The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells
Abstract
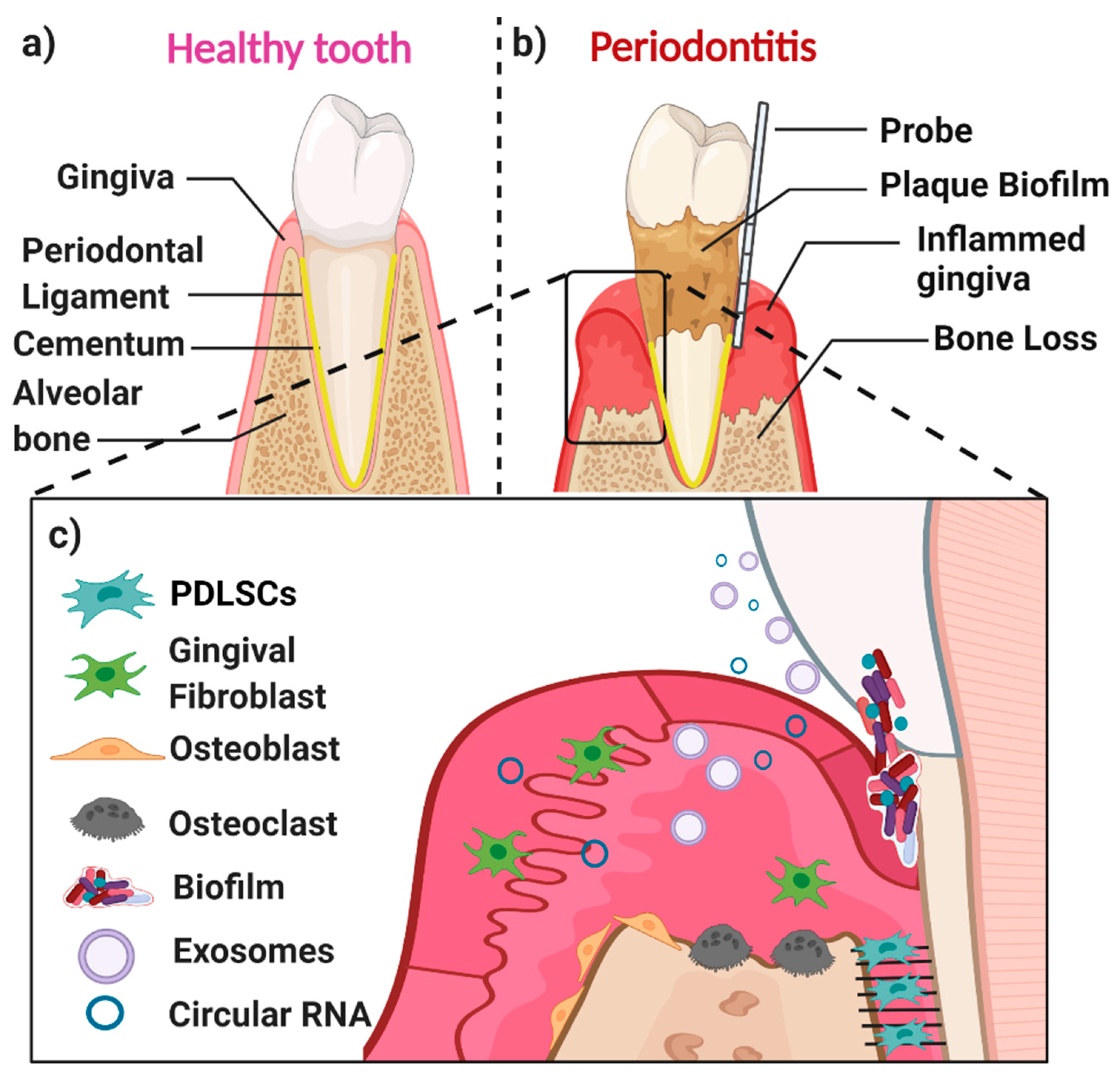
1. Periodontal Disease and Regeneration
Periodontal Disease
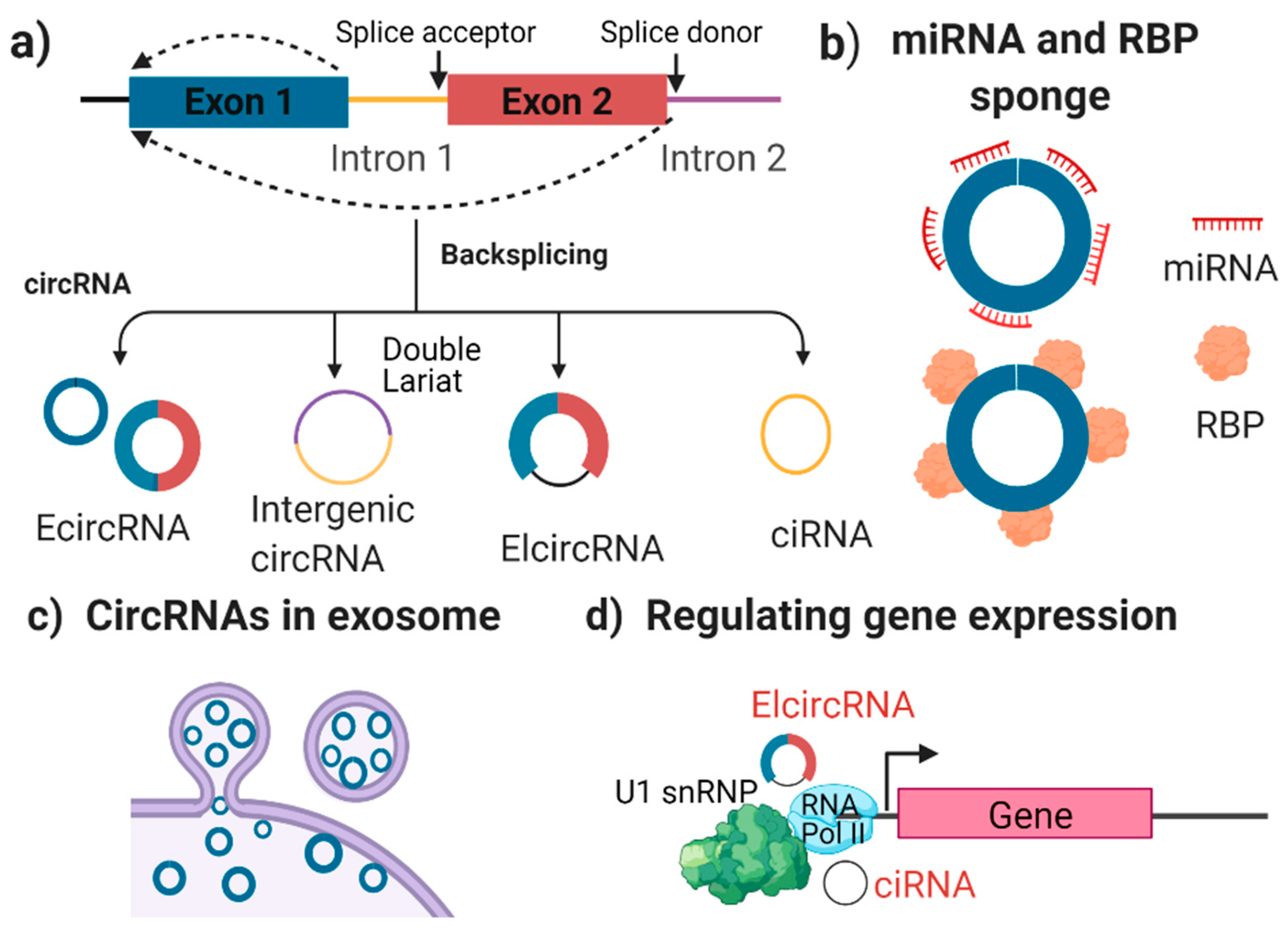
2. circRNA Biogenesis and Function
2.1. What Are circRNAs?
2.2. The Characterisation and Function of circRNAs
2.3. Methods for Detecting circRNA
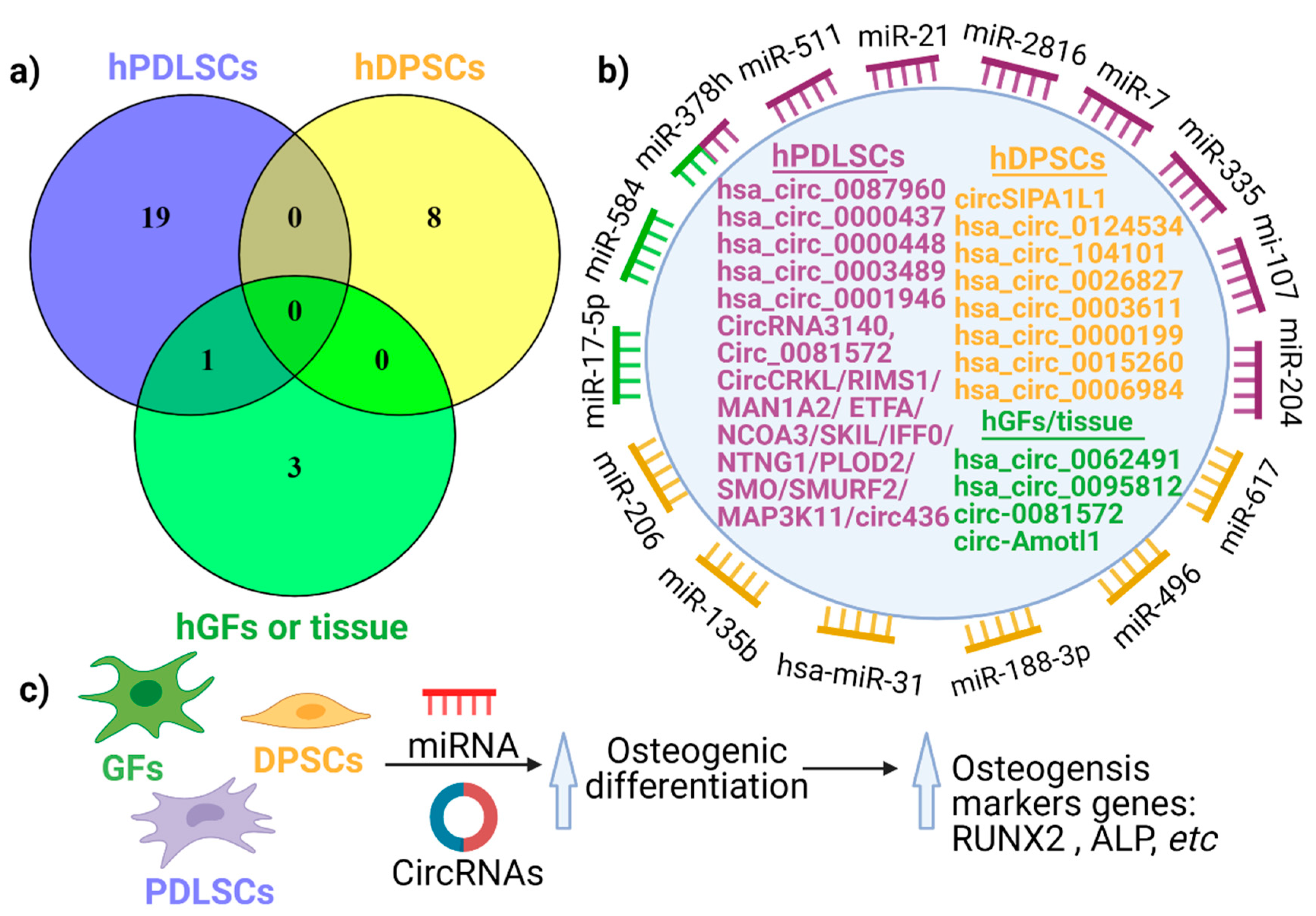
3. circRNA Expression in Periodontal Tissues
4. The Emerging Role of circRNAs in Periodontal Regeneration
4.1. Periodontal Tissue Engineering
4.2. The Role of circRNA in Human Periodontal Ligament Stem Cell Differentiation
4.3. The Impact of circRNAs in the Differentiation of Human Dental Pulp Stem Cells (hDPSCs)
4.4. circRNAs in MC3T3-E1, Rat Dental Follicle Cells (rDFCs), and Gingival Fibroblasts
5. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000 2013, 62, 203–217. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45, S130–S148. [Google Scholar] [CrossRef]
- Kinane, D.F.; Bartold, P.M. Clinical relevance of the host responses of periodontitis. Periodontology 2000 2007, 43, 278–293. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-Regression; SAGE Publications: Los Angeles, CA, USA, 2014; Volume 93, pp. 1045–1053. [Google Scholar]
- Chapple, I.L.C.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42, S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Bouziane, A.; Hamdoun, R.; Abouqal, R.; Ennibi, O. Global prevalence of aggressive periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 406–428. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontology 2000 2018, 78, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Kiel, R.A.; Anderhalden, K. Clinical and microbiological effects of subgingival restorations with overhanging or clinically perfect margins. J. Clin. Periodontol. 1983, 10, 563–578. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Mizutani, S.; Ekuni, D.; Tomofuji, T.; Azuma, T.; Kataoka, K.; Yamane, M.; Iwasaki, Y.; Morita, M. Relationship between xerostomia and gingival condition in young adults. J. Periodontal Res. 2015, 50, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. 1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Barros, S.P.; Beck, J.D. Rethinking periodontal inflammation. J. Periodontol. 2008, 79, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Gronthos, S.; Shi, S.; Bartold, P.M. Stem cells in the periodontal ligament. Oral Dis. 2006, 12, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary outer membrane vesicles and DNA methylation of small extracellular vesicles as biomarkers for periodontal status–a pilot study. Int. J. Mol. Sci. 2021, 22, 2423. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lai, A.; Salomon, C.; Ivanovski, S. Detection of salivary small extracellular vesicles associated inflammatory cytokines gene methylation in gingivitis. Int. J. Mol. Sci. 2020, 21, 5273. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Ivanovski, S. Saliva-Friend and Foe in the COVID-19 Outbreak. Diagnostics 2020, 10, 290. [Google Scholar] [CrossRef]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary small extracellular vesicles associated miRNAs in periodontal status—A pilot study. Int. J. Mol. Sci. 2020, 21, 2809. [Google Scholar] [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J. Clin. Periodontol. 2019, 46, 6–11. [Google Scholar] [CrossRef]
