Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago
Abstract
1. Introduction
2. Results
2.1. Identification of CBL-CIPK Genes in the M. sativa and M. truncatula Genome
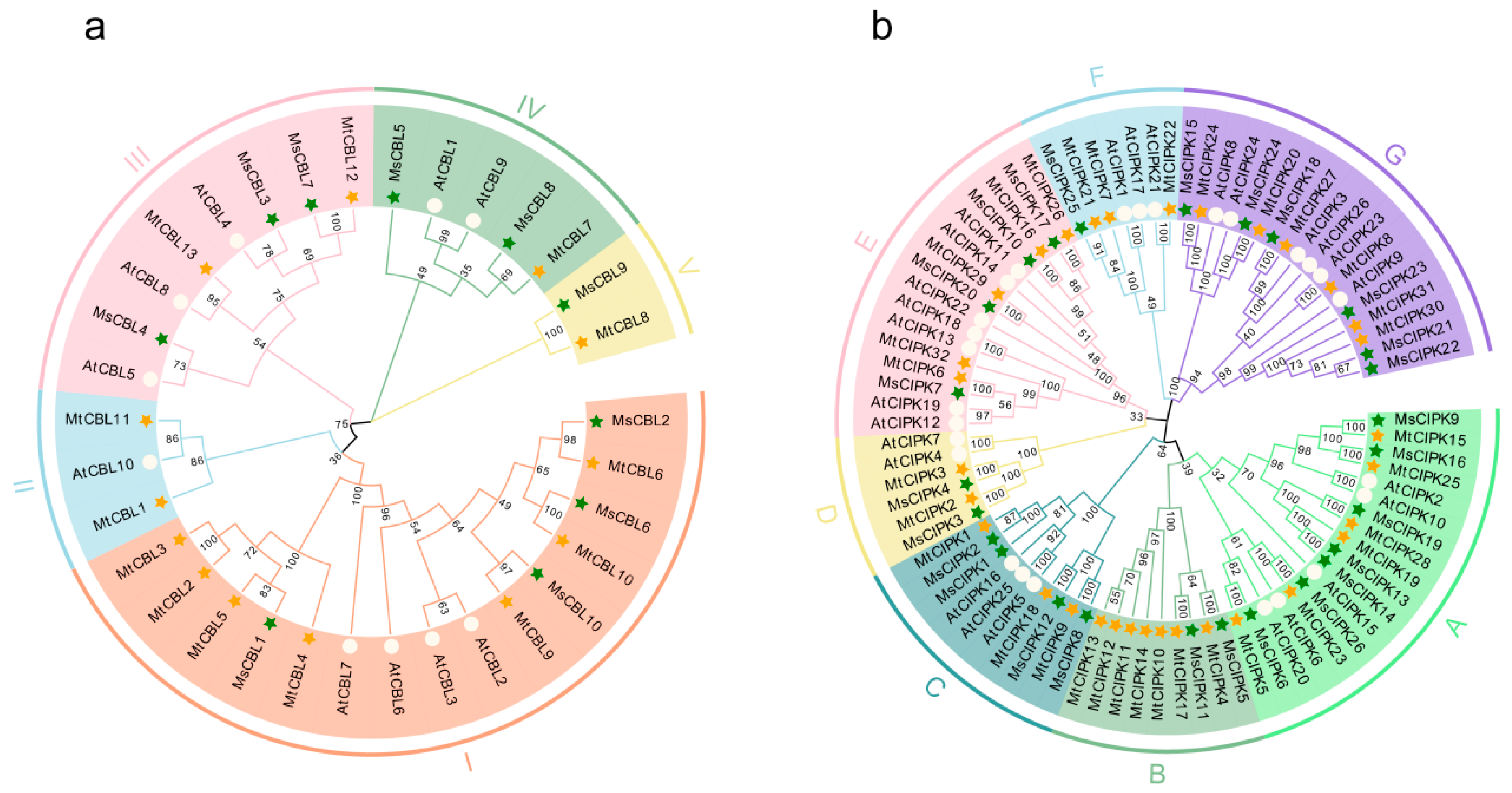
2.2. Multiple Sequence Alignment, Phylogenetic Analysis and Classification of CBL and CIPK Genes
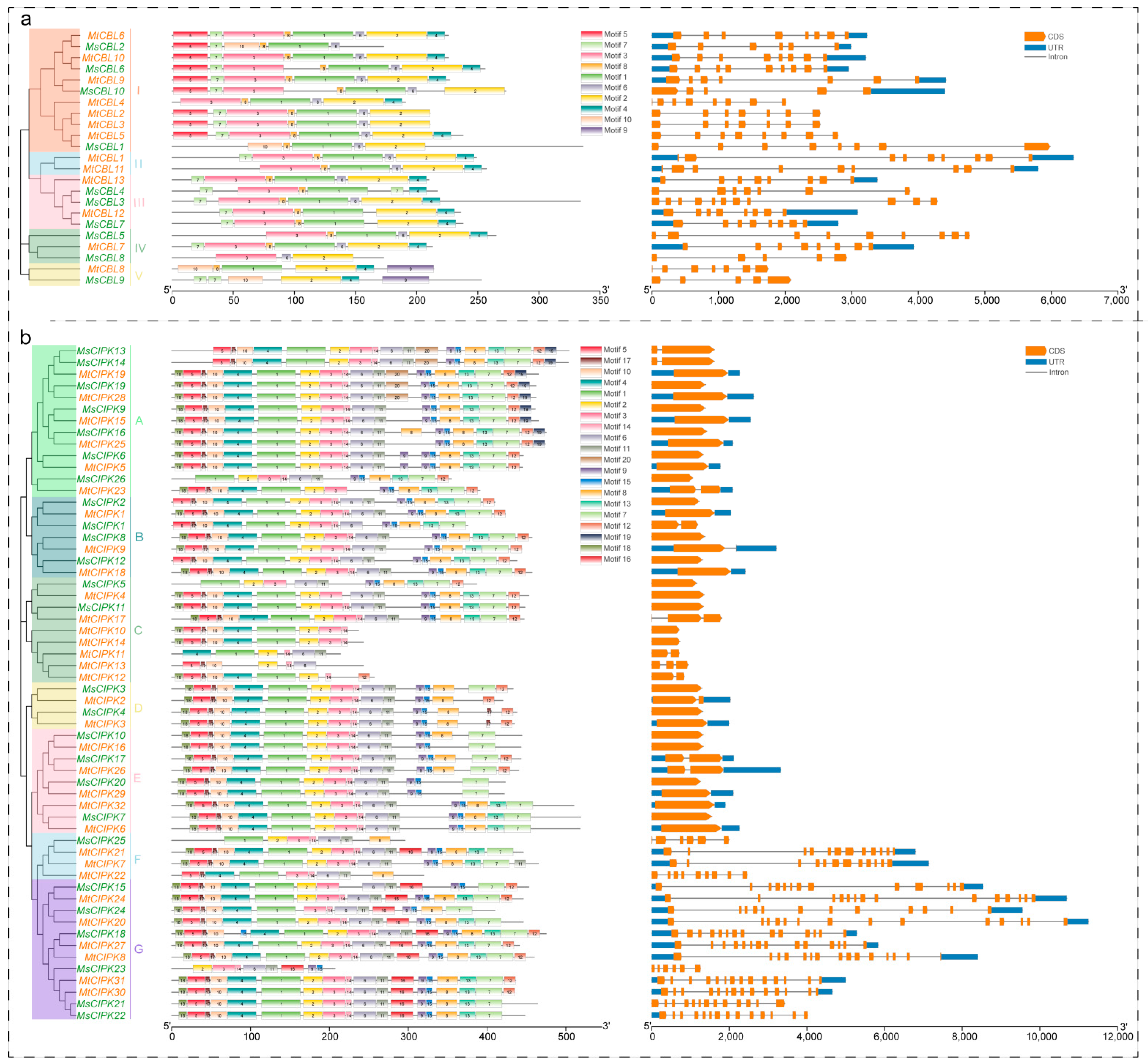
2.3. Analyses of Conserved Motif and Gene Structure
2.4. Analysis of Collinearity and Chromosome Location of CBL and CIPK Genes
2.5. Analyses of the Cis-Acting Element and Location of CBL and CIPK Genes
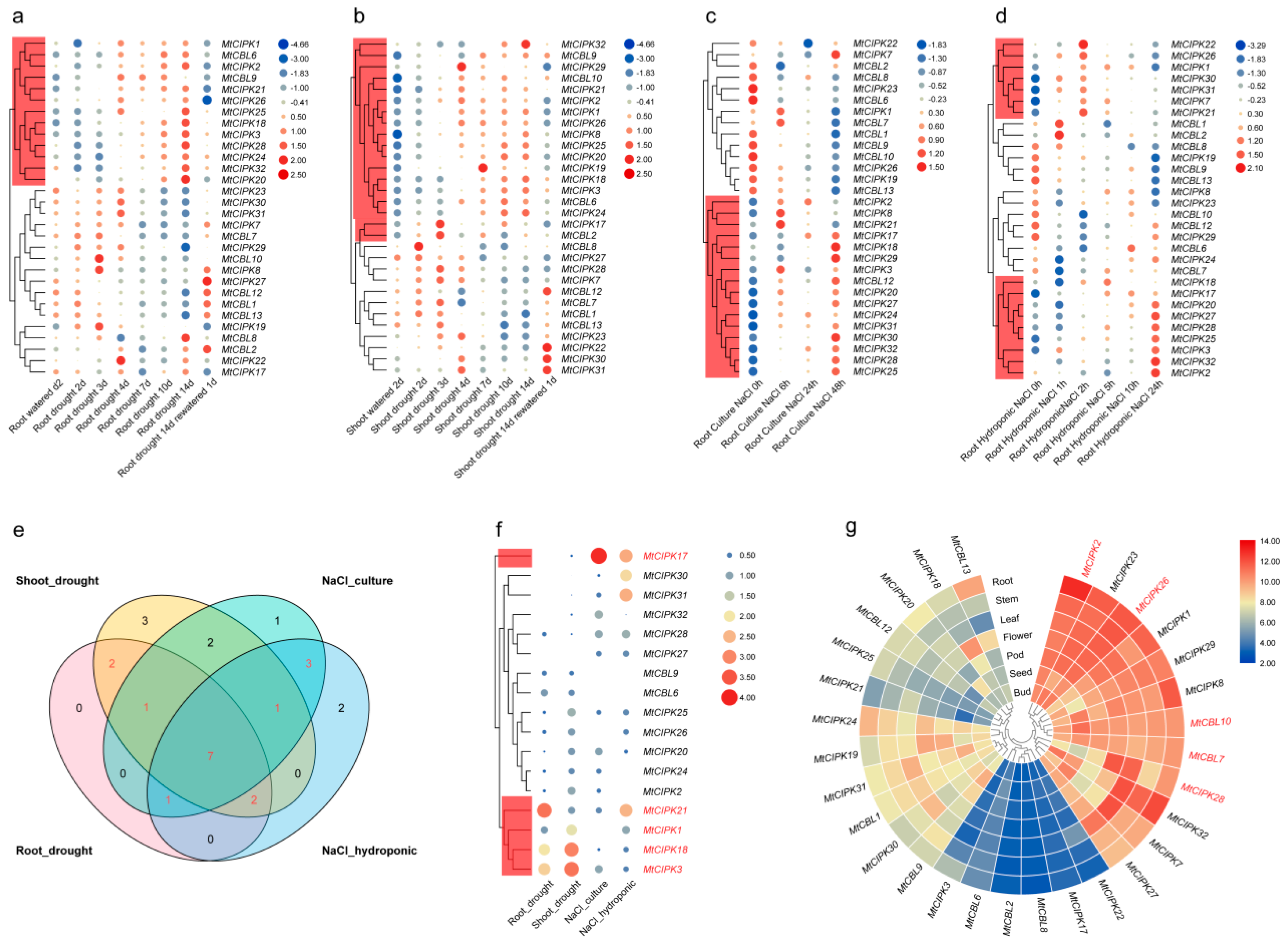
2.6. Analysis of the Expression Levels of CBL and CIPK Genes from Microarray Data
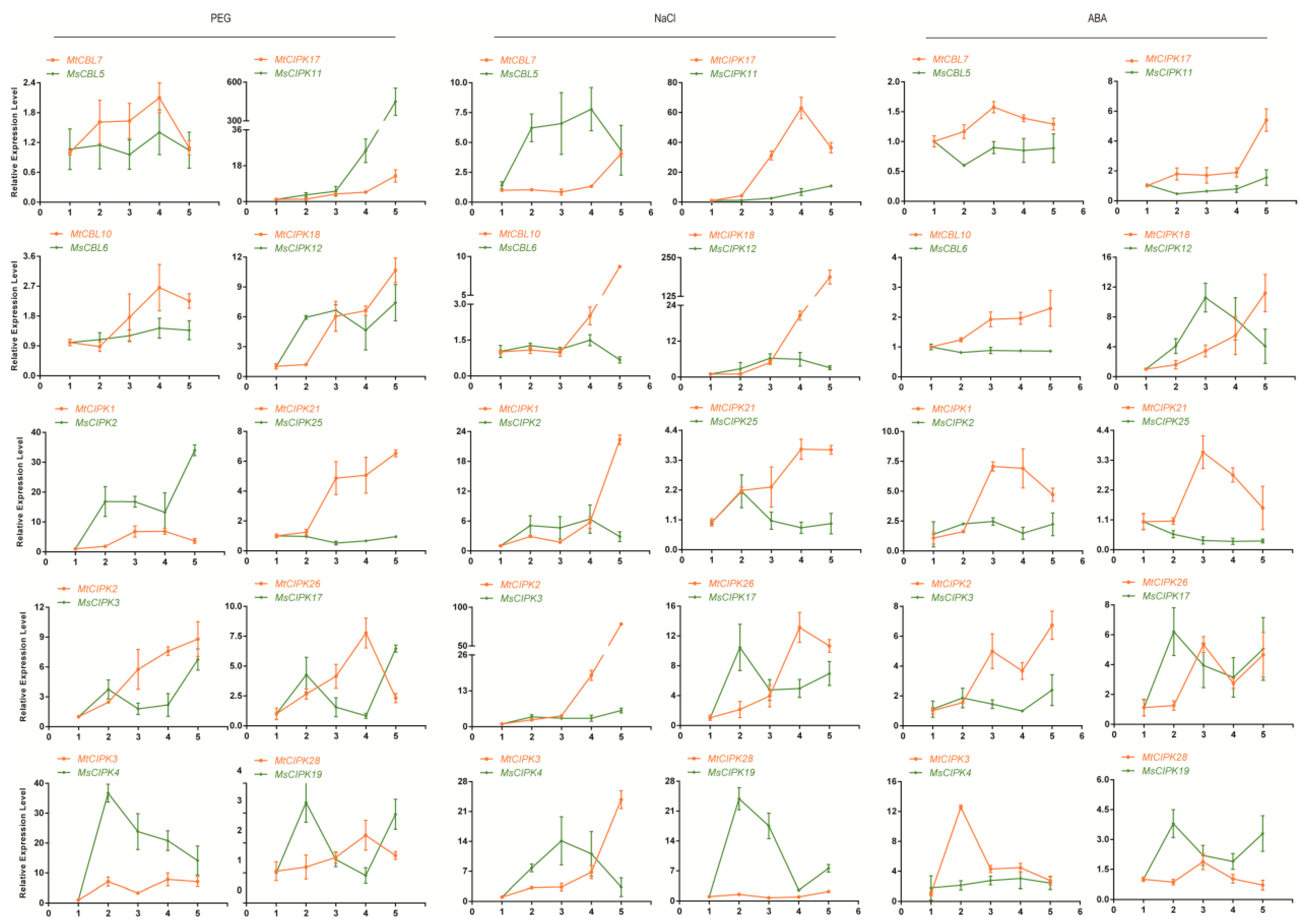
2.7. Validation of the Expression Profile of Stress-Responsive CBL and CIPK Genes by qPCR Analysis
3. Discussion
4. Materials and Methods
4.1. Identification of CBL-CIPK Genes in the Medicago Genome
4.2. Sequence Analyses and Structural Characterization of Medicago CBL and CIPK Genes
4.3. Phylogenetic Analysis and Classification of the CBL and CIPK Genes
4.4. Analysis of Collinearity and Chromosome Location
4.5. Analyses of the Cis-Acting Elements and Location of CBL and CIPK Genes in Medicago
4.6. Analysis of the Expression Levels of CBL and CIPK Genes from Genechip Data
4.7. Plant Materials and Treatments
4.8. Analysis of the Gene Expression by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, H.F.; Yang, B.; Liu, W.Z.; Li, H.W.; Wang, L.; Wang, B.Y.; Deng, M.; Liang, W.W.; Deyholos, M.K.; Jiang, Y.Q. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L.). BMC Plant Biol. 2014, 8, 1471–2229. [Google Scholar] [CrossRef]
- Kolukisaoglu, U.N.; Weinl, S.; Blazevic, D.; Batistic, O.; Kudla, J.R. Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004, 134, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Luan, S.; Lan, W.; Chul, S.L. Potassium nutrition, sodium toxicity, and calcium signaling: Connections through the CBL-CIPK network. Plant Biol. 2009, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hu, W.; Wei, S.; Zhou, S.; Zhang, F.; Han, J.; Chen, L.; Li, Y.; Feng, J.; Fang, B.; et al. TaCIPK29, a CBL-interacting protein kinase gene from wheat, confers salt stress tolerance in transgenic tobacco. PLoS ONE 2013, 8, e69881. [Google Scholar] [CrossRef]
- Nagae, M.; Nozawa, A.; Koizumi, N.; Sano, H.; Hashimoto, H.; Sato, M.; Shimizu, T. The crystal structure of the novel calcium-binding protein AtCBL2 from Arabidopsis thaliana. Biochem. Mol. Biol. 2003, 278, 42240–42246. [Google Scholar]
