (ICI 118,551) X Mechanical allodynia 5-HT1A (WAY-100635) X Thermal hyperalgesia Antagonists Delta-opioid (naltrindole hydrochloride) ↓ Mechanical allodynia Mu-opioid (β-funaltrexamine) X Thermal hyperalgesia Kappa-opioid (nor-binaltorphimine) O Mechanical allodynia or thermal hyperalgesia | Chronic treatment increased spinal monoamine serotonin and its metabolite MHPG Did not alter other monoamines/ metabolites (NA, 5-HIAA, dopamine and DOPAC) | [134] |
| Combination Study | ||||
| Male Sprague-Dawley rats | CCI + curcumin (100 mg/kg, oral, 14 days) CCI + tramadol (10 mg/kg, i.p., 14 days) CCI + chronic constriction release (CCR) + curcumin (100 mg/kg, oral, 14 days) CCI + CCR + tramadol (10 mg/kg, i.p., 14 days) CCI + curcumin (100 mg/kg, oral, 14 days) + tramadol (10 mg/kg, i.p., 14 days) | CCI + tramadol, and CCI + CCR + tramadol ↑ Thermal hyperalgesia (heat stimulus) CCI + tramadol, and CCI + CCR + tramadol ↑ Mechanical allodynia (dynamic plantar test) O Cold-induced pain (cold plate test) | ↓ Sciatic and DRG TNF-α in CCI + CCR + tramadol ↑ Sciatic IL-10 in CCI + CCR + tramadol, whereas ↑ DRG IL-10 in CCI + tramadol followed by CCI + CCR + tramadol ↑ Number of regenerated axons in CCI + CCR + curcumin and CCI + CCR + tramadol | [144] |
| Animals (Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Effects | Reference | ||
|---|---|---|---|---|---|
| Behavioral/Other | Electrophysiological/ Functional | Histopathological/ Biochemical/Molecular | |||
| Sciatic Nerve Crush (SNC) Injury | |||||
| Male Sprague-Dawley rats | SNC + curcumin dissolved in polyethylene glycol 300 at a concentration of 0.035 mg/µL, 0.2 mg/day osmotic minipump infusion, 28 days | ✓ Mechanical (von Frey), finger spacing of injured paw (visual static sciatic index), skillful walking (beam walking task test), grip strength (grip strength test) | ↑ MNCV and SNCV | ↑ Myelin sheath thickness ↑ MPZ and PMP22 expressions ↓ Neurogenic lesions ↓ Macrophage-induced production of ROS, lipid peroxidation ↑ Transcription factor Nrf2 expression | [150] |
| Female Wistar Albino rats | SNC + curcumin (100 mg/kg, nasogastric tube, 28 days) | Not tested | ↑ SFI values ✓ Amplitude values and latency time O Gastrocnemius muscle weight | O G ratios ✓ Myelin thickness, axon diameter, and nerve diameter | [154] |
| Male Sprague-Dawley rats | SNC + curcumin (50, 100, 300 mg/kg, i.p., 4 weeks) | ↑ Mechanical withdrawal threshold (von Frey) ↓ Thermal withdrawal threshold (hotplate) | ↑ SFI values ✓ NCV, CMAP latency onset and peak amplitude ↓ Atrophy of gastrocnemius muscle | ↑ Number of fluoro-gold-positive neurons ↑ Number of myelinated axons per nerve transverse section and mean diameter of nerve fibers | [152] |
| Female Sprague-Dawley rats | SNC + curcumin (100 mg/kg, gavage, 28 days) | Not tested | Not tested | ↓ Decreased volume of ganglion, mean cell volume, total volume of DRG cells (A- and B-cells), total surface of DRG cells, total number, diameter, and area of myelinated nerve fibers | [153] |
| Combination Study | |||||
| Male Wistar rats | SNC + curcumin (100 mg/kg, i.p., 4 weeks) + melatonin (10 mg/kg, i.p., 4 weeks) during light (9 am) and dark (9 pm) periods | Not tested | Light and dark curcumin ✓ SFI Dark melatonin group ✓ SFI Light and dark curcumin O Amplitudes and conduction latencies of evoked CMAP recorded in gastrocnemius muscle Curcumin ✓✓ Shortest latency and greatest amplitude Light and dark curcumin ✓✓ Smallest gastrocnemius muscle atrophy Dark melatonin ✓✓ Gastrocnemius muscle mass | Light and dark curcumin ✓✓ Lesser TOS Light and dark curcumin ✓✓ Higher color intensity of nerve myelin staining (Luxol Fast Blue staining) Dark melatonin ✓✓ Higher nerve myelin staining Light and dark curcumin ✓✓ Higher number of Schwann cells Light curcumin ✓✓ Increased number of neurofilament-positive stained areas Dark melatonin ✓✓ Better neurofilament-positive stained areas | [151] |
