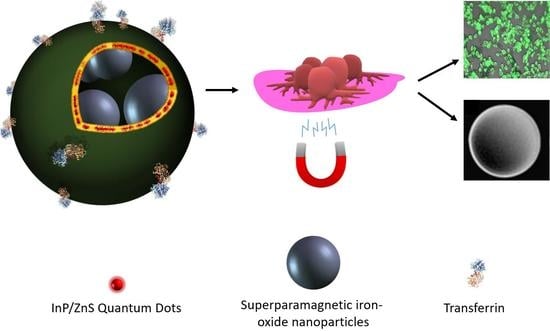
Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: Dual Targeting and Imaging of Glioma
Abstract
1. Introduction
2. Results and Discussion
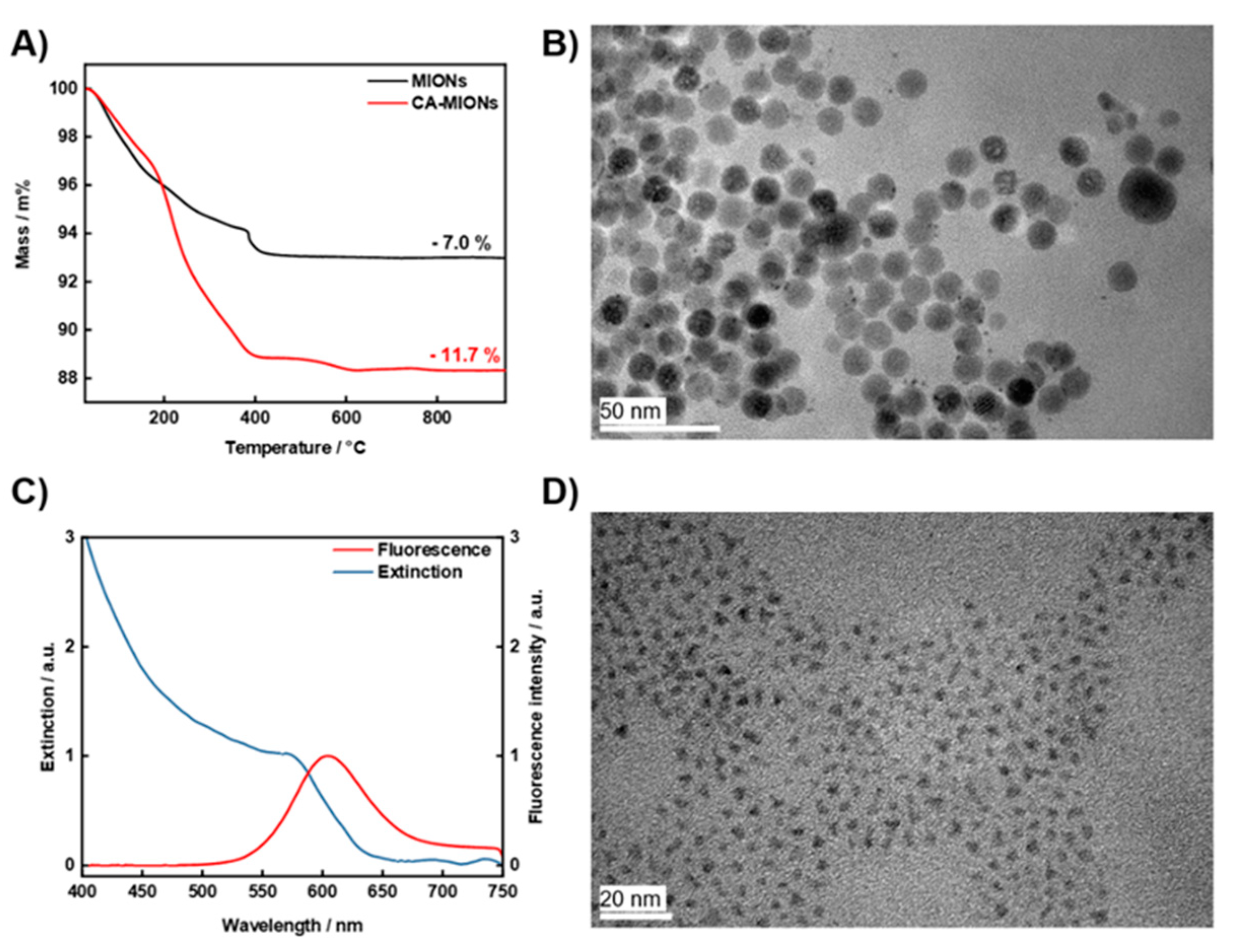
2.1. Characterization of CA-MIONs and InP/ZnS QDs
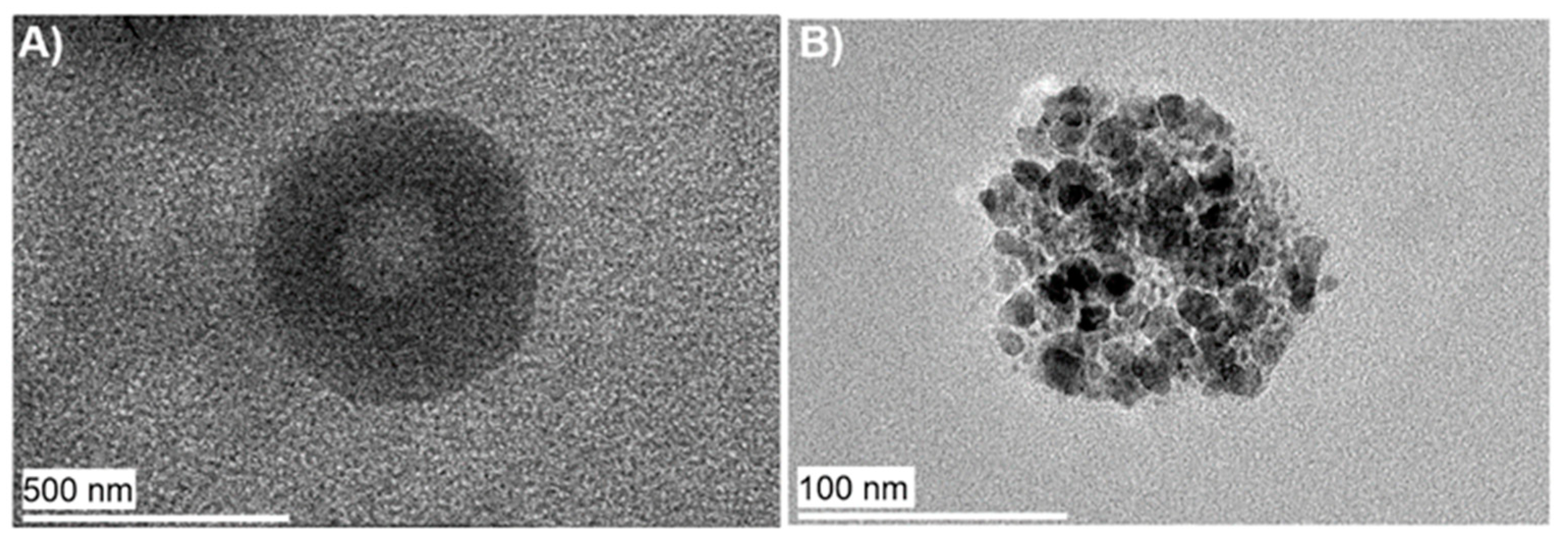
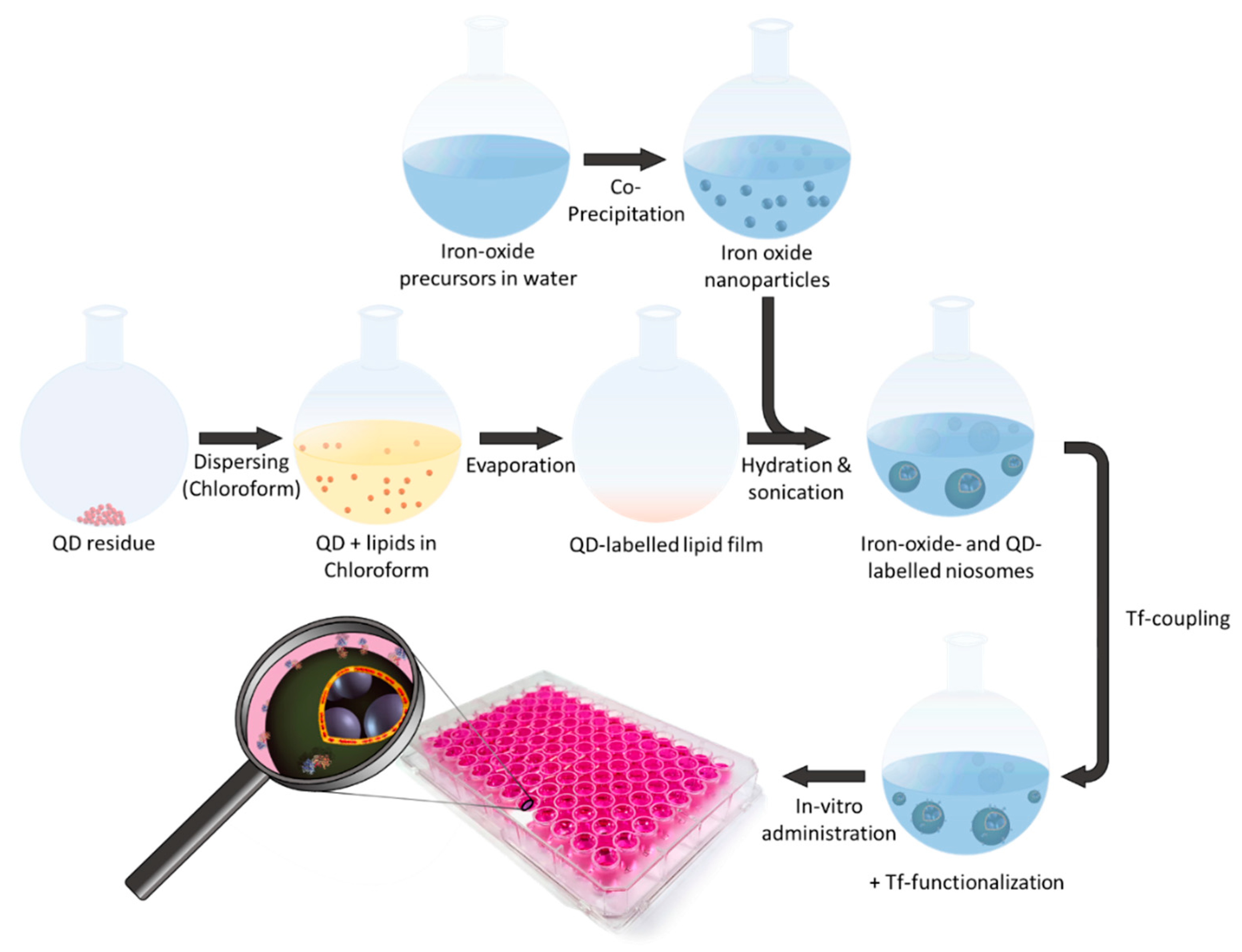
2.2. Characterization of Niosomes with Integrated InP/ZnS QDs and CA-MIONs
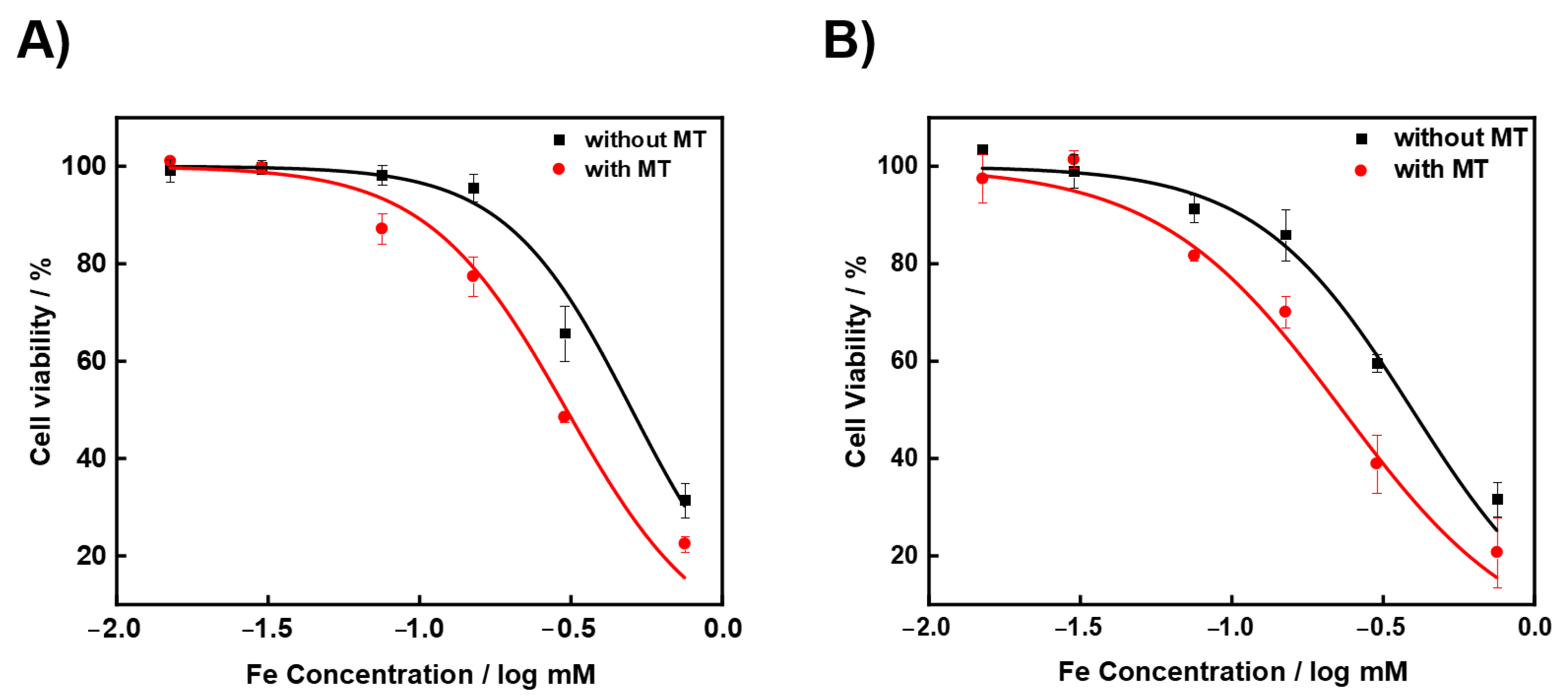
2.3. Cytotoxicity
2.4. Cellular Internalization and Uptake
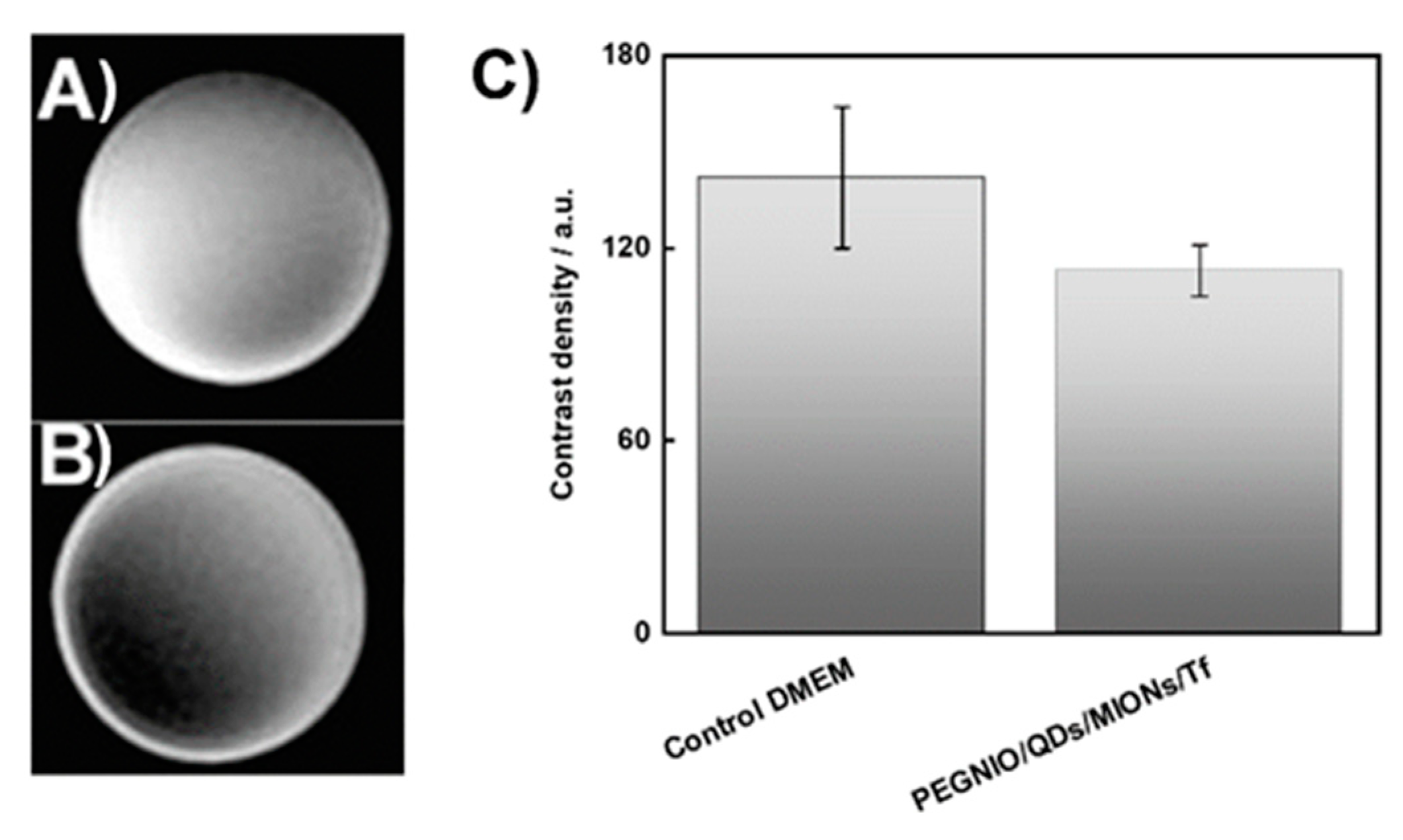
2.5. MR Imaging
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of CA-MIONs
3.3. Synthesis and Characterization of InP/ZnS QDs
3.4. Preparation of Niosomes Loaded with InP/ZnS QDs and CA-MIONs
3.5. Preparation of Transferrin-Conjugated Niosomes
3.6. Characterization of Niosomes
3.7. Cellular Uptake and Internalization
3.8. Cytotoxicity
3.9. MR Imaging
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, M.C.; Giverso, C.; Faggiano, E.; Boffano, C.; Acerbi, F.; Ciarletta, P. Towards the Personalized Treatment of Glio-blastoma. PLoS ONE 2015, 10, e0132887. [Google Scholar]
- Tamimi, A.F.; Juweid, M. Glioblastoma. In Epidemiology and Outcome of Glioblastoma; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Paolillo, M.; Boselli, C.; Schinelli, S. Glioblastoma under Siege: An Overview of Current Therapeutic Strategies. Brain Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.-S.; Zhang, J.-L.; Liu, G.; Zhang, W.-G. Application of iron oxide nanoparticles in glioma imaging and therapy: From bench to bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Stahl, F.; Scheper, T. Tumor homing and penetrating peptide-conjugated niosomes as multi-drug carriers for tumor-targeted drug delivery. RSC Adv. 2017, 7, 33378–33384. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6, 87910–87918. [Google Scholar] [CrossRef]