- Sanchez-Barrena, M.J.; Martınez-Ripoll, M.; Zhu, J.K.; Albert, A. The structure of the Arabidopsis thaliana SOS3: Molecular mechanism of sensing calcium for salt stress response. J. Mol. Biol. 2005, 345, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lin, H.; Chen, S.; Wu, Y.; Zhang, J.; Fuglsang, A.T.; Palmgren, M.G.; Wu, W.; Guo, Y. Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol. 2011, 156, 2235–2243. [Google Scholar] [CrossRef]
- Albrecht, V.A.; Ritz, O.; Linder, S.; Harter, K.; Kudla, J. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001, 20, 1051–1063. [Google Scholar] [CrossRef]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1399. [Google Scholar] [CrossRef]
- Ohta, M.; Guo, Y.; Halfter, U.; Zhu, J.K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Plant Biol. 2003, 100, 11771–11776. [Google Scholar] [CrossRef]
- Chen, X.F.; Gu, Z.M.; Liu, F.; Ma, B.J.; Zhang, H.S. Molecular analysis of rice CIPKs involved in both biotic and abiotic stress responses. Rice Sci. 2011, 18, 1–9. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Zhao, L.; Cao, S.; Cheng, Y.; Qin, Y. Genome-wide identification and expression profiling of CBL-CIPK gene family in pineapple (Ananas comosus) and the role of AcCBL1 in abiotic and biotic stress response. Biomolecules 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Sung, S.J.; Kim, B.G.; Pandey, G.K.; Cho, J.S.; Kim, K.N.; Luan, S. Constitutive overexpression of the calcium sensor CBL5 confers osmotic or drought stress tolerance in Arabidopsis. Mol. Cells 2010, 29, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Wang, Y.Y.; Tsay, Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009, 57, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Zhang, J.W.; Wei, J.H.; Wang, H.Z.; Wang, Y.Z.; Ma, R.C. Functions and mechanisms of the CBL-CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci. 2009, 19, 667–676. [Google Scholar] [CrossRef]
- Weinl, S.; Kudla, J. The CBL-CIPK Ca2+-decoding signaling network: Function and perspectives. New Phytol. 2009, 184, 517–528. [Google Scholar] [CrossRef]
- Yu, Q.Y.; An, L.J.; Li, W.L. The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014, 33, 203–214. [Google Scholar] [CrossRef]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.S.; Fujii, H.; Pan, S.Q.; Schumaker, K.S.; Grillo, S.; Zhu, J.K. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.D.; Lin, H.X.; Mendoza, I.; Zhang, Y.G.; Cao, W.H.; Yang, Y.Q.; Shang, M.; Chen, S.Y.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Loubna, F.; Pascal, D.; Jean, A.; Alain, G.; Didier, L.; Romain, K.; Florence, L.; Louis-Philippe, V.; Laurent, B. Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 2009, 329, 60–67. [Google Scholar]