- Jurdziński, K.T.; Potempa, J.; Grabiec, A.M. Epigenetic regulation of inflammation in periodontitis: Cellular mechanisms and therapeutic potential. Clin. Epigenet. 2020, 12, 186. [Google Scholar] [CrossRef]
- Benakanakere, M.R.; Finoti, L.; Palioto, D.B.; Teixeira, H.S.; Kinane, D.F. Epigenetics, inflammation, and periodontal disease. Curr. Oral Health Rep. 2019, 6, 37–46. [Google Scholar] [CrossRef]
- Larsson, L.; Castilho, R.M.; Giannobile, W.V. Epigenetics and its role in periodontal diseases: A state-of-the-art review. J. Periodontol. 2015, 86, 556–568. [Google Scholar] [CrossRef]
- Larsson, L. Current concepts of epigenetics and its role in periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293. [Google Scholar] [CrossRef] [PubMed]
- De Fraipont, F.; Gazzeri, S.; Cho, W.C.; Eymin, B. Circular RNAs and RNA splice variants as biomarkers for prognosis and therapeutic response in the liquid biopsies of lung cancer patients. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Xiao, J. Circular RNAs: Biogenesis and Functions; Springer: Singapore, 2018. [Google Scholar]
- Ma, S.; Kong, S.; Wang, F.; Ju, S.Q. CircRNAs: Biogenesis, functions, and role in drug-resistant tumours. Mol. Cancer 2020, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.-L. The Biogenesis, functions, and challenges of circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, X.K.; Chen, L.L.; Yang, L. Increased complexity of circRNA expression during species evolution. RNA Biol. 2017, 14, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tan, W.; Wang, C. The emerging roles of exosomal circRNAs in diseases. Clin. Transl. Oncol. 2020. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Li, P.; Wang, K. Emerging function and clinical significance of exosomal circRNAs in cancer. Mol. Ther. Nucleic Acids 2020, 21, 367–383. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, S.T.; Wu, Z.; Chen, L.; Wen, C.; Chen, H.; Du, W.W.; Wu, N.; Guan, D.; Lian, S.; et al. Rapid development of targeting circRNAs in cardiovascular diseases. Mol. Ther. Nucleic Acids 2020, 21, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, T.; Dieterich, C. Deep computational circular RNA analytics from RNA-seq data. Methods Mol. Biol. 2018, 1724, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Singh, A.K.; Mishra, G.; Maurya, A.; Chellappan, D.K.; Gupta, G.; Hansbro, P.M.; Dua, K. An overview of circular RNAs. Adv. Exp. Med. Biol. 2018, 1087, 3–14. [Google Scholar] [CrossRef]
- Huda, H.A.; Vijayarathna, S.; Oon, C.E.; Chen, Y.; Kanwar, J.R.; Ng, M.L.; Sasidharan, S. Functional analysis of circular RNAs. Adv. Exp. Med. Biol. 2018, 1087, 95–105. [Google Scholar] [CrossRef]
