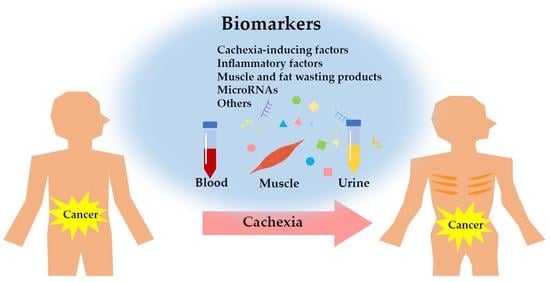
Biomarkers for Cancer Cachexia: A Mini Review
Abstract
1. Introduction
2. Cachexia-Inducing Factors
3. Inflammatory Factors
4. Muscle and Fat Wasting Products
5. MicroRNAs
6. Other Potential Biomarkers
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ActA | Activin A |
| ActRIIB | Type IIB activin receptor |
| Ang II | Angiotensin II |
| Atrogin-1 | Muscle Atrophy F-box gene |
| BAT | Brown adipose tissue |
| CNDP1 | Carnosine dipeptidase 1 |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| FGF21 | Fibroblast growth factor 21 |
| GDF15 | Growth/differentiation factor 15 |
| GI | Gastrointestinal cancer |
| HCERs | Hexosyl-ceramides |
| HNC | Head and neck cancer |
| IRMA | Immunoradiometric assay |
| LBM | Lean body mass |
| LCERs | Lactosyl-ceramides |
| LLC | Lewis Lung Carcinoma |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MPM | Malignant pleural mesothelioma |
| MS | Mass spectrometry |
| Mstn | Myostatin |
| MuRF1 | Muscle RING-finger protein-1 |
| NDPs | Neutrophil-derived proteases |
| NSCLC | Non–small-cell lung cancer |
| PaCa | Pancreatic cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| PPARγ | Peroxisome proliferator–activated receptor γ |
| PTHrP | Parathyroid Hormone release Peptide |
| qRT-PCR | Quantitative real-time reverse transcription PCR |
| TGF | Transforming growth factor |
| TIA | Thymidine Incorporation Assay |
| TIMP-1 | Tissue inhibitor of metalloproteinases-1 |
| WAT | White adipose tissue |
| ZAG | Zinc-α2-glycoprotein |
References
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Dunne, R.F.; Mustian, K.M.; Garcia, J.M.; Dale, W.; Hayward, R.; Roussel, B.; Buschmann, M.M.; Caan, B.J.; Cole, C.L.; Fleming, F.J.; et al. Research priorities in cancer cachexia: The University of Rochester Cancer Center NCI Community Oncology Research Program Research Base Symposium on Cancer Cachexia and Sarcopenia. Curr. Opin. Support. Palliat. Care 2017, 11, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Johnston, M.; Hancock, M.; Small, S.; Taylor, R.; Dalton, J.; Steiner, M. Enobosarm, a selective androgen receptor modulator (SARM) increases lean body mass (LBM) in advanced NSCLC patients: Updated results of two pivotal, international phase 3 trials. Support. Care Cancer 2014, 22 (Suppl. 1), S30. [Google Scholar]
- Crawford, J.; Dalton, J.; Hancock, M.; Johnston, M.; Steiner, M. Enobosarm, a selective androgen receptor modulator (SARM), increases lean body mass (LBM) in advanced non-small cell lung cancer patients in two pivotal, international Phase 3 trials. J. Cachexia Sarcopenia Muscle 2014, 5, 35–78. [Google Scholar]
- Laird, B.J.A.; Balstad, T.R.; Solheim, T.S. Endpoints in clinical trials in cancer cachexia: Where to start? Curr. Opin. Support. Palliat. Care 2018, 12, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.; Temel, J.; Currow, D.; Gleich, L.; Friend, J. Anamorelin HCl for the treatment of anorexia–cachexia in lung cancer: Study design and baseline characteristics of patients in the phase III clinical trial ROMANA 2 (HT-ANAM-302). J. Cachexia Sarcopenia Muscle 2013, 4, 295–343. [Google Scholar]
- Kinsey, E.; Ajazi, E.; Wang, X.; Johnston, M.A.M.; Crawford, J. Predictors of Physical and Functional Loss in Advanced-Stage Lung Cancer Patients Receiving Platinum Chemotherapy. J. Thorac. Oncol. 2018, 13, 1294–1301. [Google Scholar] [CrossRef]
- Naito, T.; Okayama, T.; Aoyama, T.; Ohashi, T.; Masuda, Y.; Kimura, M.; Shiozaki, H.; Murakami, H.; Kenmotsu, H.; Taira, T.; et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer 2017, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mediators of cachexia in cancer patients. Nutrition 2019, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef]