- Li, X.H.; Brummer, E.C. Applied genetics and genomics in alfalfa breeding. Agronomy 2012, 2, 40–61. [Google Scholar] [CrossRef]
- Barker, D.G.; Bianchl, S.; Blondon, F.; Dattde, Y.; Duc, G.; Essad, S.; Flament, P.; Gallusci, P.; Gdnier, G.; Guy, P.; et al. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol. Biol. Report. 1990, 8, 40–49. [Google Scholar] [CrossRef]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schultke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007, 52, 473–484. [Google Scholar] [CrossRef]
- Zhang, H.C.; Yin, W.L.; Xia, X.L. Calcineurin B-Like family in Populus: Comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul. 2008, 56, 129–140. [Google Scholar] [CrossRef]
- Boudet, N.; Aubourg, S.; Toffano-Nioche, C.; Kreis, M.; Lecharny, A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 2015, 11, 2101–2114. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, N.N.; Chen, F.; Cai, B.; Santo, S.D.; Tornielli, G.B.; Pezzotti, M.; Cheng, Z.M. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, Y.; Xin, H.P.; Fang, L.C.; Li, S.H. Comprehensive analysis of NAC domain transcription factor gene family in Vitis vinifera. Plant Cell Rep. 2013, 32, 61–75. [Google Scholar] [CrossRef]
- Zhu, J.K.; Chen, F.; Liu, J.Y.; Chen, X.L.; Hewezi, T.; Cheng, Z.M. Evolution of an intron-poor cluster of the CIPK gene family and expression in response to drought stress in soybean. Sci. Rep. 2016, 6, 28225. [Google Scholar] [CrossRef]
- Roy, S.W.; Penny, D. Patterns of intron loss and gain in plants: Intron loss-dominated evolution and genome-wide comparison of O. sativa and A. thaliana. Mol. Biol. Evol. 2007, 24, 171–181. [Google Scholar] [CrossRef]
- Kleist, T.J.; Spencley, A.L.; Luan, S. Comparative phylogenomics of the CBL-CIPK calcium-decoding network in the moss Physcomitrella, Arabidopsis, and other green lineages. Front. Plant Sci. 2014, 5, e187. [Google Scholar] [CrossRef]
- Vision, T.J.; Brown, D.G.; Tanksley, S.D. The origins of genomic duplications in Arabidopsis. Science 2000, 290, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. The evolution of functionally novel proteins after gene duplication. R. Soc. 1994, 256, 119–124. [Google Scholar]