- Seleci, D.A.; Maurer, V.; Stahl, F.; Scheper, T.; Garnweitner, G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. Int. J. Mol. Sci. 2019, 20, 4696. [Google Scholar] [CrossRef]
- Seleci, M.; Seleci, D.A.; Joncyzk, R.; Stahl, F.; Blume, C.; Scheper, T. Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials 2016, 17, 33–41. [Google Scholar] [CrossRef]
- Ag, D.; Bongartz, R.; Dogan, L.E.; Seleci, M.; Walter, J.-G.; Demirkol, D.O.; Stahl, F.; Ozcelik, S.; Timur, S.; Scheper, T. Bio-functional quantum dots as fluorescence probe for cell-specific targeting. Colloids Surf. B 2014, 114, 96–103. [Google Scholar] [CrossRef]
- Ag, D.; Seleci, M.; Bongartz, R.; Can, M.; Yurteri, S.; Cianga, I.; Stahl, F.; Timur, S.; Scheper, T.; Yagci, Y. From invisible struc-tures of SWCNTs toward fluorescent and targeting architectures for cell imaging. Biomacromolecules 2013, 14, 3532–3541. [Google Scholar] [CrossRef]
- Javed, Y.; Akhtar, K.; Anwar, H.; Jamil, Y. MRI based on iron oxide nanoparticles contrast agents: Effect of oxidation state and architecture. J. Nanoparticle Res. 2017, 19, 366. [Google Scholar] [CrossRef]
- Karki, K.; Ewing, J.R.; Ali, M.M. Targeting Glioma with a Dual Mode Optical and Paramagnetic Nanoprobe across the Blood-brain Tumor Barrier. J. Nanomed. Nanotechnol. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Rivas, C.; Stasiuk, G.J.; Gallo, J.; Minuzzi, F.; Rutter, G.A.; Long, N.J. Lanthanide(III) complexes of rhodamine-DO3A con-jugates as agents for dual-modal imaging. Inorg. Chem. 2013, 52, 14284–14293. [Google Scholar] [CrossRef]