- Talbert, E.E.; Lewis, H.L.; Farren, M.R.; Ramsey, M.L.; Chakedis, J.M.; Rajasekera, P.; Haverick, E.; Sarna, A.; Bloomston, M.; Pawlik, T.M. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 358–368. [Google Scholar] [CrossRef]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Morigny, P.; Zuber, J.; Haid, M.; Kaltenecker, D.; Riols, F.; Lima, J.D.C.; Simoes, E.; Otoch, J.P.; Schmidt, S.F.; Herzig, S.; et al. High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, A.; Dalgas, U.; Primdahl, H.; Johansen, J.; Overgaard, J.; Overgaard, K.; Henriksen, K.; Karsdal, M.A.; Lønbro, S. Collagen fragment biomarkers as serological biomarkers of lean body mass–a biomarker pilot study from the DAHANCA25B cohort and matched controls. J. Cachexia Sarcopenia Muscle 2015, 6, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, S.; Farneti, A.; Marucci, L.; Ganci, F.; Sacconi, A.; Strano, S.; Sanguineti, G.; Blandino, G. Non-coding RNAs as Putative Biomarkers of Cancer-Associated Cachexia. Front. Cell Dev. Biol. 2020, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, A.; Thissen, J.-P. Biomarkers of cancer cachexia. Clin. Biochem. 2017, 50, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Patel, M.S.; Lee, J.; Baz, M.; Wells, C.E.; Bloch, S.; Lewis, A.; Donaldson, A.V.; Garfield, B.E.; Hopkinson, N.S.; Natanek, A. Growth differentiation factor–15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J. Cachexia Sarcopenia Muscle 2016, 7, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Mitch, W.E. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support. Palliat. Care 2011, 5, 334–341. [Google Scholar] [CrossRef]
- Chen, J.L.; Walton, K.L.; Qian, H.; Colgan, T.D.; Hagg, A.; Watt, M.J.; Harrison, C.A.; Gregorevic, P. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Res. 2016, 76, 5372–5382. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.-P. Role of Activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef]
- Terpos, E.; Kastritis, E.; Christoulas, D.; Gkotzamanidou, M.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Papatheodorou, A.; Dimopoulos, M. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann. Oncol. 2012, 23, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Hoda, M.A.; Rozsas, A.; Lang, E.; Klikovits, T.; Lohinai, Z.; Torok, S.; Berta, J.; Bendek, M.; Berger, W.; Hegedus, B. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget 2016, 7, 13388. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Circulating Activin A predicts survival in cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, J.; Ilonen, I.; Lauri, H.; Järvinen, T.; Sutinen, E.; Ollila, H.; Rouvinen, E.; Lemström, K.; Räsänen, J.; Ritvos, O.; et al. Elevated Circulating Activin A Levels in Patients With Malignant Pleural Mesothelioma Are Related to Cancer Cachexia and Reduced Response to Platinum-based Chemotherapy. Clin. Lung Cancer 2020, 21, e142–e150. [Google Scholar] [CrossRef] [PubMed]
- Talar-Wojnarowska, R.; Wozniak, M.; Borkowska, A.; Olakowski, M.; Malecka-Panas, E. Clinical significance of activin A and myostatin in patients with pancreatic adenocarcinoma and progressive weight loss. J. Physiol. Pharmacol. 2020, 71, 1. [Google Scholar]