- Lara, A.; Rodenas, R.; Andres, Z.; Martinez, V.; Quintero, F.J.; Nieves-Cordones, M.; Botella, M.A.; Rubio, F. Arabidopsis K+ transporter HAK5-mediated high-affinity root K+ uptake is regulated by protein kinases CIPK1 and CIPK9. J. Exp. Bot. 2020, 71, 5053–5060. [Google Scholar] [CrossRef]
- Liu, L.L.; Ren, H.M.; Chen, L.Q.; Wang, Y.; Wu, W.H. A protein kinase CIPK9 interacts with calcium sensor CBL3 and regulates K+ homeostasis under low-K+ stress in Arabidopsis. Plant Physiol. 2013, 161, 266–277. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, Y.M.; Xiong, L.Z. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007, 144, 1416–1428. [Google Scholar] [CrossRef]
- Shannon, M.C.; Rhoades, J.D.; Draper, J.H.; Scardaci, S.C.; Spyres, M.D. Assessment of salt tolerance in rice cultivars in response to salinity problems in california Crop Ecology. Prod. Manag. 1998, 38, 394–398. [Google Scholar]
- Kanwar, P.; Sanyal, S.K.; Tokas, I.; Yadav, A.K.; Pandey, A.; Kapoor, S.; Pandey, G.K. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice. Cell Calcium 2014, 56, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ding, S.; Zhang, H.; Du, H.; An, L. CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci. 2011, 181, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.K.; Vishwakarma, N.K.; Tripathi, V.; Chattopadhya, D. CBL-interacting protein kinase 25 contributes to root meristem development. Soc. Exp. Biol. 2018, 70, 133–147. [Google Scholar]
- Angelo, C.D.; Weinl, S.; Batistic, O.; Pandey, G.K.; Cheong, Y.H.; Schultke, S.S.; Albrecht, V.; Ehlert, B.; Schulz, B.; Harter, K.; et al. Alternative complex formation of the Ca2+-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J. 2006, 48, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef]
- Young, N.D.; Debelle, F.; Oldroyd, G.E.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.D.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Yang, J.F.; Ma, L.; Jiang, W.B.; Yao, Y.; Tang, Y.H.; Pang, Y.Z. Comprehensive identification and characterization of abiotic stress and hormone responsive glycosyl hydrolase family 1 genes in Medicago truncatula. Plant Physiol. Biochem. 2021, 158, 21–33. [Google Scholar] [CrossRef]


| Gene Name | TIGR Locus | Homologous Gene | Chromosome Location | pI | MW | Protein Length | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| MsCBL1 | MsG0280007543.01.T01 | MtCBL5 | Chr2: 16946249–16952230 | 6.2 | 38,240.38 | 336 | Extracellular |
| MsCBL2 | MsG0380014586.01.T01 | MtCBL6 | Chr3: 56818145–56821980 | 4.9 | 19,486.31 | 173 | Plasma membrane |