- Yu, B.; Hu, J.; Li, Q.; Wang, F. CircMAP3K11 contributes to proliferation, apoptosis and migration of human periodontal ligament stem cells in inflammatory microenvironment by regulating TLR4 via miR-511 sponging. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, X.; He, Y.; Hou, S.; Liu, T.; Zhi, K.; Hou, T.; Gao, L. CircCDK8 regulates osteogenic differentiation and apoptosis of PDLSCs by inducing ER stress/autophagy during hypoxia. Ann. N. Y. Acad. Sci. 2020, 1485. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Han, Y.; Xi, S.; Wu, G. circRNA CDR1as regulated the proliferation of human periodontal ligament stem cells under a lipopolysaccharide-induced inflammatory condition. Mediat. Inflamm. 2019, 2019, 1625381. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.; Xu, L. Circ_0081572 inhibits the progression of periodontitis through regulating the miR-378h/RORA axis. Arch. Oral Biol. 2021, 124, 105053. [Google Scholar] [CrossRef]
- Li, J.; Xie, R. Circular RNA expression profile in gingival tissues identifies circ_0062491 and circ_0095812 as potential treatment targets. J. Cell. Biochem. 2019, 120, 14867–14874. [Google Scholar] [CrossRef]
- Ivanovski, S. Periodontal regeneration. Aust. Dent. J. 2009, 54, S118–S128. [Google Scholar] [CrossRef]
- Carter, S.S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive biomanufacturing: An advanced approach for periodontal tissue regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal Res. 2016, 51, 1–15. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Dan, H.X.; Vaquette, C.; Fisher, A.G.; Hamlet, S.M.; Xiao, Y.; Hutmacher, D.W.; Ivanovski, S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue engineered constructs for periodontal eegeneration: Current status and future perspectives. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Staples, R.J.; Ivanovski, S.; Vaquette, C. Fibre guiding scaffolds for periodontal tissue engineering. J. Periodontal Res. 2020, 55, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lloyd, T.; Chen, Z.; Xiao, Y. Proinflammatory cytokines regulate cementogenic differentiation of periodontal ligament cells by Wnt/Ca(2+) signaling pathway. J. Interferon Cytokine Res. 2016, 36, 328–337. [Google Scholar] [CrossRef]
- Han, P.; Ivanovski, S.; Crawford, R.; Xiao, Y. Activation of the canonical Wnt signaling pathway induces cementum regeneration. J. Bone Miner. Res. 2015, 30, 1160–1174. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/beta-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379. [Google Scholar] [CrossRef]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal tissue engineering with a multiphasic construct and cell sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.; Ren, X.; Zhang, M.; Thuaksuban, N.; Nuntanaranont, T.; Guan, Z. Alteration of circRNA and lncRNA expression profile in exosomes derived from periodontal ligament stem cells undergoing osteogenic differentiation. Arch. Oral Biol. 2021, 121, 104984. [Google Scholar] [CrossRef]
- Wang, H.; Feng, C.; Jin, Y.; Tan, W.; Wei, F. Identification and characterization of circular RNAs involved in mechanical force-induced periodontal ligament stem cells. J. Cell. Physiol. 2019, 234, 10166–10177. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Y.; Zheng, Y.; Huang, Y.; Zhang, Y.; Jia, L.; Li, W. Circular RNA CDR1as regulates osteoblastic differentiation of periodontal ligament stem cells via the miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res. Ther. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, X.; Huang, Y.; Jia, L.; Li, W. The circular RNA landscape of periodontal ligament stem cells during osteogenesis. J. Periodontol. 