- Assi, M.; Derbré, F.; Lefeuvre-Orfila, L.; Rébillard, A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Radic. Biol. Med. 2016, 91, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, A.; Scharf, G.M.; Duncker, D.; Widera, C.; Gottlieb, J.; Vogel, A.; Schmidt, S.; Brandes, G.; Heuft, H.-G.; Lichtinghagen, R. Highly specific detection of myostatin prodomain by an immunoradiometric sandwich assay in serum of healthy individuals and patients. PLoS ONE 2013, 8, e80454. [Google Scholar] [CrossRef] [PubMed]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15–GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; Isnard, S.; Lin, J.; Routy, B.; Routy, J.-P. GDF15/GFRAL Pathway as a Metabolic Signature for Cachexia in Patients with Cancer. J. Cancer 2021, 12, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Gyuris, J.; Nicoletti, R.; Gifford, J.; Krieger, B.; Jatoi, A. Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncol. Lett. 2016, 12, 4219–4223. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Schäfer, T.; Martens, A.; Kuzkina, A.; Uder, L.; Noor, S.; Garbe, C.; Harter, P.N.; Mittelbronn, M.; Wischhusen, J. High GDF-15 serum levels independently correlate with poorer overall survival of patients with tumor-free stage III and unresectable stage IV melanoma. J. Investig. Dermatol. 2016, 136, 2444–2452. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers 2021, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Bao, Y.; Jenkins, J.; Sanders, P.; Manieri, M.; Cinti, S.; Tisdale, M.J.; Trayhurn, P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc. Natl. Acad. Sci. USA 2004, 101, 2500–2505. [Google Scholar] [CrossRef]
- Mracek, T.; Stephens, N.A.; Gao, D.; Bao, Y.; Ross, J.A.; Rydén, M.; Arner, P.; Trayhurn, P.; Fearon, K.C.H.; Bing, C. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br. J. Cancer 2011, 104, 441–447. [Google Scholar] [CrossRef]
- Elattar, S.; Dimri, M.; Satyanarayana, A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018, 32, 4727–4743. [Google Scholar] [CrossRef]
- Felix, K.; Fakelman, F.; Hartmann, D.; Giese, N.A.; Gaida, M.M.; Schnölzer, M.; Flad, T.; Büchler, M.W.; Werner, J. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 2011, 88, 218–225. [Google Scholar] [CrossRef]
- Rydén, M.; Agustsson, T.; Andersson, J.; Bolinder, J.; Toft, E.; Arner, P. Adipose zinc-α2-glycoprotein is a catabolic marker in cancer and noncancerous states. J. Intern. Med. 2012, 271, 414–420. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.W.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; van der Lans, A.A.J.J.; Cheng, C.C.; Adams, A.C.; van Marken Lichtenbelt, W.D.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef]
- Franz, K.; Ost, M.; Otten, L.; Herpich, C.; Coleman, V.; Endres, A.-S.; Klaus, S.; Müller-Werdan, U.; Norman, K. Higher serum levels of fibroblast growth factor 21 in old patients with cachexia. Nutrition 2019, 63–64, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-W.; Park, J.H.; Kim, D.A.; Jang, I.-Y.; Park, S.J.; Lee, J.Y.; Lee, S.; Kim, J.H.; Yi, H.-S.; Lee, E.; et al. Association between serum FGF21 level and sarcopenia in older adults. Bone 2021, 145, 115877. [Google Scholar] [CrossRef]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef]
- Hong, N.; Yoon, H.-J.; Lee, Y.-H.; Kim, H.R.; Lee, B.W.; Rhee, Y.; Kang, E.S.; Cha, B.-S.; Lee, H.C. Serum PTHrP predicts weight loss in cancer patients independent of hypercalcemia, inflammation, and tumor burden. J. Clin. Endocrinol. Metab. 2016, 101, 1207–1214. [Google Scholar] [CrossRef]
- Penafuerte, C.A.; Gagnon, B.; Sirois, J.; Murphy, J.; MacDonald, N.; Tremblay, M.L. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br. J. Cancer 2016, 114, 680–687. [Google Scholar] [CrossRef]
- Song, Y.-H.; Li, Y.; Du, J.; Mitch, W.E.; Rosenthal, N.; Delafontaine, P. Muscle-specific expression of IGF-1 blocks angiotensin II–induced skeletal muscle wasting. J. Clin. Investig. 2005, 115, 451–458. [Google Scholar] [CrossRef]