- Mishra, A.; Pfeuffer, J.; Mishra, R.; Engelmann, J.; Mishra, A.K.; Ugurbil, K.; Logothetis, N.K. A New Class of Gd-Based DO3A-Ethylamine-Derived Targeted Contrast Agents for MR and Optical Imaging. Bioconjugate Chem. 2006, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, B.; Liu, Y.; Shen, Q.-D.; Jiang, S. Dual-Color Fluorescence Imaging of Magnetic Nanoparticles in Live Cancer Cells Using Conjugated Polymer Probes. Sci. Rep. 2016, 6, 22368. [Google Scholar] [CrossRef]
- Jang, H.; Lee, C.; Nam, G.-E.; Quan, B.; Choi, H.J.; Yoo, J.S.; Piao, Y. In vivo magnetic resonance and fluorescence dual imaging of tumor sites by using dye-doped silica-coated iron oxide nanoparticles. J. Nanoparticle Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Seleci, M.; Seleci, D.A.; Scheper, T.; Stahl, F. Theranostic Liposome-Nanoparticle Hybrids for Drug Delivery and Bioimaging. Int. J. Mol. Sci. 2017, 18, 1415. [Google Scholar] [CrossRef]
- Cui, L.; Li, C.-C.; Tang, B.; Zhang, C.-Y. Advances in the integration of quantum dots with various nanomaterials for bio-medical and environmental applications. Analyst 2018, 143, 2469–2478. [Google Scholar] [CrossRef]
- Porsiel, J.C.; Temel, B.; Schirmacher, A.; Buhr, E.; Garnweitner, G. Dimensional characterization of cadmium selenide nanocrystals via indirect Fourier transform evaluation of small-angle X-ray scattering data. Nano Res. 2019, 12, 2849–2857. [Google Scholar] [CrossRef]
- Wang, F.; Shu, L.; Wang, J.; Pan, X.; Huang, R.; Lin, Y.; Cai, X. Perspectives on the Toxicology of Cadmium-based Quantum Dots. Curr. Drug Metab. 2013, 14, 847–856. [Google Scholar] [CrossRef]
- Ulusoy, M.; Jonczyk, R.; Walter, J.-G.; Springer, S.; Lavrentieva, A.; Stahl, F.; Green, M.; Scheper, T. Aqueous Synthesis of PEGylated Quantum Dots with Increased Colloidal Stability and Reduced Cytotoxicity. Bioconjugate Chem. 2015, 27, 414–426. [Google Scholar] [CrossRef]
