Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects
Abstract
1. Introduction
2. Results
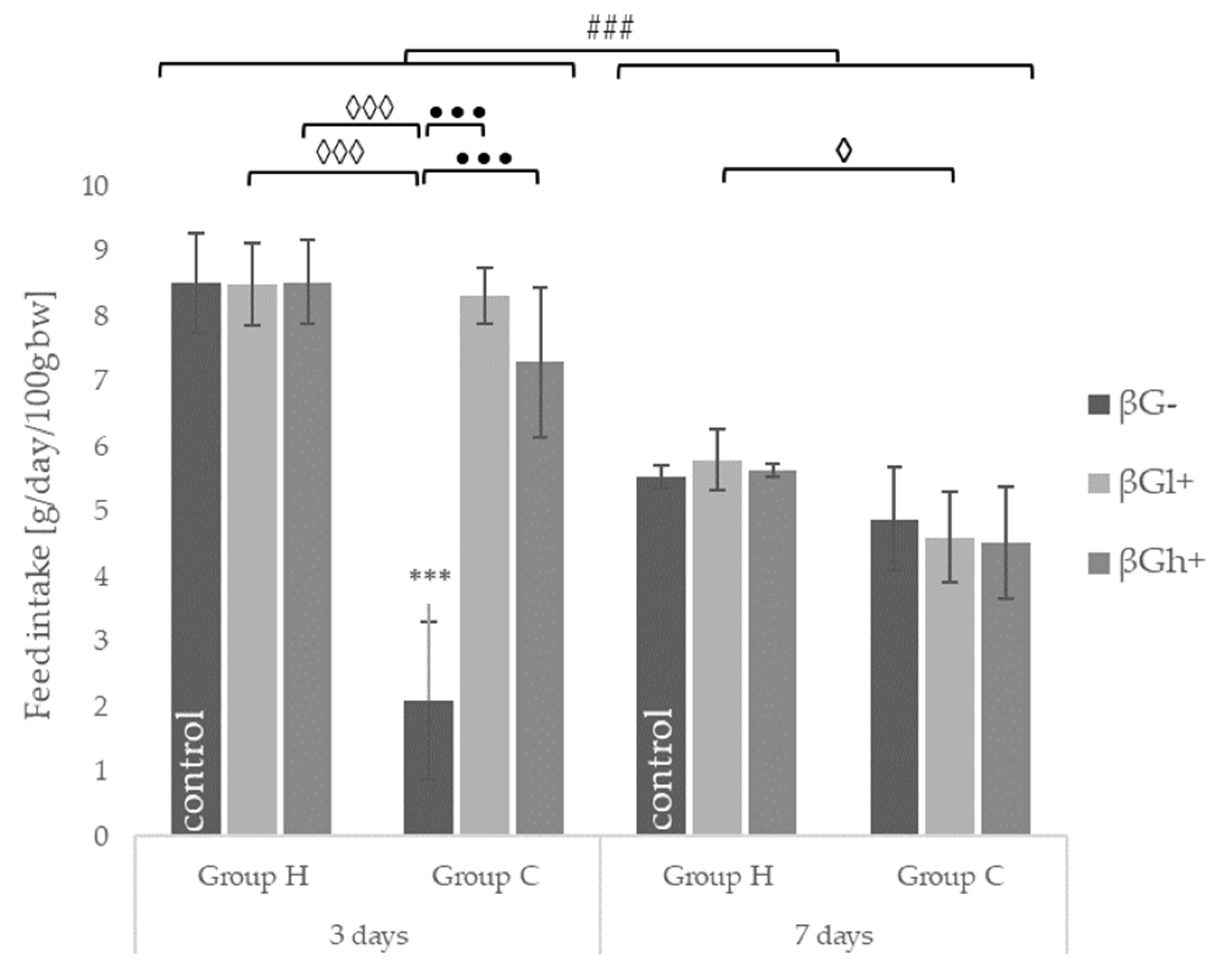
2.1. Feed Intake and Body Weight
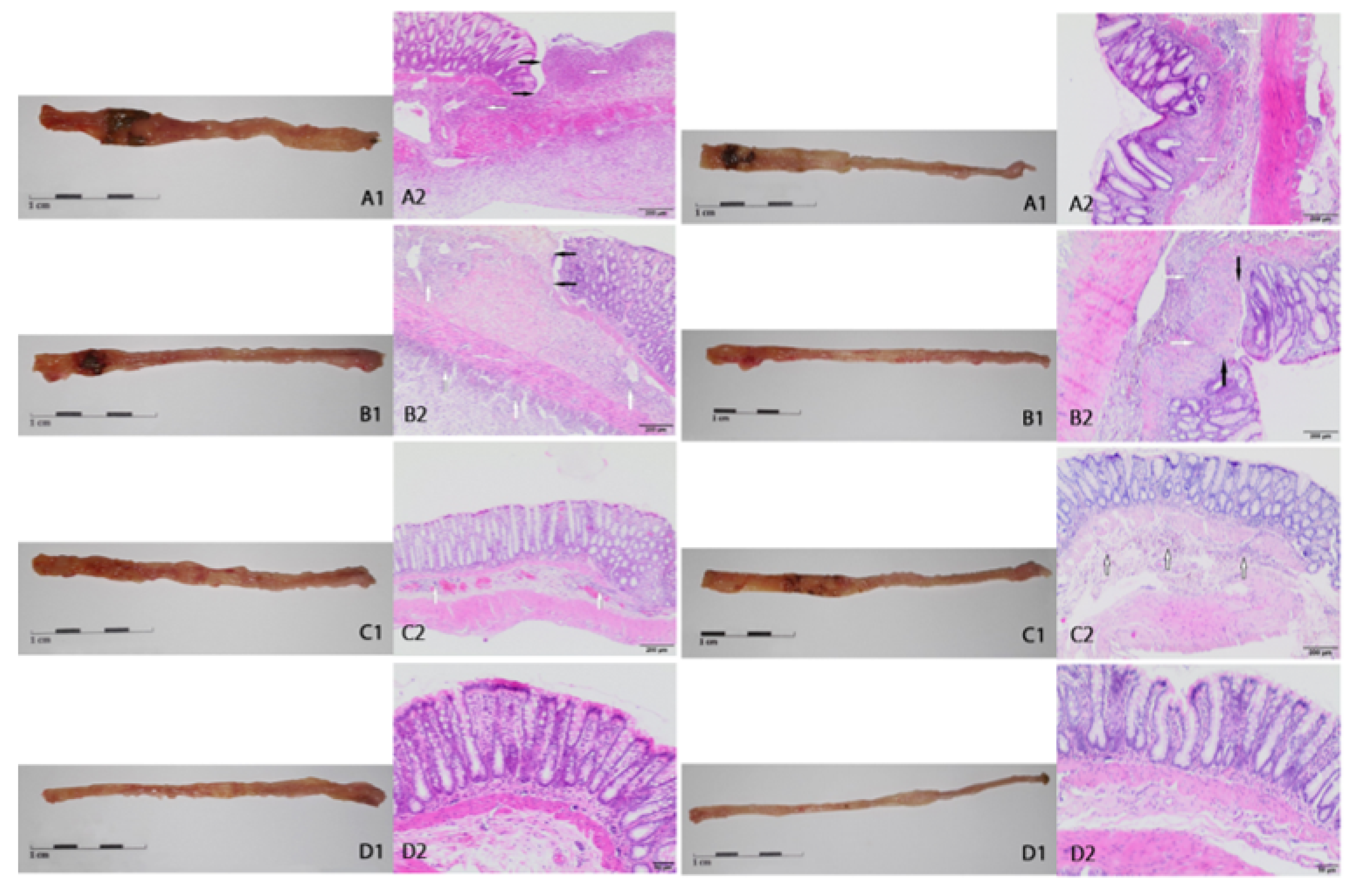
2.2. Macroscopic and Microscopic Changes
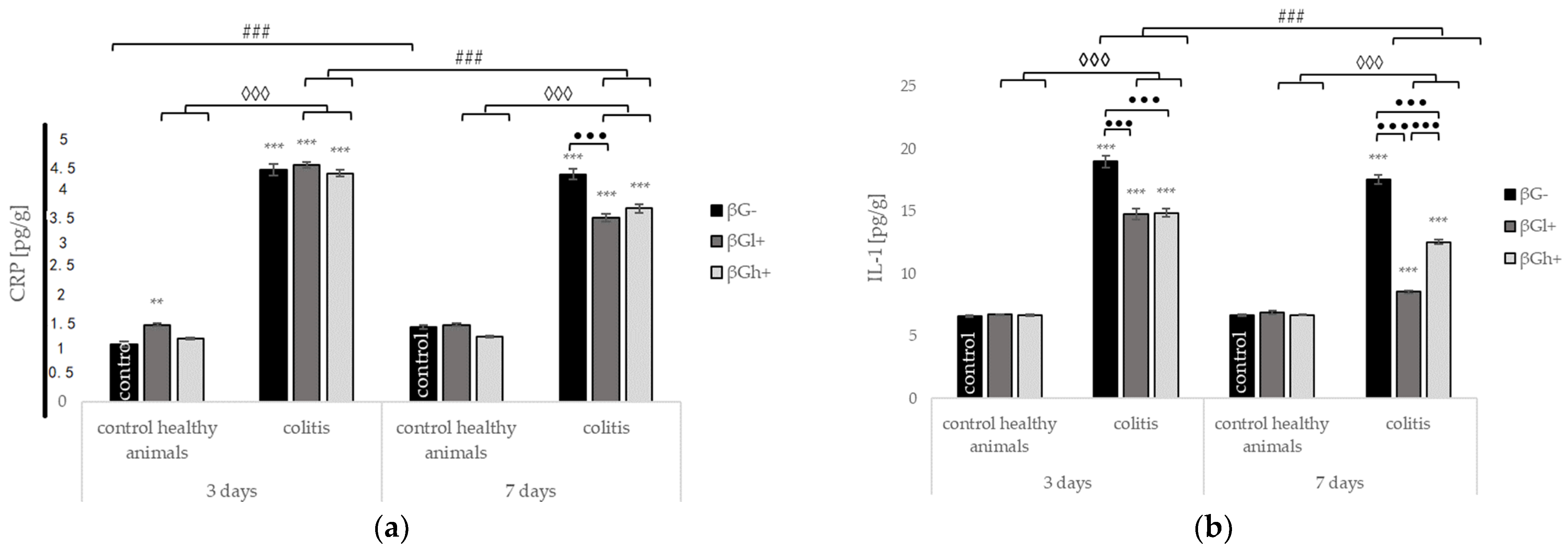
2.3. Levels of C-Reactive Protein and Selected Cytokines in the Colon Tissue
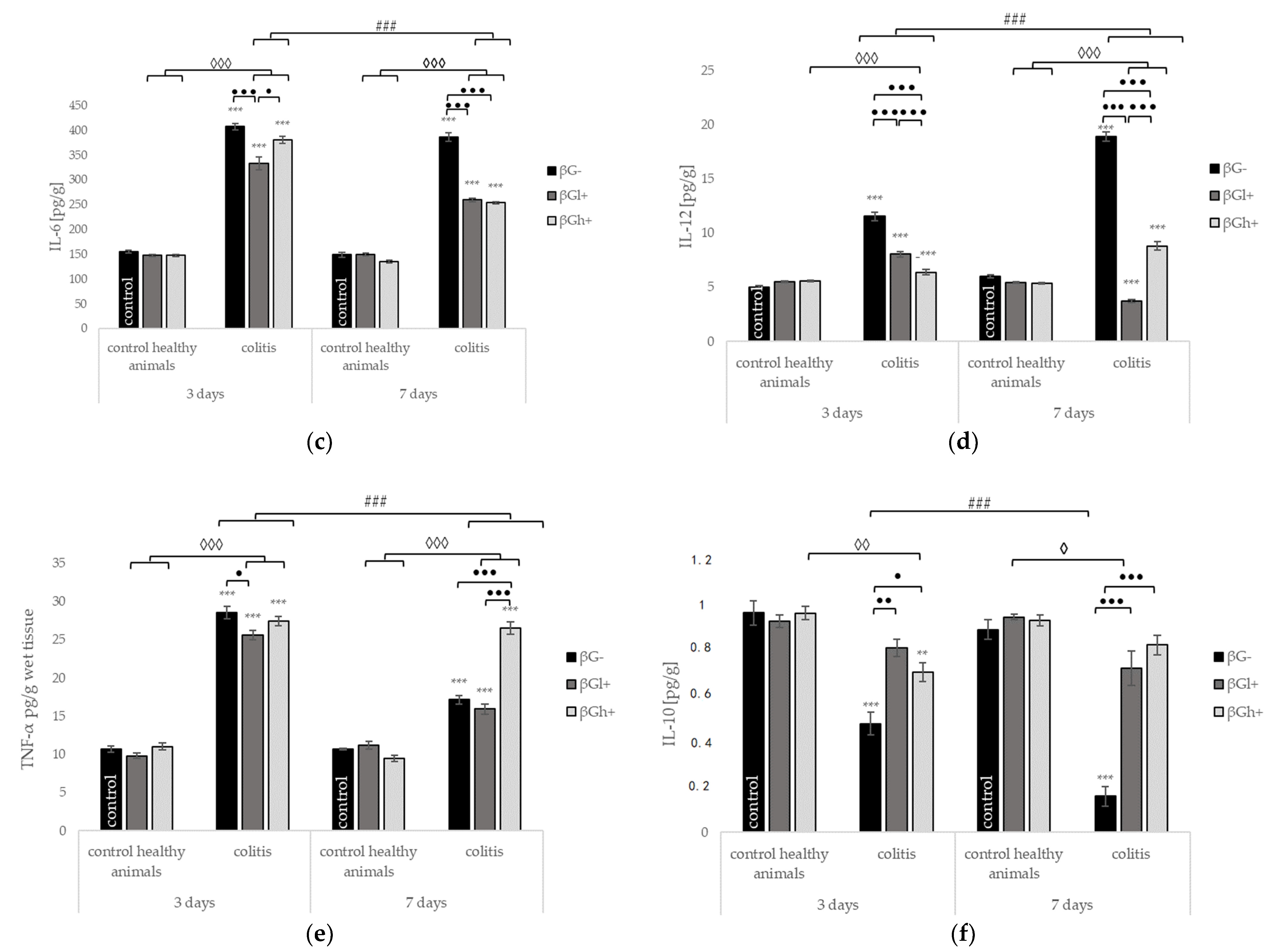
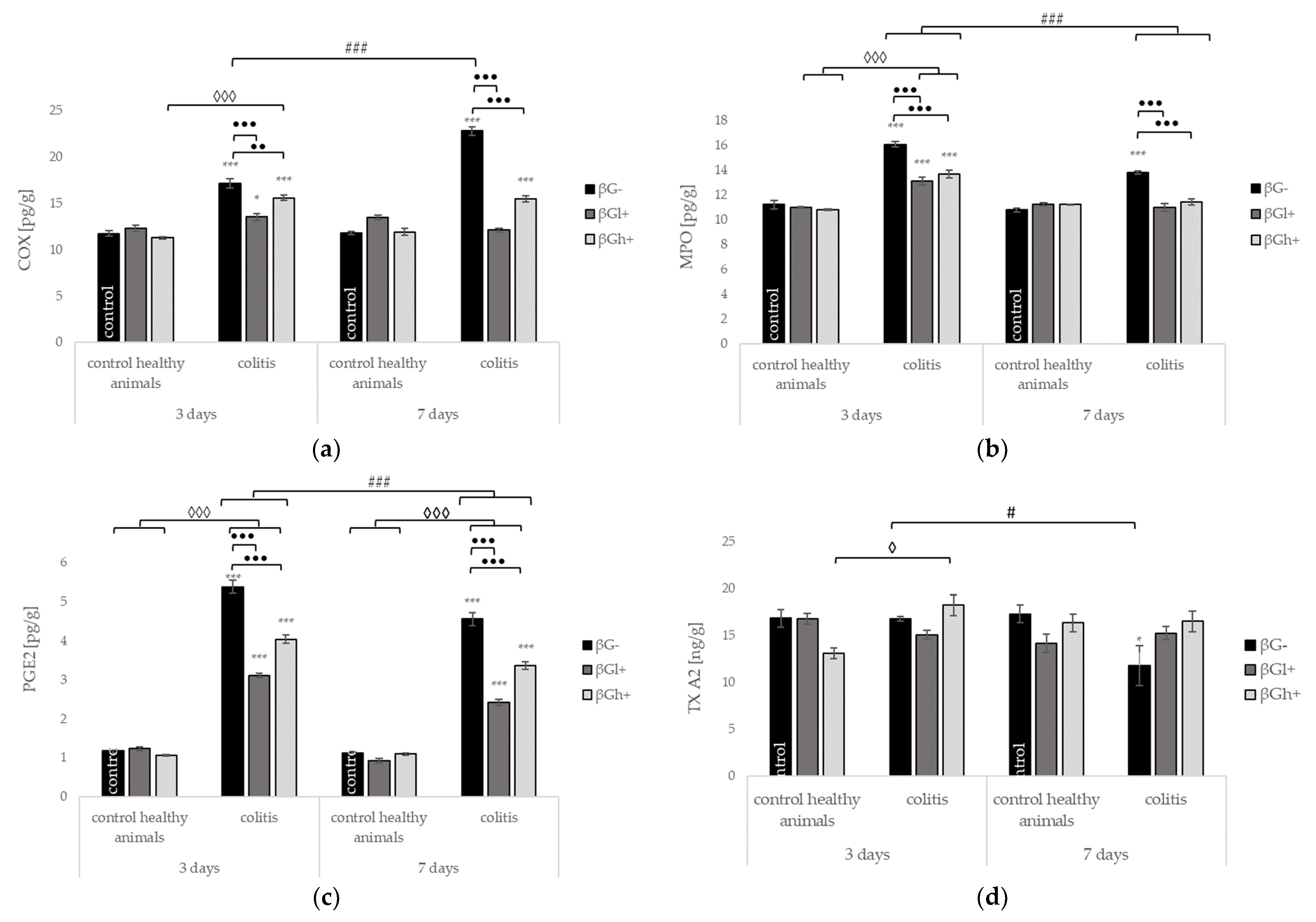
2.4. Other Selected Inflammatory Markers
2.5. Expression of Genes Encoding Inflammatory Cytokines and their Receptors
3. Discussion
4. Materials and Methods
4.1. Preparation of Deproteinated Oat Beta-Glucan Fractions
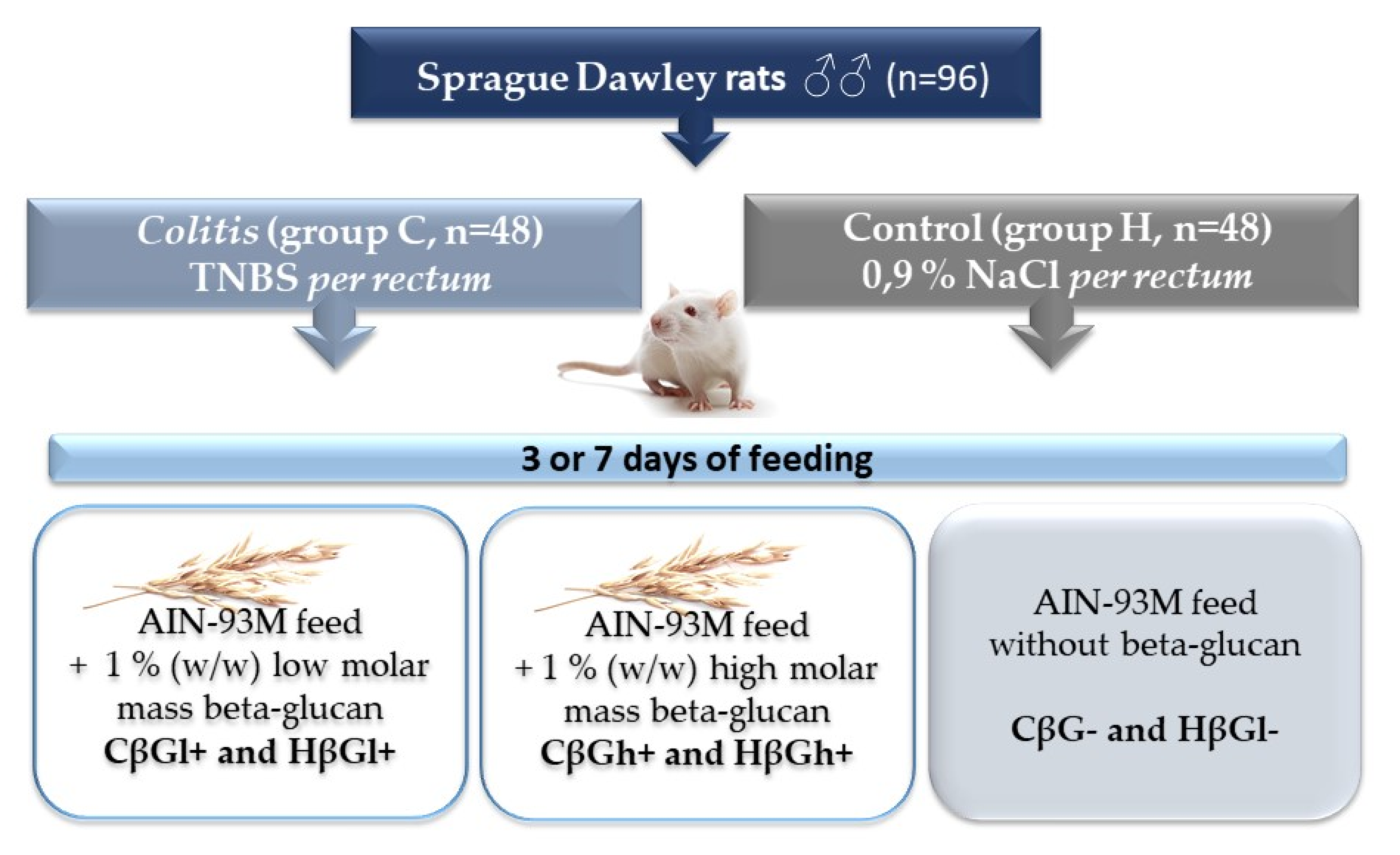
4.2. Animals and Experimental Design
4.3. Evaluation of Histopathological Changes in the Wall of the Colon
4.4. Determination of Inflammation Parameters
4.5. RNA Isolation, Reverse Transcription, and Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef]
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Tun, G.S.Z.; Cripps, S.; Lobo, A.J. Crohn’s disease: Management in adults, children and young people-concise guidance. Clin. Med. J. R. Coll. Physicians Lond. 2018, 18, 231–236. [Google Scholar] [CrossRef]
- Słowińska-Solnica, K.; Pawlica-Gosiewska, D.; Gawlik, K.; Owczarek, D.; Cibor, D.; Pocztar, H.; Mach, T.; Solnica, B. Serum inflammatory markers in the diagnosis and assessment of Crohn’s disease activity. Arch. Med. Sci. 2021, 17, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Choi, J.S. Clinical and physiological perspectives of β-glucans: The past, present, and future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, L.; Du, Y.; Mao, X.; He, J.; Yu, B.; Chen, D. The anti-inflammatory effects of low- and high-molecular-weight beta-glucans from: Agrobacterium sp. ZX09 in LPS-induced weaned piglets. Food Funct. 