- Yoshida, T.; Tabony, A.M.; Galvez, S.; Mitch, W.E.; Higashi, Y.; Sukhanov, S.; Delafontaine, P. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: Potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Ramaha, A.; Patston, P.A. Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch. Biochem. Biophys. 2002, 397, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, D.B.; Casarini, D.E.; Cristovam, P.C.; Leite, C.A.; Schor, N.; Boim, M.A. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am. J. Physiol. Ren. Physiol. 2004, 286, F1039–F1045. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jose, I.; Glab, J.; Puthalakath, H.; Osellame, L.D.; Hoogenraad, N.J. Generation of reporter cell lines for factors inducing muscle wasting in cancer cachexia. Anal. Biochem. 2020, 606, 113877. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, L.; Costelli, P.; Baccino, F.M. Humoral mediation for cachexia in tumour-bearing rats. Br. J. Cancer 1993, 67, 15–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karayiannakis, A.J.; Syrigos, K.N.; Polychronidis, A.; Pitiakoudis, M.; Bounovas, A.; Simopoulos, K. Serum Levels of Tumor Necrosis Factor-and Nutritional Status in Pancreatic Cancer Patients. Anticancer Res. 2001, 21, 1355–1358. [Google Scholar] [PubMed]
- Suh, S.-Y.; Choi, Y.S.; Yeom, C.H.; Kwak, S.M.; Yoon, H.M.; Kim, D.G.; Koh, S.-J.; Park, J.; Lee, M.A.; Lee, Y.J. Interleukin-6 but not tumour necrosis factor-alpha predicts survival in patients with advanced cancer. Supportive Care Cancer 2013, 21, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.C.; Bland, L.A.; Oettinger, C.W.; Arduino, M.J.; McAllister, S.K.; Aguero, S.M.; Favero, M.S. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993, 12, 115–120. [Google Scholar]
- Scott, H.; McMillan, D.; Crilly, A.; McArdle, C.; Milroy, R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br. J. Cancer 1996, 73, 1560–1562. [Google Scholar] [CrossRef]
- Batista, M.L.; Olivan, M.; Alcantara, P.S.M.; Sandoval, R.; Peres, S.B.; Neves, R.X.; Silverio, R.; Maximiano, L.F.; Otoch, J.P.; Seelaender, M. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine 2013, 61, 532–539. [Google Scholar] [CrossRef]
- Kayacan, O.; Karnak, D.; Beder, S.; Güllü, E.; Tutkak, H.; Senler, F.Ç.; Köksal, D. Impact of TNF-α and IL-6 levels on development of cachexia in newly diagnosed NSCLC patients. Am. J. Clin. Oncol. 2006, 29, 328–335. [Google Scholar] [CrossRef]
- Scheede-Bergdahl, C.; Watt, H.L.; Trutschnigg, B.; Kilgour, R.D.; Haggarty, A.; Lucar, E.; Vigano, A. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin. Nutr. 2012, 31, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Wang, C.-J.; Chao, Y.-J.; Chen, H.-Y.; Wang, H.-C.; Tung, H.-L.; Lin, J.-T.; Shan, Y.-S. Elevated Serum Interleukin-8 Level Correlates with Cancer-Related Cachexia and Sarcopenia: An Indicator for Pancreatic Cancer Outcomes. J. Clin. Med. 2018, 7, 502. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Strieter, R.M.; Standiford, T.J.; McDonald, R.A.; Burdick, M.D.; Kunkel, S.L.; Toews, G.B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 1995, 155, 4790–4797. [Google Scholar] [PubMed]
- Yan, L.; Nielsen, F.H.; Sundaram, S.; Cao, J. Monocyte chemotactic protein-1 deficiency attenuates and high-fat diet exacerbates bone loss in mice with Lewis lung carcinoma. Oncotarget 2017, 8, 23303–23311. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.E.; Kim, M.-S.; Kang, J.H.; Lee, J.Y.; Lee, M.S.; Kim, E.H.; Chung, N.; Jeong, Y.K. Potential role of immunological factors in early diagnosis of cancer cachexia in C26 tumor-bearing mice. Appl. Biol. Chem. 2019, 62, 3. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Bilir, C.; Engin, H.; Can, M.; Temi, Y.B.; Demirtas, D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med. Oncol. 2015, 32, 56. [Google Scholar] [CrossRef]
