Anti-Inflammatory Role of Netrin-4 in Diabetic Retinopathy
Abstract
:1. Introduction
2. Results
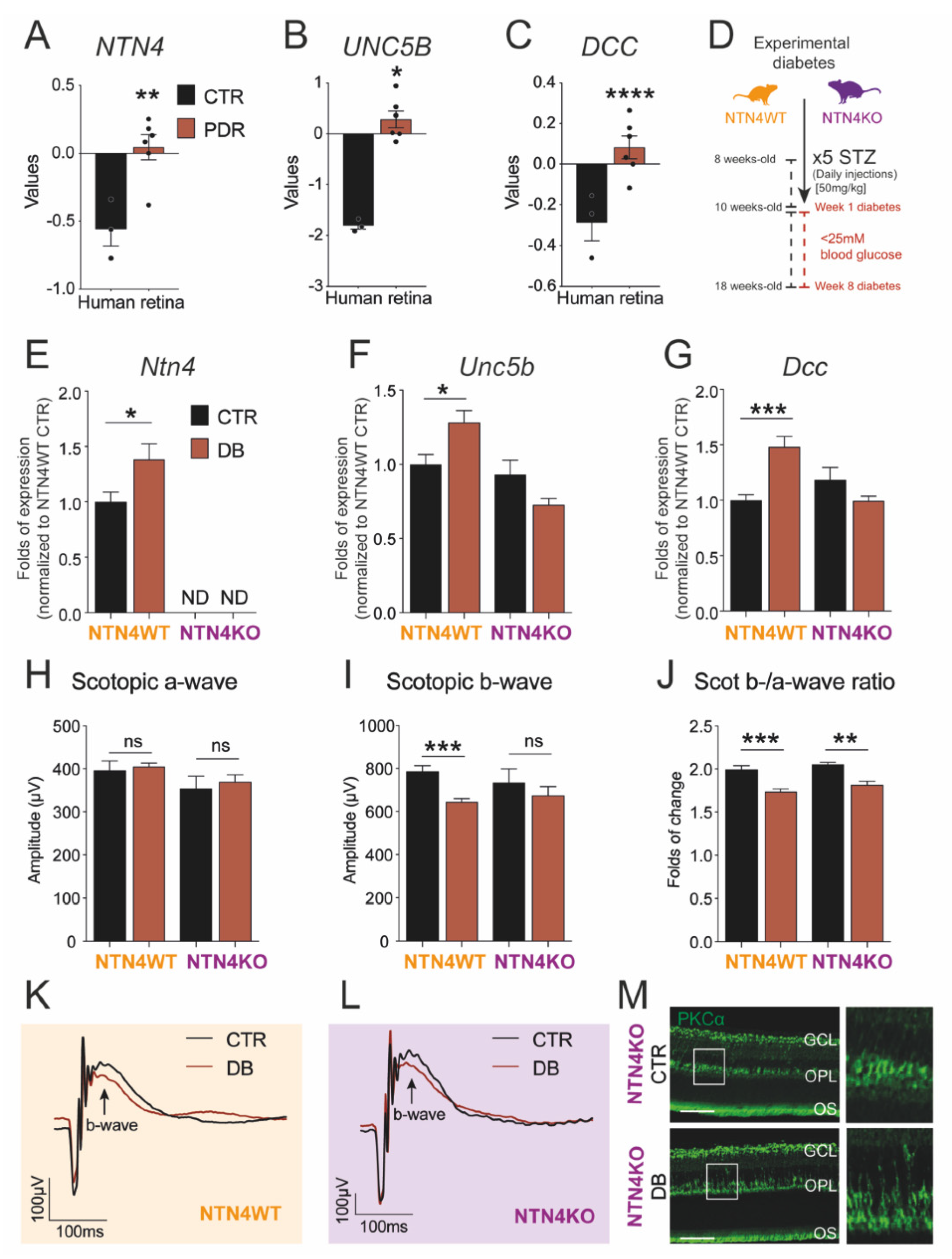
2.1. Netrin-4 Expression Increases in the Human Diabetic Retina
2.2. Netrin-4 Expression Increases in Experimental Diabetic Retinopathy
2.3. Netrin-4 Expression Does Not Alter Visual Function in Experimental Diabetic Retinopathy
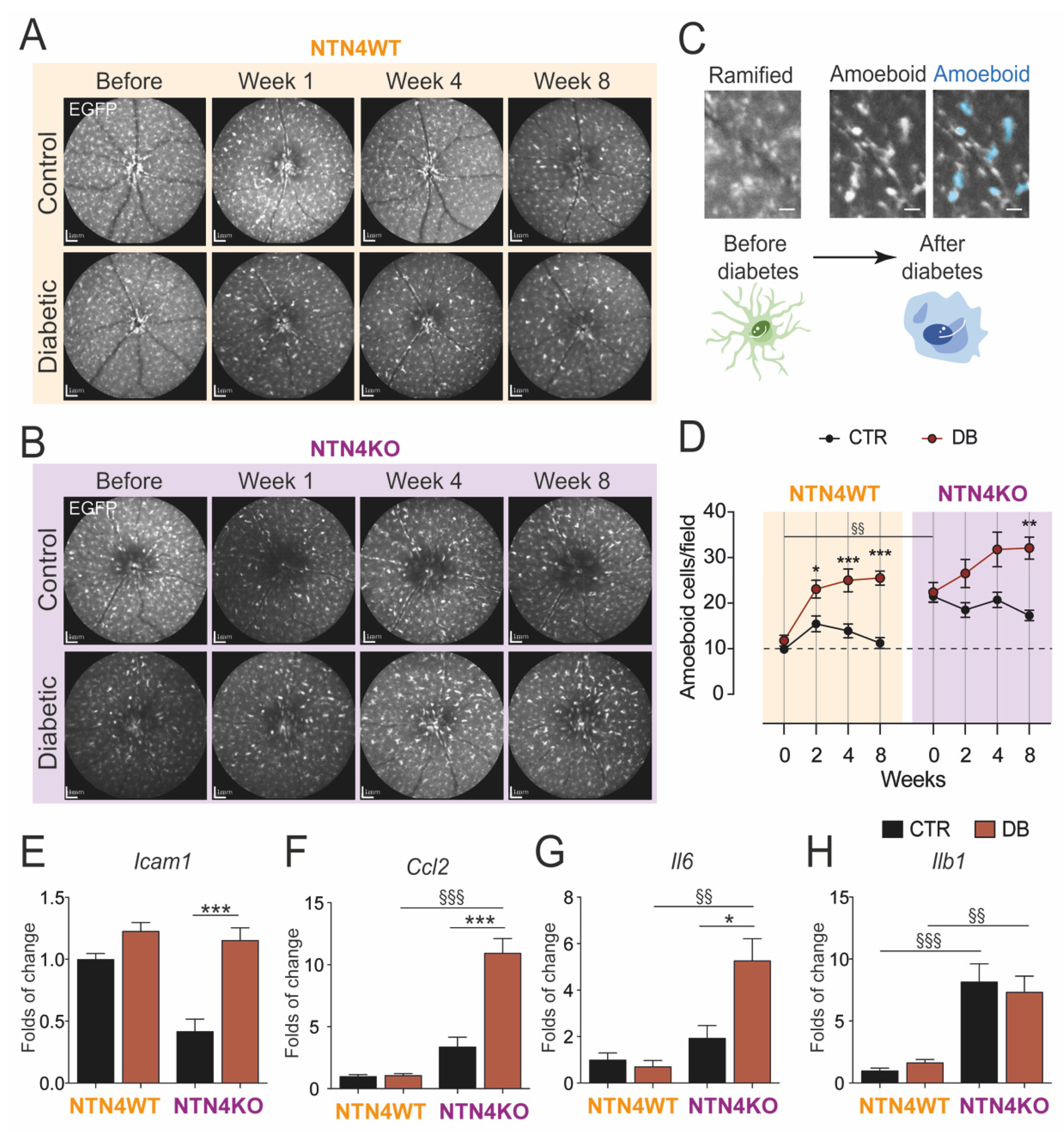
2.4. Lack of Netrin-4 Accentuates Low-Grade Inflammation in Diabetic Retinopathy
3. Discussion
4. Materials and Methods
4.1. Curation of Human Retina RNA-Sequencing Data
4.2. Animal Models
4.3. Streptozotocin-Induced Experimental Diabetes
4.4. Analysis of Mononuclear Phagocytes in the Retina In Vivo
4.5. Scotopic Electroretinography (ERG)
4.6. Immunolabeling of PKCα
4.7. Quantitative PCR
4.8. Statistical Analysis and Experimental Design
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Dev. Ophthalmol. 2017, 58, 11–20. [Google Scholar] [CrossRef]
- Roy, S.; Kim, D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog. Retin Eye Res. 2020, 100903. [Google Scholar] [CrossRef]
- Robb, J.L.; Morrissey, N.A.; Weightman Potter, P.G.; Smithers, H.E.; Beall, C.; Ellacott, K.L.J. Immunometabolic Changes in Glia—A Potential Role in the Pathophysiology of Obesity and Diabetes. Neuroscience 2020, 447, 167–181. [Google Scholar] [CrossRef]
- Lai Wing Sun, K.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar] [CrossRef] [Green Version]
- Kociok, N.; Crespo-Garcia, S.; Liang, Y.; Klein, S.V.; Nurnberg, C.; Reichhart, N.; Skosyrski, S.; Moritz, E.; Maier, A.K.; Brunken, W.J.; et al. Lack of netrin-4 modulates pathologic neovascularization in the eye. Sci. Rep. 2016, 6, 18828. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.N.; Pinzon-Duarte, G.; Dattilo, M.; Claudepierre, T.; Koch, M.; Brunken, W.J. The expression and function of netrin-4 in murine ocular tissues. Exp. Eye Res. 2012, 96, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuten, R.; Patel, T.R.; McDougall, M.; Rama, N.; Nikodemus, D.; Gibert, B.; Delcros, J.G.; Prein, C.; Meier, M.; Metzger, S.; et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat. Commun. 