- Wegner, K.D.; Dussert, F.; Truffier-Boutry, D.; Benayad, A.; Beal, D.; Mattera, L.; Ling, W.L.; Carrière, M.; Reiss, P. Influence of the Core/Shell Structure of Indium Phosphide Based Quantum Dots on Their Photostability and Cytotoxicity. Front. Chem. 2019, 7, 466. [Google Scholar] [CrossRef]
- Ulusoy, M.; Walter, J.-G.; Lavrentieva, A.; Kretschmer, I.; Sandiford, L.; le Marois, A.; Bongartz, R.; Aliuos, P.; Suhling, K.; Stahl, F.; et al. One-pot aqueous synthesis of highly strained CdTe/CdS/ZnS nanocrystals and their interac-tions with cells. RSC Adv. 2015, 5, 7485–7494. [Google Scholar] [CrossRef]
- Lin, G.; Ouyang, Q.; Hu, R.; Ding, Z.; Tian, J.; Yin, F.; Xu, G.; Chen, Q.; Wang, X.; Yong, K.-T. In vivo toxicity assessment of non-cadmium quantum dots in BALB/c mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 341–350. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Functionalized-quantum-dot-liposome hy-brids as multimodal nanoparticles for cancer. Small 2008, 4, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Kulkarni, S.A.; Raju, A.; Feng, S.-S. Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 2012, 33, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Danelon, C.; Izewska, P.; Prummer, M.; Bolinger, P.-Y.; Geissbühler, I.; Demurtas, D.; Dubochet, J.; Vogel, H. Multifunctional Lipid/Quantum Dot Hybrid Nanocontainers for Controlled Targeting of Live Cells. Angew. Chem. Int. Ed. 2006, 45, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Demır, B.; Barlas, F.B.; Gumus, Z.P.; Unak, P.; Timur, S.; Demir, B. Theranostic Niosomes as a Promising Tool for Combined Therapy and Diagnosis: “All-in-One” Approach. ACS Appl. Nano Mater. 