- Stephens, N.A.; Skipworth, R.J.E.; Gallagher, I.J.; Greig, C.A.; Guttridge, D.C.; Ross, J.A.; Fearon, K.C.H. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 2015, 6, 53–61. [Google Scholar] [CrossRef]
- Burney, B.O.; Hayes, T.G.; Smiechowska, J.; Cardwell, G.; Papusha, V.; Bhargava, P.; Konda, B.; Auchus, R.J.; Garcia, J.M. Low Testosterone Levels and Increased Inflammatory Markers in Patients with Cancer and Relationship with Cachexia. J. Clin. Endocrinol. Metab. 2012, 97, E700–E709. [Google Scholar] [CrossRef]
- Tavares, P.; Gonçalves, D.M.; Santos, L.L.; Ferreira, R. Revisiting the clinical usefulness of C-reactive protein in the set of cancer cachexia. Porto Biomed. J. 2021, 6, e123. [Google Scholar] [CrossRef]
- Ishida, S.; Hashimoto, I.; Seike, T.; Abe, Y.; Nakaya, Y.; Nakanishi, H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: A retrospective study. J. Med. Investig. 2014, 61, 361–368. [Google Scholar] [CrossRef]
- Han, J.; Lu, C.; Meng, Q.; Halim, A.; Yean, T.J.; Wu, G. Plasma concentration of interleukin-6 was upregulated in cancer cachexia patients and was positively correlated with plasma free fatty acid in female patients. Nutr. Metab. 2019, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Agustsson, T.; Wikrantz, P.; Rydén, M.; Brismar, T.; Isaksson, B. Adipose tissue volume is decreased in recently diagnosed cancer patients with cachexia. Nutrition 2012, 28, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Agustsson, T.; Rydén, M.; Hoffstedt, J.; van Harmelen, V.; Dicker, A.; Laurencikiene, J.; Isaksson, B.; Permert, J.; Arner, P. Mechanism of Increased Lipolysis in Cancer Cachexia. Cancer Res. 2007, 67, 5531–5537. [Google Scholar] [CrossRef]
- Dahlman, I.; Mejhert, N.; Linder, K.; Agustsson, T.; Mutch, D.M.; Kulyte, A.; Isaksson, B.; Permert, J.; Petrovic, N.; Nedergaard, J.; et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br. J. Cancer 2010, 102, 1541–1548. [Google Scholar] [CrossRef]
- Riccardi, D.M.d.R.; das Neves, R.X.; de Matos-Neto, E.M.; Camargo, R.G.; Lima, J.D.C.C.; Radloff, K.; Alves, M.J.; Costa, R.G.F.; Tokeshi, F.; Otoch, J.P.; et al. Plasma Lipid Profile and Systemic Inflammation in Patients With Cancer Cachexia. Front. Nutr. 2020, 7, 4. [Google Scholar] [CrossRef]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar] [CrossRef]
- Sandri, M.; Coletto, L.; Grumati, P.; Bonaldo, P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci. 2013, 126, 5325–5333. [Google Scholar] [CrossRef]
- Skipworth, R.J.E.; Stewart, G.D.; Bhana, M.; Christie, J.; Sturgeon, C.M.; Guttridge, D.C.; Cronshaw, A.D.; Fearon, K.C.H.; Ross, J.A. Mass spectrometric detection of candidate protein biomarkers of cancer cachexia in human urine. Int. J. Oncol. 2010, 36, 973–982. [Google Scholar]
- Stephens, N.A.; Gallagher, I.J.; Rooyackers, O.; Skipworth, R.J.; Tan, B.H.; Marstrand, T.; Ross, J.A.; Guttridge, D.C.; Lundell, L.; Fearon, K.C.; et al. Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med. 2010, 2, 1. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5760. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, S.; Chen, F.; Fu, Z.; Yin, H.; Lu, X.; Yu, J.; Lu, C. Mammary fat of breast cancer: Gene expression profiling and functional characterization. PLoS ONE 2014, 9, e109742. [Google Scholar] [CrossRef]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2019, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell Metab. 2018, 28, 631–643.e3. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Stroud, A.M.; Argilés, J.M. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E191–E202. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Zuijdgeest-van Leeuwen, S.D.; van den Berg, J.W.O.; Wattimena, J.D.; van der Gaast, A.; Swart, G.R.; Wilson, J.P.; Dagnelie, P.C. Lipolysis and lipid oxidation in weight-losing cancer patients and healthy subjects. Metab. Clin. Exp. 2000, 49, 931–936. [Google Scholar] [CrossRef]