2017, 88, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, M.; Jin, Y.; Liu, D.; Wei, F. Identification and integrated analysis of differentially expressed lncRNAs and circRNAs reveal the potential ceRNA networks during PDLSC osteogenic differentiation. BMC Genet. 2017, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Pan, J.; Shen, Z.; Yang, Z.; Wang, J.; Bai, X.; Tao, J. The circular RNA circRNA124534 promotes osteogenic differentiation of human dental pulp stem cells through modulation of the miR-496/β-catenin pathway. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Y.; Zeng, J.; Deng, Z.; Wu, B. circRNA expression profile in dental pulp stem cells during odontogenic differentiation. Stem Cells Int. 2020, 2020, 5405931. [Google Scholar] [CrossRef]
- Ji, F.; Zhu, L.; Pan, J.; Shen, Z.; Yang, Z.; Wang, J.; Bai, X.; Lin, Y.; Tao, J. hsa_circ_0026827 promotes osteoblast differentiation of human dental pulp stem cells through the Beclin1 and RUNX1 signaling pathways by sponging miR-188-3p. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Xie, L.; Guan, Z.; Zhang, M.; Lyu, S.; Thuaksuban, N.; Kamolmattayakul, S.; Nuntanaranont, T. Exosomal circLPAR1 promoted osteogenic differentiation of homotypic dental pulp stem cells by competitively binding to hsa-miR-31. Biomed. Res. Int. 2020, 2020, 6319395. [Google Scholar] [CrossRef]
- Ge, X.; Li, Z.; Zhou, Z.; Xia, Y.; Bian, M.; Yu, J. Circular RNA SIPA1L1 promotes osteogenesis via regulating the miR-617/Smad3 axis in dental pulp stem cells. Stem Cell Res. Ther. 2020, 11, 364. [Google Scholar] [CrossRef]
- Zhang, B.; Huo, S.; Cen, X.; Pan, X.; Huang, X.; Zhao, Z. circAKT3 positively regulates osteogenic differentiation of human dental pulp stromal cells via miR-206/CX43 axis. Stem Cell Res. Ther. 2020, 11, 531. [Google Scholar] [CrossRef]
- Li, C.; Jiang, H. Altered expression of circular RNA in human dental pulp cells during odontogenic differentiation. Mol. Med. Rep. 2019, 20, 871–878. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Y.; Wei, S.; Zhang, X.; Guo, Y.; Han, B. Comprehensive circRNA expression profile and function network in osteoblast-like cells under simulated microgravity. Gene 2021, 764, 145106. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ren, W.; Zheng, Z.; Huang, Z.; Liang, T.; Li, F.; Shi, Z.; Jiang, Q.; Yang, X.; Guo, L. Mmu_circ_003795 regulates osteoblast differentiation and mineralization in MC3T3‑E1 and MDPC23 by targeting COL15A1. Mol. Med. Rep. 2020, 22, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Meng, X.; Han, X.; Li, J. Exosomes derived from circRNA Rtn4-modified BMSCs attenuate TNF-α-induced cytotoxicity and apoptosis in murine MC3T3-E1 cells by sponging miR-146a. Biosci. Rep. 2020, 40, BSR20193436. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, Y.; Chen, L.; Yan, C.; Endo, Y.; Liu, Y.; Liu, J.; Hu, L.; Hu, Y.; Sun, Y.; et al. CircRNA AFF4 promotes osteoblast cells proliferation and inhibits apoptosis via the Mir-7223-5p/PIK3R1 axis. Aging 2019, 11, 11988–12001. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, J.; Hou, Y.; Chen, C.; Long, W.; Jiang, H. Alteration of circular RNA expression in rat dental follicle cells during osteogenic differentiation. J. Cell Biochem. 2019, 120, 13289–13301. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Awan, F.M.; Du, W.W.; Zeng, Y.; Lyu, J.; Wu, D.; Gupta, S.; Yang, W.; Yang, B.B. The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol. Ther. 2017, 25, 2062–2074. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Wu, W.-P.; Xiong, X.-D. Circular RNAs: Promising biomarkers for age-related diseases. Aging Dis. 2020, 11, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Lin, X.; Mo, C. Integrated analysis of circRNA-miRNA-mRNA regulatory network identifies potential diagnostic biomarkers in diabetic foot ulcer. Noncoding Rna Res. 2020, 5, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Pu, C.; Li, Y.; Qi, B. Construction of a circRNA-miRNA-mRNA network based on competitive endogenous RNA reveals the function of circRNAs in osteosarcoma. Cancer Cell Int. 2020, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.-w.; Cao, D.; Tang, Y.-f.; Shu, L.; Zuo, Z.; Zhang, L.-y. Identification of circRNA–miRNA–mRNA regulatory network in gastrointestinal stromal tumor. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
| Reference | circRNA Name (Gene Name), Genome Location and Length | circRNA Target or Pathway | CircRNA Detection Method | Cell (Tissue) Details | Key Findings |
|---|---|---|---|---|---|
| Yu et al. 2021 [37] | circMAP3K11 (MAP3K11) | miR-511 | RT-qPCR10 | PDL tissues from 10 healthy and 20 periodontitis cases. hPDLSCs from healthy donors. Donor age—unclear. Passage 3. | Higher expression levels of circMAP3K11 and TLR4, and lower expression levels of miR-511-3p were found in periodontitis affected PDL tissues, compared to healthy controls. circMAP3K11 enhanced hPDLSCs proliferation, migration and osteogenic differentiation, and reduced the apoptosis of hPDLSCs in vitro through a circMAP3K11/ miR-511-3p/TLR4 axis. In vivo—silencing circMAP3K11 can prevent periodontitis development in mice, with decreased cell proliferation and increased apoptosis. |
| Zheng et al. 2021. [38] | hsa_circ_0003489 (circCDK8) chr13:26974589-26975761; 1172 bp | mTOR signalling pathway | RT-qPCR | PDL tissues from 6 healthy and 6 mild/moderate chronic periodontitis patients hPDLSCs; Healthy PDL tissues (third molars); Passage 3 to 5. |
CircCDK8 and HIF-1α were increased in PDL tissues from periodontitis patients. Overexpression of CircCDK8 decreased osteogenic differentiation of hPDLSCs through the mTOR pathway under hypoxia. |
| Wang et al. 2019. [39] | hsa_circ_0001946; CDR1as (or CiRS-7) chrX:139865339-139866824; 1485 bp | miR-7 ERK/MAPK signal pathway | RT-qPCR | PDL tissue from 10 periodontitis and 11 healthy cases. hPDLSCs from healthy teeth and chronic periodontitis tissue; passage 3. Donor age 30–40 yrs. | circCDR1as was significantly downregulated in PDL tissues from periodontitis patients. circRNA CDR1as inhibited hPDLSCs proliferation through the miR-7 and ERK/MAPK pathway |
| Wang et al. 2021. [40] | circ_0081572 (GRHL2) chr8:102564942-102571040; 6098 bp | miR-378h | RT-qPCR | Gingival tissues from 21 healthy and 21 periodontitis cases, Human periodontal ligament cells (hPDLCs). Unclear donor age and cell passage number. | Circ_0081572 was downregulated in the gingival tissues of periodontitis, compared to healthy gingival tissues. Overexpression of circ_0081572 could alleviate LPS-induced PDLCs injury via circ_0081572/miR-378h/RORA axis. |