2020, 11, 585–595. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Damiano, S.; Montanino, C.; Del Buono, A.; La Rosa, G.; Guida, B.; Santillo, M. Effect of beta- and alpha-glucans on immune modulating factors expression in enterocyte-like Caco-2 and goblet-like LS 174T cells. Int. J. Biol. Macromol. 2020, 153, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Błaszczyk, K.; Wilczak, J.; Harasym, J.; Gudej, S.; Suchecka, D.; Kr, T.; Lange, E.; Gromadzka-Ostrowska, J. Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with LPS induced enteritis. Food Hydrocoll. 2015, 51, 272–280. [Google Scholar] [CrossRef]
- Suchecka, D.; Harasym, J.; Wilczak, J.; Gromadzka-Ostrowska, J. Hepato- and gastro- protective activity of purified oat 1–3, 1–4-β-d-glucans of different molecular weight. Int. J. Biol. Macromol. 2016, 91, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef]
- Roberts, S.E.; Thorne, K.; Thapar, N.; Broekaert, I.; Benninga, M.A.; Dolinsek, J.; Mas, E.; Miele, E.; Orel, R.; Pienar, C.; et al. A systematic review and meta-analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J. Crohn’s Colitis 2020, 14, 1119–1148. [Google Scholar] [CrossRef]
- Te Velde, A.A.; Verstege, M.I.; Hommes, D.W. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm. Bowel Dis. 2006, 12, 995–999. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.S.; Lopes, A.H.; Carreira, E.U.; Feitosa, M.R.; Cunha, F.Q.; Garcia, S.B.; Cunha, T.M.; Da Rocha, J.J.R.; Féres, O. Hyperbaric oxygen therapy ameliorates TNBS-induced acute distal colitis in rats. Med. Gas Res. 2015, 5, 6. [Google Scholar] [CrossRef]