| Spared Nerve Injury (SNI) | |||||
| Male Sprague-Dawley rats | SNI + curcumin (100 mg/kg, i.p., 4 weeks) SNI + PI3K inhibitor LY294002 (30 mg/kg, i.p., 10 min before curcumin administration) + curcumin (100 mg/kg, i.p., 4 weeks) SNI + siNGF (1ng in 5µL, i.t., 10 min before curcumin administration) + curcumin (100 mg/kg, i.p., 4 weeks) In vitro: 30 mM | Not tested | Not tested | ✓ PC-12 neurons against H2O2-induced apoptosis by ↑ TrkA, Akt and ↓ p17 ↓ pro-NGF but ↑ mature NGF level PI3K/Akt inhibition ↑ Apoptotic rate by decreasing p17, Ki67, and cyclin D1 NGF suppression and PI3K inhibition ↑ Neuron cell death by increasing proNGF and decreasing mNGF, Akt, TrkA, p75NTR, and p17 | [158] |
| Male BALB/c mice | SNI or sham + curcumin (30, 60, 120 mg/kg, i.p., twice daily from day 1 until day 7 after surgery) | ↓ Mechanical (von Frey) and cold (acetone test) allodynia | Not tested | X IL-1β protein level X NALP1 inflammasome aggregation and JAK2-STAT3 cascade activation in astrocytes | [159] |
| Spinal Nerve Ligation (SNL) | |||||
| Female Wistar rats | SNL or sham + curcumin (30, 100, 200, and 300 μg, i.t., 14 days after surgery) SNL or sham + curcumin (10, 100, 310 mg/kg, oral, 14 days after surgery) | X SNL-induced mechanical allodynia (von Frey) Curcumin + Inhibitors-NO synthase (L-NAME), guanylyl cyclase (ODQ), and ATP-sensitive K+ channel (glibenclamide) X anti-allodynic activity | Not tested | [160] | |
| Sprague-Dawley rats, sex not specified | SNL + curcumin (200 μg, i.t., 7th, 8th, 9th, 10th, 15th, and 20th day after surgery) | ↓ SNL-induced mechanical allodynia (von Frey) from 10th day post-treatment | Not tested | Not tested | [161] |
| Animals (Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Effects | Reference | |
|---|---|---|---|---|
| Behavioral/Other | Histopathological/ Biochemical/Molecular | |||
| Postoperative Pain | ||||
| Male Sprague-Dawley rats | Incision + curcumin (0.01, 0.03, or 0.1 mg, i.t.) | ↓ Mechanical hypersensitivity (von Frey) Antagonists GABA-A (bicuculline) and GABA-B (saclofen) X antinociceptive activity Antagonists mu (CTOP), delta (naltrindole), and kappa (GNTI) opioid receptor X antinociceptive activity | ↑ mRNA expressions of GABA-A and GABA-B in incised spinal cord O mRNA expressions of opioid receptors in incised spinal cord | [173] |
| Male C57BL/6 mice | Incision + curcumin (50 mg/kg, i.p., 4 days) | ↓ Mechanical hypersensitivity (von Frey) ↓ Thermal hypersensitivity (Hargreaves test) ↓ Prostaglandin-induced hyperalgesic priming O Paw edema (laser sensor technique) and hindpaw temperature (fine wire thermocouple) O Morphine-induced place preference (affective component of incision measured by conditioned CPP) X Functional abnormalities in gait indices (gait analysis) | O IL-1β, IL-6, macrophage inflammatory protein-1α at peri-incisional level O IL-10 at peri-incisional level ↑ TGF-β levels at peri-incisional level | [171] |
| Male Sprague-Dawley rats | Acute treatment: Incision + curcumin (10–40 mg/kg, oral, 1 day after surgery) Repeated treatment: Incision + curcumin (10–40 mg/kg, oral, 20 min before to surgery and twice daily for 7 days) Repeated treatment before surgery: Curcumin (10–40 mg/kg, oral, twice daily for 7 days before surgery) + incision | Acute treatment ↓ Mechanical hyperalgesia (von Frey) Repeated treatment before surgery O Mechanical hyperalgesia Repeated treatment ✓ Mechanical hyperalgesia Repeated treatment ✓ Recovery from surgery Repeated treatment before surgery O recovery rate O Locomotor activity (YLS-1B apparatus) | Not tested | [170] |
| Preemptive Analgesia | ||||
| Common crossbred swine | Curcumin [(130 mg/kg, oral, 3 days prior to CPB and extracorporeal support surgery | Not tested | ↓ Concentrations of IL-6, TNF-α, and ICAM-1 | [174] |
| Male Wistar Bratislava Albino rats | Nitroglycerin (NTG) (1 mg/100 g body weight i.p.) + curcumin (10 mg/100 g body weight, i.p., 14 days before NTG administration) | ↓ Number of flinches and shakes (formalin test) | ↓ Blood pressure ↓ MDA, NO, TOS, and thiol compound ↑ TAC | [175] |