| Li et al. 2019. [41] | hsa_circ_0062491 chr22:23063339-23180200; 116861 bp | miR-584 | RNA sequencing RT-qPCR | Gingival tissues from 4 healthy and 4 chronic periodontitis cases. THP1 cells treated with P. gingivalis. | 1, 304 circRNAs were significantly differentially expressed in the gingival tissues of periodontitis patients (n = 4). Decreased circ_0062491 and increased circ_0095812 found in periodontitis-gingival tissues compared to healthy tissues (n = 30) using RT-qPCR. Circ_0062491 function as a miR-584 sponge in THP1 cells. |
| Reference | circRNA Name (Gene Name), Genome Location and Length | circRNA Target or Pathway | CircRNA Detection Method | Cell (Tissue) Details | Key Findings |
|---|---|---|---|---|---|
| Xie et al. 2021. [54] | hsa_circ_0087960 (LPAR1) chr9:113734352-113735838; 1486 bp hsa_circ_0000437 (CORO1C) chr12:109046047-109048186; 2139 bp hsa_circ_0000448 (GCN1L1) chr12:120592773-120593523; 750 bp | miRNA TGF/β, MAPK, mTOR, and FOXO1 pathways | RNA sequencing RT-qPCR | Human Periodontal Ligament Stem Cells (hPDLSCs) from third molar tissue; Donor ages 18–30 yrs; Passage 3. | 69–557 exosomal circRNAs were detected after 5 and 7 days of osteogenic differentiation of hPDLSCs. Exosomal circRNA-LPAR1 was increased and has_circ_0000448 was decreased in hPDLSCs after 5 and 7-days osteogenesis. Function as miRNA sponge and modulate TGF- β, MAPK, mTOR, and FOXO1 pathways. |
| Wang et al. 2018. [55] | CircRNA3140 CircRNA436 | miR-21 miRNA-107 miRNA-335 | RT-qPCR RNA sequencing | hPDLSCs; Middle third root; Healthy PDL cells culture; age 14–16 passage 3 | 1191 cricrRNAs in hPDLSC were enhanced by mechanical force-induced osteogenic differentiation. Potential functions of circRNAs through circRNAs–miRNAs networks. For instance, circRNA3140 targets miR-21; circRNA436 targets miRNA-107 and miRNA-335. |
| Li et al. 2018. [56] | CDR1as hsa_circ_0001946; chrX:139865339-139866824; 1485 bp | miR-7; TGF-β/Smad and MAPK pathway | RT-qPCR | hPDLSCs PDL tissue from healthy premolars; Passage 4 | CircRNA CDR1as inhibits osteogenic differentiation of hPDLSCs via inhibiting miR-7, TGF-β/Smad and MAPK pathways; In vivo knockdown of CDR1as reduced bone formation in a mouse calvarial defect model. |
| Zheng et al. 2017. [57] | CircCRKL, CircRIMS1, CircMAN1A2, CircETFA | miRNA sponge | RT-qPCR RNA sequencing | hPDLSCs (n = 3) PDL tissue from a healthy premolar; Donor age: 12–18; Passage 4 | 12,693 circRNA transcripts were detected in hPDCSc osteogenic differentiation with a time-specific expression. Four circRNAs were increased in hPDLSCs osteogenesis by RNA-seq and RT-qPCR; circRNA-miRNA-mRNA network is the potential regulatory role of circRNA. |
| Gu et al. 2017. [58] | Upregulated: CDR1as, circNCOA3 and circSKIL; Downregulated: circIFF01, circNTNG1, circPLOD2, circSMO and circSMURF2 | miRNA34a and miRNA146a; MAPK and Wnt pathway | RT-qPCR RNA sequencing | hPDLSCs Donor age:18–20, Passage 3 | 1456 circRNAs were differentially expressed after 7-day osteogenic differentiated hPDLSCs. CDR1as, circNCOA3 and circSKIL upregulated during osteogenesis of hPDLSCs; CircRNA-miRNA-mRNA network is the potential function of circRNA in hPDLSCs osteogenic differentiation. For instance, circRNA BANP and circRNA ITCH were predicted to interact with miRNA34a and miRNA146a to regulate PDLSC osteogenic differentiation via the MAPK pathway. |