2018, 1, 2827–2835. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable Synthesis of a Smart Multifunctional Nanoscale Met-al-Organic Framework for Magnetic Resonance/Optical Imaging and Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Xu, H.-L.; Yang, J.-J.; ZhuGe, D.-L.; Lin, M.-T.; Zhu, Q.-Y.; Jin, B.-H.; Tong, M.-Q.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Glio-ma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilen-gitide for Dual-Imaging Guiding Cancer Surgery. Adv. Healthc. Mater. 2018, 7, e1701130. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Chin, P.X.; Phang, Y.L.; Cheah, J.Y.; Ooi, S.C.; Mak, K.-K.; Pichika, M.R.; Kesharwani, P.; Hussain, Z.; et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: A review of recent advancements and emerging trends. Drug Deliv. Transl. Res. 2018, 8, 1545–1563. [Google Scholar] [CrossRef]
- Calzolari, A.; LaRocca, L.M.; Deaglio, S.; Finisguerra, V.; Boe, A.; Raggi, C.; Ricci-Vitani, L.; Pierconti, F.; Malavasi, F.; De Maria, R.; et al. Transferrin Receptor 2 Is Frequently and Highly Expressed in Glioblastomas. Transl. Oncol. 2010, 3, 123–134. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Rubab, F.; Rao, A.K.; Kiritsy, M.C.; Pirollo, K.F.; Wang, S.; Weiner, L.M.; Chang, E.H. The clinical po-tential of targeted nanomedicine: Delivering to cancer stem-like cells. Mol. Ther. 2014, 22, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Sonali; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef]
- Jiang, W.; Xie, H.; Ghoorah, D.; Shang, Y.; Shi, H.; Liu, F.; Yang, X.; Xu, H. Conjugation of Functionalized SPIONs with Transferrin for Targeting and Imaging Brain Glial Tumors in Rat Model. PLoS ONE 2012, 7, e37376. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.; Leung, C.; Chan, K.; Chan, W.; Zhong, W.; Pong, P. Effect of synthesis conditions on the properties of citric-acid coated iron oxide nanoparticles. Microelectron. Eng. 2013, 110, 329–334. [Google Scholar] [CrossRef]