- Santos, J.M.O.; Peixoto da Silva, S.; Gil da Costa, R.M.; Medeiros, R. The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia. Cancers 2020, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Yao, L.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Yin, C.; Omura, Y.; Ide, S.; Kitajima, T.; Shimura, T.; et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 2018, 39, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. miRNA-130a significantly improves accuracy of SGA nutritional assessment tool in prediction of malnutrition and cachexia in radiotherapy-treated head and neck cancer patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer (Review). Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef]
- He, W.A.; Calore, F.; Londhe, P.; Canella, A.; Guttridge, D.C.; Croce, C.M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529. [Google Scholar] [CrossRef]
- Hur, K.; Toiyama, Y.; Okugawa, Y.; Ide, S.; Imaoka, H.; Boland, C.R.; Goel, A. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut 2017, 66, 654–665. [Google Scholar] [CrossRef]
- Arner, P.; Henjes, F.; Schwenk, J.M.; Darmanis, S.; Dahlman, I.; Iresjö, B.-M.; Naredi, P.; Agustsson, T.; Lundholm, K.; Nilsson, P.; et al. Circulating Carnosine Dipeptidase 1 Associates with Weight Loss and Poor Prognosis in Gastrointestinal Cancer. PLoS ONE 2015, 10, e0123566. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, R.L.H.; Williams, B.J.; Carroll, J.L.; Daves, L.K.; Cardelli, J.A. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res. Treat. 2009, 117, 31–44. [Google Scholar] [CrossRef]
- Gong, Y.; Scott, E.; Lu, R.; Xu, Y.; Oh, W.K.; Yu, Q. TIMP-1 Promotes Accumulation of Cancer Associated Fibroblasts and Cancer Progression. PLoS ONE 2013, 8, e77366. [Google Scholar] [CrossRef]
- Bloomston, M.; Shafii, A.; Zervos, E.E.; Rosemurgy, A.S. TIMP-1 Overexpression in Pancreatic Cancer Attenuates Tumor Growth, Decreases Implantation and Metastasis, and Inhibits Angiogenesis. J. Surg. Res. 2002, 102, 39–44. [Google Scholar] [CrossRef]
- Song, T.; Dou, C.; Jia, Y.; Tu, K.; Zheng, X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget 2015, 6, 12061–12079. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Wu, T.-L.; Tsao, K.-C.; Sun, C.-F. Serum TIMP-1 in gastric cancer patients: A potential prognostic biomarker. Ann. Clin. Lab. Sci. 2006, 36, 23–30. [Google Scholar] [PubMed]
- Ylisirniö, S.; Höyhtyä, M.; Turpeenniemi-Hujanen, T. Serum matrix metalloproteinases-2, -9 and tissue inhibitors of metalloproteinases-1, -2 in lung cancer—TIMP-1 as a prognostic marker. Anticancer Res. 2000, 20, 1311–1316. [Google Scholar] [PubMed]
- Wu, Z.-S.; Wu, Q.; Yang, J.-H.; Wang, H.-Q.; Ding, X.-D.; Yang, F.; Xu, X.-C. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int. J. Cancer 2008, 122, 2050–2056. [Google Scholar] [CrossRef]
- Meng, C.; Yin, X.; Liu, J.; Tang, K.; Tang, H.; Liao, J. TIMP-1 is a novel serum biomarker for the diagnosis of colorectal cancer: A meta-analysis. PLoS ONE 2018, 13, e0207039. [Google Scholar] [CrossRef]