- Catana, C.S.; Magdas, C.; Tabaran, F.A.; Craciun, E.C.; Deak, G.; Magdas, V.A.; Cozma, V.; Gherman, C.M.; Berindan-Neagoe, I.; Dumitrascu, D.L. Comparison of two models of inflammatory bowel disease in rats. Adv. Clin. Exp. Med. 2018, 27, 599–607. [Google Scholar] [CrossRef]
- Brenna, Ø.; Furnes, M.W.; Drozdov, I.; van Beelen Granlund, A.; Flatberg, A.; Sandvik, A.K.; Zwiggelaar, R.T.M.; Mårvik, R.; Nordrum, I.S.; Kidd, M.; et al. Relevance of TNBS-Colitis in Rats: A Methodological Study with Endoscopic, Historical and Transcripttomic Characterization and Correlation to IBD. PLoS ONE 2013, 8, e54543. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Chamouard, P.; Richert, Z.; Meyer, N.; Rahmi, G.; Baumann, R. Diagnostic Value of C-Reactive Protein for Predicting Activity Level of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-Reactive Protein as a Marker for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Cytokines and feeding. Int. J. Obes. 2001, 25, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Grisham, M.; Hodge, J.; Telliez, J.-B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: A hub for multiple inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G155–G162. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Orlando, A.; Cottone, M. Anti-interleukin-12 and anti-interleukin-23 agents in Crohn’s disease. Expert Opin. Biol. Ther. 2019, 19, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.Q.; Shen, H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef]
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, ijfs.14971. [Google Scholar] [CrossRef]