| MsCBL3 | MsG0380016524.01.T01 | MtCBL7 | Chr3: 84678194–84682482 | 4.9 | 38,012.36 | 334 | Plasma membrane |
| MsCBL4 | MsG0480023701.01.T01 | MtCBL9 | Chr4: 88741570–88745444 | 4.55 | 24,633.18 | 217 | Plasma membrane |
| MsCBL5 | MsG0580024593.01.T01 | MtCBL7 | Chr5: 6687437–6692205 | 4.77 | 30,599.99 | 265 | Plasma membrane |
| MsCBL6 | MsG0580030002.01.T01 | MtCBL10 | Chr5: 104956837–104960723 | 5.02 | 29,541.79 | 256 | Plasma membrane |
| MsCBL7 | MsG0880043002.01.T01 | MtCBL12 | Chr8: 17451660–17454557 | 4.82 | 27,195.04 | 238 | Plasma membrane |
| MsCBL8 | MsG0880046091.01.T01 | MtCBL7 | Chr8: 68926482–68929407 | 4.99 | 20,024.43 | 173 | Plasma membrane |
| MsCBL9 | MsG0880046710.01.T01 | MtCBL8 | Chr8: 77208147–77210234 | 4.79 | 26,744.53 | 253 | Extracellular |
| MsCBL10 | MsG0880047760.01.T01 | MtCBL9 | Chr8: 90474978–90479379 | 5.64 | 31,753.31 | 273 | Plasma membrane |
| MtCBL1 | MtrunA17_Chr1g0151621 | MsCBL3 | Chr1: 4424275–4430607 | 4.68 | 28,348.38 | 249 | Plasma membrane |
| MtCBL2 | MtrunA17_Chr2g0290751 | MsCBL6 | Chr2: 10098104–10100632 | 4.8 | 24,076.53 | 211 | Plasma membrane |
| MtCBL3 | MtrunA17_Chr2g0290761 | MsCBL6 | Chr2: 10120014–10122541 | 5.02 | 24,212.82 | 211 | Plasma membrane |
| MtCBL4 | MtrunA17_Chr2g0290771 | MsCBL6 | Chr2: 10131045–10133055 | 4.44 | 21,889.77 | 191 | Plasma membrane |
| MtCBL5 | MtrunA17_Chr2g0290781 | MsCBL6 | Chr2: 10140913–10143707 | 4.76 | 27,082.64 | 238 | Plasma membrane |
| MtCBL6 | MtrunA17_Chr3g0102821 | MsCBL6 | Chr3: 27325210–27329496 | 4.7 | 25,954.5 | 226 | Plasma membrane |
| MtCBL7 | MtrunA17_Chr4g0055421 | MsCBL5 | Chr4: 49038532–49043130 | 4.74 | 24,483.05 | 213 | Plasma membrane |
| MtCBL8 | MtrunA17_Chr4g0063121 | MsCBL9 | Chr4: 54777738–54779483 | 4.54 | 23,911.69 | 214 | Plasma membrane |
| MtCBL9 | MtrunA17_Chr4g0076551 | MsCBL6 | Chr4: 64369731–64374408 | 4.75 | 25,972.49 | 227 | Plasma membrane |
| MtCBL10 | MtrunA17_Chr5g0446931 | MsCBL6 | Chr5: 43351610–43355760 | 4.71 | 26,064.69 | 226 | Plasma membrane |
| MtCBL11 | MtrunA17_Chr8g0341941 | MsCBL3 | Chr8: 5383017–5389089 | 4.78 | 29,496.87 | 257 | Plasma membrane |
| MtCBL12 | MtrunA17_Chr8g0346911 | MsCBL7 | Chr8: 9926428–9929518 | 4.99 | 27,094.98 | 236 | Plasma membrane |
| MtCBL13 | MtrunA17_Chr8g0388331 | MsCBL3 | Chr8: 46203348–46207118 | 4.97 | 24,028.73 | 210 | Plasma membrane |
| Gene Name | TIGR Locus | Homologous Gene | Chromosome Location | pI | MW | Protein Length | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| MsCIPK1 | MsG0180000513.01.T01 | MtCIPK1 | Chr1: 7219758–7220941 | 8.93 | 42,516.31 | 376 | Plasma membrane |