- Poruk, K.E.; Firpo, M.A.; Scaife, C.L.; Adler, D.G.; Emerson, L.L.; Boucher, K.M.; Mulvihill, S.J. Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas 2013, 42, 193–197. [Google Scholar] [CrossRef]
- Narasimhan, A.; Shahda, S.; Kays, J.K.; Perkins, S.M.; Cheng, L.; Schloss, K.N.H.; Schloss, D.E.I.; Koniaris, L.G.; Zimmers, T.A. Identification of Potential Serum Protein Biomarkers and Pathways for Pancreatic Cancer Cachexia Using an Aptamer-Based Discovery Platform. Cancers 2020, 12, 3787. [Google Scholar] [CrossRef]
- Freire, P.P.; Fernandez, G.J.; de Moraes, D.; Cury, S.S.; Dal Pai-Silva, M.; dos Reis, P.P.; Rogatto, S.R.; Carvalho, R.F. The expression landscape of cachexia-inducing factors in human cancers. J. Cachexia Sarcopenia Muscle 2020, 11, 947–961. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Thibaut, M.M.; Joudiou, N.; Sboarina, M.; Neyrinck, A.M.; Cani, P.D.; Claus, S.P.; Delzenne, N.M.; Bindels, L.B. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J. Cachexia Sarcopenia Muscle 2021, 12, 456–475. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Brossel, H.; Sboarina, M.; Catry, E.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M.; Bindels, L.B. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018, 8, 12321. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: let’s talk about sex. Biol. Sex. Differ. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.L.; Grundmann, O.; Williams, J.J.; Gordan, L.; George, T.J., Jr. Body composition changes differ by gender in stomach, colorectal, and biliary cancer patients with cachexia: Results from a pilot study. Cancer Med. 2018, 7, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Greenman, A.C.; Albrecht, D.M.; Halberg, R.B.; Diffee, G.M. Sex differences in skeletal muscle alterations in a model of colorectal cancer. Physiol. Rep. 2020, 8, e14391. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Caillet, P.; Liuu, E.; Raynaud Simon, A.; Bonnefoy, M.; Guerin, O.; Berrut, G.; Lesourd, B.; Jeandel, C.; Ferry, M.; Rolland, Y.; et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin. Nutr. 2017, 36, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2017, 13, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.J.; Murphy, K.T.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G.; et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Re Cecconi, A.D.; Forti, M.; Chiappa, M.; Zhu, Z.; Zingman, L.V.; Cervo, L.; Beltrame, L.; Marchini, S.; Piccirillo, R. Musclin, A Myokine Induced by Aerobic Exercise, Retards Muscle Atrophy During Cancer Cachexia in Mice. Cancers 2019, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.A.; Pillai, V.B.; Gupta, M.P. Skeletal muscle-specific over-expression of the nuclear sirtuin SIRT6 blocks cancer-associated cachexia by regulating multiple targets. JCSM Rapid Commun. 2021, 4, 40–56. [Google Scholar] [CrossRef]

| Biomarker | Potential Source | Detection Method | Sample Format | Cancer Type | Median Level in Cachexia | Median Level in Non-Cachexia | Ref. |
|---|---|---|---|---|---|---|---|
| ActA | Tumor, host | ELISA | Serum | NSCLC, MPM | 1.179 ng/mL | 0.634 ng/mL | [26] |
| Plasma | PDAC | 1.997 ng/mL | 1.027 ng/mL | [27] | |||
| CRC, lung | 0.558 ng/mL | 0.397 ng/mL | [22] | ||||
| Mstn | Tumor, host | ELISA | Plasma | CRC, lung | 1.371 ng/mL | 2.109 ng/mL | [22] |
| GDF15 | Tumor | ELISA | Plasma | Lung | 2 ng/mL | 1 ng/mL | [33] |
| Lung, GI | 2.3 ng/mL | 1.8 ng/mL | [32] | ||||
| PTHrP | Tumor | IRMA | Serum | Lung, liver, PaCa, GI | 5.7 pmol/L | Undetectable | [48] |
| ELISA | NSCLC, CRC | 205 pg/mL | Undetectable | [46] | |||
| ZAG | Tumor, host | ELISA | Serum | PaCa | 40.3 µg/mL | 28.9 µg/mL | [39] |
| Ang II | Host | ELISA | Plasma | PaCa, lung, breast | 17 pg/mL | 7.5 pg/mL | [49] |