| Reference | circRNA Name (Gene Name), Genome Location and Length | circRN Target or Pathway | CircRNA Detection Method | Cell (Tissue) Details | Key Findings |
|---|---|---|---|---|---|
| Ji et al. 2020. [59] | circRNA124534/ hsa_circ_0124534 (FRMD4B) chr3:69247848-69265490; 17,642 bp | As a miRNA sponge; miR-496/β-catenin pathway | RT-PCR | hDPSCs healthy pulp tissues (3 male, 3 female); Donor age: females: 22–33; males: 26–41 passage 4 | CircRNA124534 enhanced in vitro osteogenic differentiation in hDPSCs via the miR-496/β-catenin pathway. Over-expression of circRNA124534 in vivo increased bone formation in a mouse subcutaneous model. |
| Chen et al. 2020. [60] | hsa_circRNA_104101 | Wnt and the TGF-β signalling pathway | RT-qPCR Microarray Electro-phoresis | hDPSCs donor age: 18–25 yrs; passages 3–5 | 43 upregulated and 144 downregulated circRNAs were detected in hDPSCs during odontogenic differentiation. hsa_circRNA_104101 promoted hDPSCs odontogenic differentiation. |
| Ji et al. 2020. [61] | hsa_circ_0026827 (RPL41); chr12:56510373-56511616; 1243 bp | miR-188-3p; Beclin1& RUNX1 pathway | Microarray RT-qPCR | hDPSCs | has_circ_0026827 was upregulated during osteogenic differentiation in hDPSCs. has_circ_0026827 targets the miR-188-3p via Beclin1 & RUNX1 pathway. Overexpression of has_circ_0026827 promoted in vivo bone formation. circRNA–miRNA–mRNA networks may operate during odontogenic differentiation in hDPSCs via the Wnt and TGF-β signalling pathways. |
| Xie et al. 2020. [62] | circLPAR1 (hsa_circ_0003611) chr9:113703700-113735838; 32,138 bp | hsa-miR-31 SATB2 and RUNX2 | RNA-seq RT-PCR | hDPSCs (Exosomes) from one healthy donor (age: 20 yrs); Passage 2 | Exosomal crcLPAR1 enhanced osteogenic differentiation of hDPSCs by binding to has-miR-31. |
| Ge et al. 2020. [63] | circSIPA1L1 | miR-617 Smad3 | RT-PCR | hDPSCs Third molar from a healthy donor aged 18–25 yrs; Passage is unclear | CircSIPA1L1 promoted osteogenesis via regulating the miR-617 and Smad3 pathway in hDPSCs. |
| Zhang et al. 2020. [64] | circAKT3 (hsa_circ_0000199) chr1:243708811-243736350; 27,539 bp | miR-206; CX34 | qPT-PCR RNA sequencing |
hDPSCs premolars and third molars; age:14–25; passages 3 to 5 | 29 circRNAs were down-regulated and 57 circRNAs were upregulated during hDPSCs osteogenesis. CircAKT3 promoted osteogenesis in hDPSCs by binding to miR-206. In vivo—silencing circAKT3 inhibited bone formation in a mouse subcutaneous model. |
| Li et al. 2019. [65] | hsa_circ_0015260 (C1orf9), chr1:172520652-172548407; 27,755 bp hsa_circ_0006984 (ZNF79) chr9:130206308-130207528; 1220 bp | miR-135b; MAPK pathway | RT-qPCR RNA-seq | Human dental pulp cells (hDPCs) from healthy premolars and third molars (3 males and 5 females; 12–25 yrs); Passage 3 | 1341 increased circRNAs and 1780 decreased circRNAs were identified in hDPCs during odontogenic differentiation. Has_circ_0015260 and has_circ_0006984 were up-regulated during osteogenesis of hDPCs via miR-135b and the MAPK pathway. |
| Reference | circRNA Name (Gene Name), Genome Location and Length | circRNA Target or Pathway | CircRNA Detection Method | Cell (Tissue) Details | Key Findings |