- Christophi, G.P.; Rong, R.; Holtzapple, P.G.; Massa, P.T.; Landas, S.K. Immune markers and differential signaling networks in ulcerative colitis and Crohnʼs disease. Inflamm. Bowel Dis. 2012, 18, 2342–2356. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhang, X. Meta-analysis of macrophage migration inhibitory factor (MIF) gene-173G/C polymorphism and inflammatory bowel disease (IBD) risk. Int. J. Clin. Exp. Med. 2015, 8, 9570. [Google Scholar]
- Cui, G.; Olsen, T.; Christiansen, I.; Vonen, B.; Florholmen, J.; Goll, R. Improvement of real-time polymerase chain reaction for quantifying TNF-α mRNA expression in inflamed colorectal mucosa: An approach to optimize procedures for clinical use. Scand. J. Clin. Lab. Investig. 2006, 66, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L.; Simmons, J.G.; Jobin, C.; Lund, P.K. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 2005, 280, 36099–36109. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Tomiyasu, N.; Takaki, K.; Masuda, J.; Yamasaki, H.; Kuwaki, K.; Takeda, T.; Kitazaki, S.; Tsuruta, O.; Sata, M. Interleukin-10 in the Pathophysiology of Inflammatory Bowel Disease: Increased Serum Concentrations During the Recovery Phase. Mediat. Inflamm. 2006, 2006, 026875. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm. Bowel Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef]
- Żyła, E.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Gromadzka-Ostrowska, J. Beneficial effects of oat beta-glucan dietary supplementation in colitis depend on its molecular weight. Molecules 2019, 24, 3591. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Capozzi, V.; Rascón, A.; Felis, G.E.; Spano, G.; Fiocco, D. Combinations of cereal β-glucans and probiotics can enhance the anti-inflammatory activity on host cells by a synergistic effect. J. Funct. Foods 2016, 23, 12–23. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Q.; Yang, T.; Nie, Y.; Li, X.; Liu, B.; Shen, J.; Liang, Y.; Tang, Y.; Luo, F. Oral administration of: Lentinus edodes β-glucans ameliorates DSS-induced ulcerative colitis in mice via MAPK-Elk-1 and MAPK-PPARγ pathways. Food Funct. 2016, 7, 4614–4627. [Google Scholar] [CrossRef]
- Liu, B.; Lin, Q.; Yang, T.; Zeng, L.; Shi, L.; Chen, Y.; Luo, F. Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct. 2015, 6, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Crespo, H.; Guillén, H.; de Pablo-Maiso, L.; Gómez-Arrebola, C.; Rodríguez, G.; Glaria, I.; de Andrés, D.; Reina, R. Lentinula edodes β-glucan enriched diet induces pro-and anti-inflammatory macrophages in rabbit. Food Nutr. Res. 2017, 61, 1412791. [Google Scholar] [CrossRef][Green Version]
- Mikkelsen, M.S.; Jespersen, B.M.; Mehlsen, A.; Balling Engelsen, S.; Frøkiaer, H. Cereal β-glucan immune modulating activity depends on the polymer fine structure. FRIN 2014, 62, 829–836. [Google Scholar] [CrossRef]
- Kopiasz, Ł.; Dziendzikowska, K.; Gajewska, M.; Oczkowski, M.; Majchrzak-Kuligowska, K.; Królikowski, T.; Gromadzka-ostrowska, J. Effects of dietary oat beta-glucans on colon apoptosis and autophagy through tlrs and dectin-1 signaling pathways—crohn’s disease model study. Nutrients 2021, 13, 321. [Google Scholar] [CrossRef]
- Peterson, C.G.B.; Eklund, E.; Taha, Y.; Raab, Y.; Carlson, M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: Establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2002, 97, 1755–1762. [Google Scholar] [CrossRef]
- Masoodi, I.; Dutta, U.; Vaiphei, K.; Kochhar, R.; Vaishnavi, C.; Hussain, S.; Prasad, K.K.; Singh, K. Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig. Dis. Sci. 2012, 57, 1336–1340. [Google Scholar] [CrossRef]