| MsCIPK2 | MsG0180000521.01.T01 | MtCIPK1 | Chr1: 7356600–7357829 | 8.99 | 46,413.89 | 410 | Plasma membrane |
| MsCIPK3 | MsG0180001670.01.T01 | MtCIPK2 | Chr1: 24938893–24940191 | 8.85 | 48,636.26 | 433 | Plasma membrane |
| MsCIPK4 | MsG0180004166.01.T01 | MtCIPK3 | Chr1: 74311950–74313263 | 8.86 | 48,883.24 | 438 | Plasma membrane |
| MsCIPK5 | MsG0180005611.01.T01 | MtCIPK4 | Chr1: 93961932–93963092 | 8.09 | 44,450.35 | 387 | Extracellular |
| MsCIPK6 | MsG0280006904.01.T01 | MtCIPK5 | Chr2: 7792269–7793606 | 8.87 | 51,100.97 | 446 | Plasma membrane |
| MsCIPK7 | MsG0280006906.01.T01 | MtCIPK6 | Chr2: 7819514–7821070 | 6.56 | 57,894.45 | 519 | Plasma membrane |
| MsCIPK8 | MsG0280011471.01.T01 | MtCIPK9 | Chr2: 84572890–84574260 | 9.15 | 51,553.44 | 457 | Plasma membrane |
| MsCIPK9 | MsG0380014945.01.T01 | MtCIPK15 | Chr3: 62899825–62901207 | 8.58 | 52,512.51 | 461 | Plasma membrane |
| MsCIPK10 | MsG0380014950.01.T01 | MtCIPK16 | Chr3: 62944869–62946200 | 8.45 | 50,073.61 | 444 | Plasma membrane |
| MsCIPK11 | MsG0380015546.01.T01 | MtCIPK17 | Chr3: 71653648–71654991 | 8.94 | 51,591.75 | 448 | Plasma membrane |
| MsCIPK12 | MsG0480018187.01.T01 | MtCIPK18 | Chr4: 1337477–1338790 | 8.58 | 50,031.2 | 438 | Plasma membrane |
| MsCIPK13 | MsG0480019352.01.T01 | MtCIPK19 | Chr4: 18884311–18885931 | 8.55 | 56,612.39 | 504 | Plasma membrane |
| MsCIPK14 | MsG0580027748.01.T01 | MtCIPK19 | Chr5: 67945209–67946826 | 8.36 | 56,525.14 | 503 | Plasma membrane |
| MsCIPK15 | MsG0580028307.01.T01 | MtCIPK24 | Chr5: 78145226–78153759 | 6.37 | 51,712.85 | 453 | Plasma membrane |
| MsCIPK16 | MsG0580028588.01.T01 | MtCIPK25 | Chr5: 82921999–82923423 | 9.05 | 53,886.01 | 475 | Plasma membrane |
| MsCIPK17 | MsG0580028590.01.T01 | MtCIPK26 | Chr5: 82973828–82975942 | 7.14 | 49,482.95 | 443 | Plasma membrane |
| MsCIPK18 | MsG0580029551.01.T01 | MtCIPK27 | Chr5: 98438716–98444881 | 6.45 | 53,945 | 475 | Plasma membrane |
| MsCIPK19 | MsG0780039388.01.T01 | MtCIPK28 | Chr7: 63273062–63274447 | 8.02 | 52,396.38 | 462 | Plasma membrane |
| MsCIPK20 | MsG0780039391.01.T01 | MtCIPK29 | Chr7: 63319880–63321148 | 8.95 | 47,583.98 | 423 | Plasma membrane |
| MsCIPK21 | MsG0880042910.01.T01 | MtCIPK30 | Chr8: 15785566–15788993 | 9.03 | 52,865.88 | 464 | Plasma membrane |
| MsCIPK22 | MsG0880042931.01.T01 | MtCIPK30 | Chr8: 16193214–16197233 | 8.99 | 50,776.14 | 448 | Plasma membrane |