| Female Wistar Albino rats | Curcumin (400 mg/kg, oral, 45 min before formalin injection) | ↓ Thermal pain (hotplate test) ↓ Number of flinches (formalin test) | Not tested | [176] |
| Animals (Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Effects | Reference | ||
|---|---|---|---|---|---|
| Behavioral Evaluation/ Other Parameters | Electrophysiological/ Functional Evaluation | Histopathological/ Biochemical/Molecular Parameters | |||
| Diabetic Neuropathy | |||||
| Curcumin Derivative | |||||
| Female Swiss Webster mice or diabetic rats (strain and sex were not specified) | STZ − 90 mg/kg, i.p. STZ + phenyl hydrazide derivative J147 (10, 50 mg/kg, i.p. oral, 20 weeks) | ↓ Blood glucose and HbA1c levels ↑ Paw thermal response (Hargreaves test) ↓ Tactile allodynia (von Frey) O Sensorimotor function (rotarod test) | ↓ MNCV | ↓ TNFR1, TNFR2, and type I diabetes mellitus signaling pathways ↑ AMPK, and ephrin receptor signaling pathways ↓ Protein levels of TNF-α, TSPO, iNOS or GFAP and peripheral inflammation marker C-reactive protein | [27] |
| Male SPF rats | STZ − 50 mg/kg, i.p. STZ + J147 (10 or 100 μM of at 10 mg/kg weight, 5 days) In vitro: J147 (10 and 100 μM) | ↓ Mechanical withdrawal threshold (von Frey) | O Cell viability and apoptosis of RSC96 cells ↑ AMPK mRNA and protein expression levels ↓ TRPA1 mRNA and protein expression levels ↓ Calcium reaction level in AITCR treated RSC96 cells | [33] | |
| Nanoparticle-Encapsulated Curcumin | |||||
| Male Sprague-Dawley | STZ − 30 mg/kg, i.p. STZ + nanoparticle-encapsulated curcumin, 16 mg/kg, sublingual vein, 7th, and 8th week | ↓ Mechanical (electronic mechanical stimulator) and thermal (thermal paw stimulator) hyperalgesia | Interacted perfectly with P2Y12 receptor agonist-binding pocket ↓ mRNA and protein expressions of P2Y12 in DRG ↓ Co-localization of glutamine synthetase (a marker of SGCs) in DRG ↓ mRNA and protein expression of IL-1β and Cx43 expressions in DRG X AKT activation | [28] | |
| Self-Nano Emulsifying Drug Delivery System (SNEDDS) Curcumin | |||||
| Male Sprague–Dawley rats | STZ − 55 mg/kg, i.p. STZ + naïve curcumin (30, 100 and 300 mg/kg, oral, 2 weeks) STZ + SNEDDS curcumin (30, 100 and 300 mg/kg, oral, 2 weeks) | O Body weight and plasma glucose level ✓ Thermal hyperalgesia (tail flick test) in both hot and cold immersion ✓ Mechanical hyperalgesia (von Frey and Randall Sellitto tests) | Naïve and SNEDDS ↓ MNCV and NBF | ↓ MDA levels SNEDDS ↓ NF-κB protein expression SNEDDS X IKK-β phosphorylation expression SNEDDS ↓ Protein expression of NF-κB positive cells in nerves SNEDDS ↓ COX-2 and iNOS protein level Naïve and SNEDDS ↓ IL-6 level in sciatic nerves SNEDDS ↓ TNF-a level in sciatic nerves | [30] |
| CCI | |||||
| Curcumin (Cur) Loaded with Lipid Nanocapsules (Cur@LNCs) | |||||
| Female Sprague-Dawley rats | CCI + Cur@LNCs, 400 µL inject, 7 days | ↓ Thermal hyperalgesia (hotplate) | ↓ Sciatic nerve damages | [29] | |
| Curcumin Prodrug-Curcumin Diglutaric Acid | |||||
| Male ICR mice | CCI + curcumin diglutaric acid (CurDG) (25, 50, 100, and 200 mg/kg, oral, 14 days) | ✓ Mechanical allodynia (von Frey), and thermal hyperalgesia (plantar test) O Motor performance (rotarod test) | ↓ Overexpression of TNF-α and IL-6 levels in both sciatic nerve and spinal cord | [32] | |
| Curcumin-Loaded Poly (d, l-lactide-co-glycolide) Nanovesicles | |||||
| Male CD1 mice | CCI + curcumin (20 mg/kg, intravenous or 0.0005 and 0.025 mg, i.t.) CCI + PLGA CUR (0.045 mg curcumin/mg of nanoparticles, 20 mg/kg, intravenous) CCI + PLGA CUR (0.045 mg curcumin/mg of nanoparticles, 0.0005 and 0.025 mg, i.t.) | Low and high PLGA-CUR, i.t. ↓ Mechanical allodynia (dynamic plantar aesthesiometer test) and thermal hyperalgesia (plantar test) High curcumin, i.t. ↓ allodynia and hyperalgesia | High PLGA-CUR, i.t. ↓ IL-1β, IL-6,TNF-α and BDNF levels in spinal cord | [34] | |