- Pattison, D.; Davies, M. Reactions of Myeloperoxidase-Derived Oxidants with Biological Substrates:Gaining Chemical Insight into Human Inflammatory Diseases. Curr. Med. Chem. 2006, 13, 3271–3290. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Hahn, J.; Maueröder, C.; Czegley, C.; Munoz, L.E.; Herrmann, M.; Hoffmann, M.H.; Mokhir, A. Chemical tools for targeted amplification of reactive oxygen species in neutrophils. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Harasym, J.; Zyła, E.; Dziendzikowska, K.; Gromadzka-Ostrowska, J. Proteinaceous residue removal from oat β-glucan extracts obtained by alkalinewater extraction. Molecules 2019, 24, 1729. [Google Scholar] [CrossRef] [PubMed]
- Heinsbroek, S.E.M.; Williams, D.L.; Welting, O.; Meijer, S.L.; Gordon, S.; de Jonge, W.J. Orally delivered β-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutr. Res. 2015, 35, 1106–1112. [Google Scholar] [CrossRef]
- Ganda Mall, J.-P.; Casado-Bedmar, M.; Winberg, M.E.; Brummer, R.J.; Schoultz, I.; Keita, Å. V A β-Glucan-Based Dietary Fiber Reduces Mast Cell-Induced Hyperpermeability in Ileum From Patients With Crohn’s Disease and Control Subjects. Inflamm. Bowel Dis. 2017, 24, 166–178. [Google Scholar] [CrossRef]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 620189. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Anti-infectious and Anti-tumor Activities of β-glucans. Anticancer Res. 2020, 40, 3139–3145. [Google Scholar] [CrossRef]
- Choromanska, A.; Kulbacka, J.; Harasym, J.; Oledzki, R.; Szewczyk, A.; Saczko, J. High- and low-Molecular Weight oat Beta-Glucan Reveals Antitumor Activity in Human Epithelial Lung Cancer. Pathol. Oncol. Res. 2018, 24, 583–592. [Google Scholar] [CrossRef]