| Biomarker | Potential Source | Detection Method | Sample Format | Cancer Type | Median Level in Cachexia | Median Level in Non-Cachexia | Ref. |
|---|---|---|---|---|---|---|---|
| TNF-α | Tumor, host | ELISA | Serum | PaCa | 5.6 pg/mL | Undetectable | [56] |
| GE, PaCa, CRC | 15.9 pg/mL | 12 pg/mL | [68] | ||||
| Plasma | GI | 72.5 pg/mL | 13.8 pg/mL | [60] | |||
| IL-6 | Tumor, host | ELISA | Plasma | GI | 160 pg/mL | 30.3 pg/mL | [60] |
| TIA | Serum | NSCLC | 18 U/mL | 2 U/mL | [59] | ||
| ELISA | Plasma | Stomach, CRC | 8.16 pg/mL | 4.88 pg/mL | [73] | ||
| ELISA | Serum | PaCa | 207.8 pg/mL | 162.3 pg/mL | [63] | ||
| IL-1β | Tumor, host | BioPlex cytokine assay | Plasma | GI, NSCLC | 90.58 pg/mL | 57.45 pg/mL | [62] |
| IL-8 | Tumor, host | ELISA | Serum | PaCa | 460.9 pg/mL | 326.5 pg/mL | [63] |
| MCP-1 | Host | ELISA | Plasma | PaCa | 700 pg/mL | 400 pg/mL | [11] |
| CRP | Host | ELISA | Serum | GI, PaCa, CRC | 83 mg/L | 4 mg/L | [68] |
| ELISA | Plasma | GI | 24.9 mg/L | 14.9 mg/L | [60] | ||
| Turbidimetric method | Plasma | GI, Lung | 35 mg/L | 17.6 mg/mL | [70] | ||
| Albumin | Host | NM | Plasma | GI, Lung | 3.4 g/dL | 3.8 g/dL | [70] |
| Biomarker | Potential Source | Detection Method | Sample Format | Cancer Type | Median Level in Cachexia | Median Level in Non-Cachexia | Ref. |
|---|---|---|---|---|---|---|---|
| β-dystroglycan | Host | WB | Muscle | GI | NA | NA | [69] |
| Glycerol | Host | NM | Plasma | GI | 6.9 μmol·L−1·kg−1 fat | 3.9 μmol·L−1·kg−1 fat | [76] |
| 6.2 μmol·L−1 ·kg−1 fat | 3.1 μmol·L−1·kg−1 fat | [77] | |||||
| 7.0 μmol·L−1·kg−1 fat | 3.4 μmol·L−1·kg−1 fat | [78] | |||||
| Free Glycerol Reagent kit | Plasma | GI | 4 µg/mL | 3 µg/mL | [79] | ||
| FFA | Host | NM | Plasma | GI | 53.8 μmol·L−1·kg−1 fat | 32.5 μmol·L−1·kg−1 fat | [76] |
| 62 μmol·L−1·kg−1 fat | 27 μmol·L−1·kg−1 fat | [77] | |||||
| 80 μmol·L−1·kg−1 fat | 40 μmol·L−1·kg−1 fat | [78] | |||||
| HCERs | Host | MS | Plasma | GI | 4 nmol/mL | 3 nmol/mL | [13] |
| LCERs | Host | MS | Plasma | GI | 4 nmol/mL | 3.2 nmol/mL | [13] |
| Biomarker | Potential Source | Detection Method | Sample Format | Cancer Type | Median Level in Cachexia | Median Level in Non-Cachexia | Ref. |
|---|---|---|---|---|---|---|---|
| MicroRNA-21 | Tumor | qRT-PCR | Serum | CRC | NA | NA | [92] |
| MicroRNA-203 | Unknown | qRT-PCR | Serum | CRC | NA | NA | [93] |
| MicroRNA-130a | Unknown | qRT-PCR | Plasma | HNC | NA | NA | [94] |
| Biomarker | Potential Source | Detection Method | Sample Format | Cancer Type | Median Level in Cachexia | Median Level in Non-Cachexia | Ref. |
|---|---|---|---|---|---|---|---|
| CNDP1 | Host | SBA, SIA | Plasma | GI | 1500 MIF | 2000 MIF | [98] |
| TIMP-1 | Tumor | ELISA | Plasma | PDAC | 860 ng/mL | 550 ng/mL | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for Cancer Cachexia: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4501. https://doi.org/10.3390/ijms22094501
Cao Z, Zhao K, Jose I, Hoogenraad NJ, Osellame LD. Biomarkers for Cancer Cachexia: A Mini Review. International Journal of Molecular Sciences. 2021; 22(9):4501. https://doi.org/10.3390/ijms22094501
Chicago/Turabian StyleCao, Zhipeng, Kening Zhao, Irvin Jose, Nick J. Hoogenraad, and Laura D. Osellame. 2021. "Biomarkers for Cancer Cachexia: A Mini Review" International Journal of Molecular Sciences 22, no. 9: 4501. https://doi.org/10.3390/ijms22094501
APA StyleCao, Z., Zhao, K., Jose, I., Hoogenraad, N. J., & Osellame, L. D. (2021). Biomarkers for Cancer Cachexia: A Mini Review. International Journal of Molecular Sciences, 22(9), 4501. https://doi.org/10.3390/ijms22094501