| MsCIPK23 | MsG0880042932.01.T01 | MtCIPK31 | Chr8: 16202211–16203469 | 4.65 | 23,360.42 | 207 | Plasma membrane |
| MsCIPK24 | MsG0880046785.01.T01 | MtCIPK20 | Chr8: 78103239–78112793 | 9.29 | 46,753.34 | 410 | Plasma membrane |
| MsCIPK25 | MsG0880047252.01.T01 | MtCIPK21 | Chr8: 83803682–83805677 | 9.18 | 33,282.91 | 296 | Plasma membrane |
| MsCIPK26 | MsG0880047603.01.T01 | MtCIPK23 | Chr8: 88629631–88630695 | 8.59 | 40,180.45 | 355 | Plasma membrane |
| MtCIPK1 | MtrunA17_Chr1g0150321 | MsCIPK2 | Chr1: 3363660–3365695 | 6.51 | 57,997.54 | 518 | Plasma membrane |
| MtCIPK2 | MtrunA17_Chr1g0166101 | MsCIPK3 | Chr1: 15531311–15533332 | 8.71 | 48,428.7 | 435 | Plasma membrane |
| MtCIPK3 | MtrunA17_Chr1g0188031 | MsCIPK4 | Chr1: 37868206–37870202 | 7.6 | 49,372.84 | 440 | Plasma membrane |
| MtCIPK4 | MtrunA17_Chr1g0205421 | MsCIPK5 | Chr1: 50641418–50642779 | 9.44 | 28,820.94 | 243 | Plasma membrane |
| MtCIPK5 | MtrunA17_Chr2g0284011 | MsCIPK6 | Chr2: 4993431–4995206 | 8.77 | 50,920.75 | 445 | Plasma membrane |
| MtCIPK6 | MtrunA17_Chr2g0284031 | MsCIPK7 | Chr2: 5004740–5007006 | 8.92 | 53,811.89 | 474 | Plasma membrane |
| MtCIPK7 | MtrunA17_Chr2g0302431 | MsCIPK22 | Chr2: 19969671–19976812 | 7.56 | 36,077.73 | 320 | Plasma membrane |
| MtCIPK8 | MtrunA17_Chr2g0304111 | MsCIPK18 | Chr2: 21918963–21927365 | 8.71 | 44,042.03 | 391 | Plasma membrane |
| MtCIPK9 | MtrunA17_Chr2g0333821 | MsCIPK8 | Chr2: 51523898–51527942 | 7.99 | 52,575.69 | 462 | Plasma membrane |
| MtCIPK10 | MtrunA17_Chr3g0091281 | MsCIPK11 | Chr3: 12548682–12549395 | 8.9 | 48,125.84 | 423 | Plasma membrane |
| MtCIPK11 | MtrunA17_Chr3g0091291 | MsCIPK11 | Chr3: 12550018–12550736 | 8.44 | 57,285.85 | 510 | Plasma membrane |
| MtCIPK12 | MtrunA17_Chr3g0091311 | MsCIPK11 | Chr3: 12582134–12582970 | 8.95 | 47,560 | 422 | Plasma membrane |
| MtCIPK13 | MtrunA17_Chr3g0091411 | MsCIPK11 | Chr3: 12692385–12693326 | 9.27 | 30,232.13 | 257 | Plasma membrane |
| MtCIPK14 | MtrunA17_Chr3g0091421 | MsCIPK11 | Chr3: 12693793–12694524 | 8.93 | 52,506.01 | 457 | Plasma membrane |
| MtCIPK15 | MtrunA17_Chr3g0107011 | MsCIPK9 | Chr3: 30606080–30609128 | 9.05 | 52,009.48 | 447 | Plasma membrane |
| MtCIPK16 | MtrunA17_Chr3g0107051 | MsCIPK10 | Chr3: 30640386–30641717 | 9.22 | 47,462.77 | 420 | Plasma membrane |
| MtCIPK17 | MtrunA17_Chr3g0114511 | MsCIPK11 | Chr3: 35974723–35976525 | 8.67 | 50,488.22 | 446 | Plasma membrane |
| MtCIPK18 | MtrunA17_Chr4g0001331 | MsCIPK12 | Chr4: 900468–902885 | 9.27 | 28,716.18 | 243 | Plasma membrane |