2016, 7, 13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejmi, E.; Leconte, L.; Pedron-Mazoyer, S.; Ropert, S.; Raoul, W.; Lavalette, S.; Bouras, I.; Feron, J.G.; Maitre-Boube, M.; Assayag, F.; et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc. Natl. Acad. Sci. USA 2008, 105, 12491–12496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Vreeken, D.; Leuning, D.G.; Bruikman, C.S.; Junaid, A.; Stam, W.; de Bruin, R.G.; Sol, W.; Rabelink, T.J.; van den Berg, B.M.; et al. Netrin-4 expression by human endothelial cells inhibits endothelial inflammation and senescence. Int. J. Biochem. Cell Biol. 2021, 105960. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shao, Y.; Liu, T.T.; Li, S.M.; Li, W.; Liu, Z.G. Therapeutic effects of topical netrin-4 in a corneal acute inflammatory model. Int. J. Ophthalmol. 2015, 8, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.G.; Jeung, I.C.; Heo, S.C.; Song, J.; Kim, W.; Hwang, B.; Kwon, M.G.; Kim, Y.G.; Lee, J.; Park, J.G.; et al. Ischemia-induced Netrin-4 promotes neovascularization through endothelial progenitor cell activation via Unc-5 Netrin receptor B. FASEB J. 2020, 34, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Garcia, S.; Reichhart, N.; Wigdahl, J.; Skosyrski, S.; Kociok, N.; Strauss, O.; Joussen, A.M. Lack of netrin-4 alters vascular remodeling in the retina. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2179–2184. [Google Scholar] [CrossRef]
- Li, A.S.; Veerappan, M.; Mittal, V.; Do, D.V. Anti-VEGF agents in the management of diabetic macular edema. Expert Rev. Ophthalmol. 2020, 15, 285–296. [Google Scholar] [CrossRef]
- Zewdie, K.A.; Ayza, M.A.; Amare Tesfaye, B.; Yimer, E.M. Targeting Netrin-1 and -4 as a Novel Diagnostic Parameter and Treatment Option for Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 1741–1747. [Google Scholar] [CrossRef]
- Cao, B.; Meng, X.; Fu, Y.; Liu, P.; Lun, Y.; Wang, Y. Neuron-derived netrin-1 and netrin-4 proteins are additional effective targets in diabetic retinopathy beyond VEGF. Int. J. Clin. Exp. Pathol. 2017, 10, 8174–8186. [Google Scholar] [PubMed]