| Curcumin Derivative | |||||
| Male ICR mice | Curcumin derivative KMS4034 (10 mg/kg, i.p., 120 min post-injection) In vitro: 10 µM KMS4034 | ↑ Mechanical thresholds (von Frey) | X ICAP and Iheat of TRPV1-expressing HEK293 cells | ↓ CGRP expression in lamina I–II of lumbar dorsal horns | [31] |
| Participants and Study Design | Dose and Duration | Effects | Reference | |
|---|---|---|---|---|
| Pain-Related | Other/Cardiometabolic | |||
| Diabetic Sensorimotor Polyneuropathy (DSPN) | ||||
| Patients with T2D (n = 80); RCT (placebo-controlled and double-blind) | Nano curcumin (72% curcumin, 80 mg) or placebo capsules/day for eight weeks | ↓ Score of total neuropathies, reflex score, and temperature in curcumin vs. placebo | ↓ HbA1c and FBS in curcumin vs. placebo | [211] |
| Chemotherapy-Induced Peripheral Neuropathy (CIPN) | ||||
| Patients undergoing cancer chemo- and radiotherapy (n = 160); RCT (placebo-controlled and double-blind) | Lecithinized curcumin (Meriva: 500 mg) or placebo for 60 days from first cycle of chemo- or radiotherapy | ↓ Local pain rating based on VAS due to radiotherapy in curcumin vs. placebo group | ↓ Chemotherapy side effects in curcumin vs. placebo group | [212] |
| Peripheral Neuropathy (PN) | ||||
| Patients with chronic PN and lumbar disc herniation and/or lumbar canal stenosis or carpal tunnel syndrome; (n = 135); RCT, open | Three formulations as follows: (1) Dex (800 mg) + Lipicur [lipoic acid (800 mg) + curcumin phytosome (800 mg) + piperine (8 mg)]; (2) Dex + lipoic acid (800 mg) and (3) Dex only (800 mg) capsules/day for eight weeks | ↓ Neuropathic pain in patients with lumbar sciatica and carpal tunnel syndrome in Lipicur group vs. others | ↓ Use of Dex in the Lipicur group vs. others | [215] |
| Postoperative Pain | ||||
| Patients undergoing oral surgery for periodontitis (n = 15); RCT (placebo-controlled) | Curcumin mucoadhesive film (0.5% extract) or placebo mucoadhesive film placed on gingiva after surgery for seven days | ↓ Pain score rating and swelling in curcumin vs. placebo group | ↓ Use of oral analgesics in postoperative period in curcumin vs. placebo group | [210] |
| Patients following laparoscopic gynecologic surgery (n = 60); RCT, open | Curcuminoids extract (1000 mg) or standard analgesia on postoperative days one to three | ↓ VAS pain scores following surgery in curcumin vs. standard group | N/A | [214] |
| Patients undergoing oral surgery for impacted third molars (n = 90); RCT (placebo-controlled) | Curcumin (200 mg) + amoxicillin (500 mg) or control (amoxicillin 500 mg + 500 mg mefenamic acid) three times for 24 h | ↓ Pain score rating in curcumin vs. placebo group | N/A | [213] |
| Patients undergoing laparoscopic cholecystectomy (n = 50); RCT (placebo-controlled and double-blind) | Curcumin (500 mg) or placebo once every six hours/day for three weeks | ↓ Pain score rating in curcumin vs. placebo group | ↓ Fatigue score and the use of oral analgesics in postoperative period in curcumin vs. placebo group | [209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, P.; Maier, C.; Basu, A. Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4666. https://doi.org/10.3390/ijms22094666
Basu P, Maier C, Basu A. Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review. International Journal of Molecular Sciences. 2021; 22(9):4666. https://doi.org/10.3390/ijms22094666
Chicago/Turabian StyleBasu, Paramita, Camelia Maier, and Arpita Basu. 2021. "Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review" International Journal of Molecular Sciences 22, no. 9: 4666. https://doi.org/10.3390/ijms22094666
APA StyleBasu, P., Maier, C., & Basu, A. (2021). Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review. International Journal of Molecular Sciences, 22(9), 4666. https://doi.org/10.3390/ijms22094666