| MtCIPK19 | MtrunA17_Chr4g0013131 | MsCIPK14 | Chr4: 10571450–10575122 | 9.1 | 49,421.81 | 435 | Plasma membrane |
| MtCIPK20 | MtrunA17_Chr4g0063881 | MsCIPK24 | Chr4: 55303349–55314602 | 8.53 | 49,753.07 | 436 | Plasma membrane |
| MtCIPK21 | MtrunA17_Chr4g0069301 | MsCIPK18 | Chr4: 59217861–59224659 | 8.85 | 51,572.49 | 460 | Plasma membrane |
| MtCIPK22 | MtrunA17_Chr4g0072831 | MsCIPK24 | Chr4: 61783649–61786108 | 9.05 | 27,643.06 | 237 | Cytoplasmic |
| MtCIPK23 | MtrunA17_Chr4g0074111 | MsCIPK26 | Chr4: 62835031–62837115 | 9.34 | 25,004.1 | 214 | Plasma membrane |
| MtCIPK24 | MtrunA17_Chr5g0428031 | MsCIPK15 | Chr5: 29699497–29710190 | 9.26 | 50,104.11 | 444 | Plasma membrane |
| MtCIPK25 | MtrunA17_Chr5g0432471 | MsCIPK16 | Chr5: 33183949–33187482 | 8.97 | 50,856.92 | 446 | Plasma membrane |
| MtCIPK26 | MtrunA17_Chr5g0432491 | MsCIPK17 | Chr5: 33195433–33198761 | 6.82 | 50,243.62 | 441 | Plasma membrane |
| MtCIPK27 | MtrunA17_Chr5g0441011 | MsCIPK18 | Chr5: 39527588–39534318 | 6.72 | 50,665.32 | 446 | Plasma membrane |
| MtCIPK28 | MtrunA17_Chr7g0244521 | MsCIPK19 | Chr7: 33731410–33735051 | 8.92 | 52,017.29 | 453 | Plasma membrane |
| MtCIPK29 | MtrunA17_Chr7g0244561 | MsCIPK20 | Chr7: 33768481–33770580 | 5.69 | 52,186.74 | 465 | Plasma membrane |
| MtCIPK30 | MtrunA17_Chr8g0346071 | MsCIPK21 | Chr8: 9057047–9061702 | 8.58 | 53,028.89 | 465 | Plasma membrane |
| MtCIPK31 | MtrunA17_Chr8g0346081 | MsCIPK21 | Chr8: 9065393–9070388 | 9.22 | 50,033.79 | 443 | Plasma membrane |
| MtCIPK32 | MtrunA17_Chr8g0379091 | MsCIPK7 | Chr8: 39906567–39908530 | 8.93 | 52,494.6 | 465 | Plasma membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Yang, J.; Ma, L.; Su, Q.; Pang, Y. Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago. Int. J. Mol. Sci. 2021, 22, 4634. https://doi.org/10.3390/ijms22094634
Du W, Yang J, Ma L, Su Q, Pang Y. Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago. International Journal of Molecular Sciences. 2021; 22(9):4634. https://doi.org/10.3390/ijms22094634
Chicago/Turabian StyleDu, Wenxuan, Junfeng Yang, Lin Ma, Qian Su, and Yongzhen Pang. 2021. "Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago" International Journal of Molecular Sciences 22, no. 9: 4634. https://doi.org/10.3390/ijms22094634
APA StyleDu, W., Yang, J., Ma, L., Su, Q., & Pang, Y. (2021). Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago. International Journal of Molecular Sciences, 22(9), 4634. https://doi.org/10.3390/ijms22094634