- Masthoff, I.-C.; Kraken, M.; Menzel, D.; Litterst, F.J.; Garnweitner, G. Study of the growth of hydrophilic iron oxide nano-particles obtained via the non-aqueous sol–gel method. J. Sol Gel Sci. Technol. 2016, 77, 553–564. [Google Scholar] [CrossRef]
- Sahoo, Y.; Goodarzi, A.; Swihart, M.T.; Ohulchanskyy, T.Y.; Kaur, N.; Furlani, A.E.P.; Prasad, P.N. Aqueous Ferrofluid of Magnetite Nanoparticles: Fluorescence Labeling and Magnetophoretic Control. J. Phys. Chem. B 2005, 109, 3879–3885. [Google Scholar] [CrossRef]
- Maurer, V.; Altin, S.; Seleci, D.A.; Zarinwall, A.; Temel, B.; Vogt, P.M.; Strauß, S.; Stahl, F.; Scheper, T.; Bucan, V.; et al. In-Vitro Application of Magnetic Hybrid Niosomes: Targeted siRNA-Delivery for Enhanced Breast Cancer Therapy. Pharmaceutics 2021, 13, 394. [Google Scholar] [CrossRef]
- Dufès, C.; Al Robaian, M.M.M.; Somani, S. Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Ther. Deliv. 2013, 4, 629–640. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nano-particles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Jhaveri, A.; Luther, E.; Torchilin, V. The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures ofglioblastoma: Implications for targeting tumour-initiating cells. J. Drug Target 2019, 27, 601–613. [Google Scholar] [CrossRef]