|---|---|---|---|---|---|
| Cao et al. 2021. [66] | circ_014154 | miR-145a-5p and let-7a-5p; MAPK pathway | RT-qPCR RNA-seq | MC3T3-E1 | 232 upregulated and 95 down-regulated circRNAs were found during osteogenic differentiation of MC3T3-E1 cells under microgravity; Circ_014154 was upregulated in MC3T3-E1 cells with osteogenic differentiation induced by microgravity via miR-145a-5p, let-7a-5p and the MAPK pathway. |
| Wu et al. 2020. [67] | mmu_circ_003795 | mmu‑miR‑1249‑5p COL15A1 | RT-qPCR nucleic acid electro-phoresis Micro-array | MC3T3‑E1 and MDPC23 cells; 24–72 h after transfection | mmu_circ_003795 was increased after 72 h osteogensis in MC3T3-E1 and MDPC23 cells by RT-qPCR via mmu‑miR‑ 1249‑5p by targeting COL 15A1. |
| Cao et al. 2020. [68] | circ-Rtn4 | miR-146a | RT-qPCR Luciferase reporter assay | exosomes from circRtn4-modified BMSCs MC3T3-E1 | circRtn4 as miR-146a sponge to regulate exosomes from BMSCs reduced TNF-α induced cytotoxicity and inhibited apoptosis of MC3T3-E1. Luciferase reporter assay validated the binding between circRtn4 and mi-146a. |
| Mi et al. 2019. [69] | CircRNA AFF4 Mmu_circ_0000262 chr11:53182161-53194174; 12,013 bp | miR-7223-5p; (PIK3R1) | RT-qPCR Luciferase reporter assay; micro-CT | MC3T3-E1 | In vitro—CircRNA AFF4 stimulated MC3T3-E1 proliferation and inhibited apoptosis via binding to miR-7223-5p, and its downstream target is PIK3R1. In vivo—circRNA AFF4 enhanced fracture healing in a mouse femur fracture model. |
| Du et al. 2019. [70] | circFgfr2 | miR-133 and BMP6 (bone morphogenetic protein-6); MAPK, TGF-β | RT-qPCR RNA-seq | Rat dental follicle cells (rDFCs) and tissues; passage 3 to 4. | CircFgfr2 promotes osteogenic differentiation of rDFCs via miR-133/BMP6. In situ hybridization identified that circFgfr2 was upregulated in mandible dental follicle tissues at days 1 to 11 postnatally in Sprague-Dawley rats, while miR-133 was decreased. |
| Yang et al. 2017. [71] | circ-Amotl1 | miR-17-5p Stat3, Dnmt3a and fibronectin | RT-qPCR | Human gingival fibroblast cell line CRL-2014; NIH 3T3 fibroblast cell line. | Circular-Amolt promoted in vivo skin wound healing. Circular-Amolt enhanced hGFs and NIH 3T3 cell migration; Circular-Amolt enhanced STAT3, Dnmt3a and fibronectin while suppressing the expression of miR-17-5p. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, K.; Walsh, L.J.; Ivanovski, S.; Han, P. The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. Int. J. Mol. Sci. 2021, 22, 4636. https://doi.org/10.3390/ijms22094636
Jiao K, Walsh LJ, Ivanovski S, Han P. The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. International Journal of Molecular Sciences. 2021; 22(9):4636. https://doi.org/10.3390/ijms22094636
Chicago/Turabian StyleJiao, Kexin, Laurence J. Walsh, Sašo Ivanovski, and Pingping Han. 2021. "The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells" International Journal of Molecular Sciences 22, no. 9: 4636. https://doi.org/10.3390/ijms22094636
APA StyleJiao, K., Walsh, L. J., Ivanovski, S., & Han, P. (2021). The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. International Journal of Molecular Sciences, 22(9), 4636. https://doi.org/10.3390/ijms22094636