- Choromanska, A.; Kulbacka, J.; Rembialkowska, N.; Pilat, J.; Oledzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat beta-glucan—An in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef]
- Pan, W.; Hao, S.; Zheng, M.; Lin, D.; Jiang, P.; Zhao, J.; Shi, H.; Yang, X.; Li, X.; Yu, Y. Oat-Derived β-Glucans Induced Trained Immunity Through Metabolic Reprogramming. Inflammation 2020, 43, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Olędzki, R. The Mutual Correlation of Glucose, Starch, and Beta-Glucan Release During Microwave Heating and Antioxidant Activity of Oat Water Extracts. Food Bioprocess Technol. 2018, 11, 874–884. [Google Scholar] [CrossRef]
- Harasym, J.; Suchecka, D.; Gromadzka-Ostrowska, J. Effect of size reduction by freeze-milling on processing properties of beta-glucan oat bran. J. Cereal Sci. 2015, 61, 119–125. [Google Scholar] [CrossRef]
- Gálvez, J.; Coelho, G.; Crespo, M.E.; Cruz, T.; Rodríguez-Cabezas, M.E.; Concha, A.; Gonzalez, M.; Zarzuelo, A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001, 15, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
| Time (days) | Healthy Group | Colitis Group | ||||
|---|---|---|---|---|---|---|
| HβG− | HβGl+ | HβGh+ | CβG− | CβGl+ | CβGh+ | |
| 3 | 327.63 ± 6.64 | 326.38 ± 4.51 | 333.25 ± 3.65 | 304.5 ± 9.22 | 322.13 ± 9.86 | 317.5 ± 8.32 |
| 7 | 427.75 ± 5.14 | 431.5 ± 5.43 | 422.0 ± 3.96 | 406.25 ± 19.48 | 403.5 ± 11.5 | 397.88 ± 7.48 |
| Time (days) | Healthy Group | Colitis Group | ||||
|---|---|---|---|---|---|---|
| HβG− | HβGl+ | HβGh+ | CβG− | CβGl+ | CβGh+ | |
| 3 | 0 | 0 | 0 | 3 | 2 | 2 |
| 7 | 0 | 0 | 0 | 2 | 2 | 2 |
| Time (days) | Healthy Group | Colitis Group | ||||