- Sheykhzadeh, S.; Luo, M.; Peng, B.; White, J.; Abdalla, Y.; Tang, T.; Mäkilä, E.; Voelcker, N.H.; Tong, W.Y. Transfer-rin-targeted porous silicon nanoparticles reduce glioblastoma cell migration across tight extracellular space. Sci. Rep. 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Devatha, G.; Roy, S.; Rao, A.; Mallick, A.; Basu, S.; Pillai, P.P. Electrostatically driven resonance energy transfer in “cationic” biocompatible indium phosphide quantum dots. Chem. Sci. 2017, 8, 3879–3884. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C.; et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473. [Google Scholar] [CrossRef]
- He, G.; Lin, W. Peptide-Functionalized Nanoparticles-Encapsulated Cyclin-Dependent Kinases Inhibitor Seliciclib in Transferrin Receptor Overexpressed Cancer Cells. Nanomaterials 2021, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.; Chang, Y.; Zhao, L.; Zhang, K.; Zhao, Y.; Gao, F.; Gao, X. Ultrasmall Superparamagnetic Iron Oxide Na-noparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28959–28966. [Google Scholar] [CrossRef] [PubMed]
- Khaniabadi, P.M.; Shahbazi-Gahrouei, D.; Jaafar, M.S.; Majid, A.M.S.A.; Khaniabadi, B.M.; Shahbazi-Gahrouei, S. Magnetic Iron Oxide Nanoparticles as T2 MR Imaging Contrast Agent for Detection of Breast Cancer (MCF-7) Cell. Avicenna J. Med. Biotechnol. 2017, 9, 181–188. [Google Scholar]
- Sharma, A.; Baral, D.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Biocompatible capped iron oxide nanoparticles for Vibrio cholerae detection. Nanotechnology 2015, 26, 175302. [Google Scholar] [CrossRef]
- Ellis, M.A.; Grandinetti, G.; Fichter, K.M.; Fichter, K.M. Synthesis of Cd-free InP/ZnS Quantum Dots Suitable for Biomed-ical Applications. JoVE 2016, 108, e53684. [Google Scholar]
- Ge, X.; Wei, M.; He, S.; Yuan, W. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Saavedra-Alonso, S.; Zapata-Benavides, P.; Chavez-Escamilla, A.K.; Manilla-Muñoz, E.; Zamora-Avila, D.E.; Franco-Molina, M.A.; Rodríguez-Padilla, C. WT1 shRNA delivery using transferrin-conjugated PEG liposomes in an in vivo model of melanoma. Exp. Ther. Med. 2016, 12, 3778–3784. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Srinivasan, R.; Miskimins, R.; Sykes, A.G. A simple aza-crown ether containing an anthraquinone fluorophore for the selective detection of Mg(II) in living cells. Tetrahedron 2016, 72, 205–209. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ag Seleci, D.; Maurer, V.; Barlas, F.B.; Porsiel, J.C.; Temel, B.; Ceylan, E.; Timur, S.; Stahl, F.; Scheper, T.; Garnweitner, G. Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: Dual Targeting and Imaging of Glioma. Int. J. Mol. Sci. 2021, 22, 4556. https://doi.org/10.3390/ijms22094556
Ag Seleci D, Maurer V, Barlas FB, Porsiel JC, Temel B, Ceylan E, Timur S, Stahl F, Scheper T, Garnweitner G. Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: Dual Targeting and Imaging of Glioma. International Journal of Molecular Sciences. 2021; 22(9):4556. https://doi.org/10.3390/ijms22094556
Chicago/Turabian StyleAg Seleci, Didem, Viktor Maurer, Firat Baris Barlas, Julian Cedric Porsiel, Bilal Temel, Elcin Ceylan, Suna Timur, Frank Stahl, Thomas Scheper, and Georg Garnweitner. 2021. "Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: Dual Targeting and Imaging of Glioma" International Journal of Molecular Sciences 22, no. 9: 4556. https://doi.org/10.3390/ijms22094556
APA StyleAg Seleci, D., Maurer, V., Barlas, F. B., Porsiel, J. C., Temel, B., Ceylan, E., Timur, S., Stahl, F., Scheper, T., & Garnweitner, G. (2021). Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: Dual Targeting and Imaging of Glioma. International Journal of Molecular Sciences, 22(9), 4556. https://doi.org/10.3390/ijms22094556