|---|---|---|---|---|---|---|
| HβG− | HβGl+ | HβGh+ | CβG− | CβGl+ | CβGh+ | |
| 3 | 0 | 0.5 | 0.5 | 5 | 5 | 3.5 |
| 7 | 1 | 1 | 1 | 3 | 3 | 3 |
| BG Fraction | Molar Mass (g/mol) | Purity (%) | TPC (GAE/ 1 g d.b) | DPPH (μmol of Trolox) | Soluble Proteins (%/db) | Nitrogen × 5.83 (%/db) |
|---|---|---|---|---|---|---|
| low | 5.9 × 104 ± 0.3 × 104 | 99.1 ± 0.3 | 0.20 | 5.41 | 0.09 ± 0.01 | 1.14 ± 0.14 |
| high | 1.7 × 106 ± 0.05 × 106 | 97.4 ± 0.7 | 0.48 | 6.03 | 0.70 ± 0.14 | 1.17 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyła, E.; Dziendzikowska, K.; Kamola, D.; Wilczak, J.; Sapierzyński, R.; Harasym, J.; Gromadzka-Ostrowska, J. Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects. Int. J. Mol. Sci. 2021, 22, 4485. https://doi.org/10.3390/ijms22094485
Żyła E, Dziendzikowska K, Kamola D, Wilczak J, Sapierzyński R, Harasym J, Gromadzka-Ostrowska J. Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects. International Journal of Molecular Sciences. 2021; 22(9):4485. https://doi.org/10.3390/ijms22094485
Chicago/Turabian StyleŻyła, Ewa, Katarzyna Dziendzikowska, Dariusz Kamola, Jacek Wilczak, Rafał Sapierzyński, Joanna Harasym, and Joanna Gromadzka-Ostrowska. 2021. "Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects" International Journal of Molecular Sciences 22, no. 9: 4485. https://doi.org/10.3390/ijms22094485
APA StyleŻyła, E., Dziendzikowska, K., Kamola, D., Wilczak, J., Sapierzyński, R., Harasym, J., & Gromadzka-Ostrowska, J. (2021). Anti-Inflammatory Activity of Oat Beta-Glucans in a Crohn’s Disease Model: Time- and Molar Mass-Dependent Effects. International Journal of Molecular Sciences, 22(9), 4485. https://doi.org/10.3390/ijms22094485