- Larrieu-Lahargue, F.; Welm, A.L.; Thomas, K.R.; Li, D.Y. Netrin-4 induces lymphangiogenesis in vivo. Blood 2010, 115, 5418–5426. [Google Scholar] [CrossRef] [Green Version]
- Hoang, S.; Liauw, J.; Choi, M.; Choi, M.; Guzman, R.G.; Steinberg, G.K. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J. Cereb Blood Flow Metab. 2009, 29, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Prieto, C.P.; Casas, B.S.; Falcon, P.; Villanueva, A.; Lois, P.; Lattus, J.; Palma, V. Downregulation of the Netrin-1 Receptor UNC5b Underlies Increased Placental Angiogenesis in Human Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, J.; Yafai, Y.; Noack, A.; Yang, X.M.; Munk, A.B.; Krohn, S.; Iandiev, I.; Wiedemann, P.; Reichenbach, A.; Eichler, W. The axon guidance molecule Netrin-4 is expressed by Muller cells and contributes to angiogenesis in the retina. Glia 2012, 60, 1567–1578. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, J.G.; Cardona, S.M.; Pouw, C.E.; Muniz, A.; Mendiola, A.S.; Tsin, A.T.; Allen, D.M.; Cardona, A.E. The role of microglia in diabetic retinopathy. J. Ophthalmol. 2014, 2014, 705783. [Google Scholar] [CrossRef] [Green Version]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.Y.; Green, W.R.; Tso, M.O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demircan, N.; Safran, B.G.; Soylu, M.; Ozcan, A.A.; Sizmaz, S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye 2006, 20, 1366–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleva-Georgieva, D.N.; Sivkova, N.P.; Terzieva, D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med. (Plovdiv) 2011, 53, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Sonoda, K.H.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, K.; Yoshida, S.; Kobayashi, Y.; Zhou, Y.; Nakama, T.; Nakao, S.; Sassa, Y.; Oshima, Y.; Niiro, H.; Akashi, K.; et al. Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 932–946. [Google Scholar] [CrossRef] [Green Version]
- Sasmono, R.T.; Oceandy, D.; Pollard, J.W.; Tong, W.; Pavli, P.; Wainwright, B.J.; Ostrowski, M.C.; Himes, S.R.; Hume, D.A. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 2003, 101, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Garcia, S.; Reichhart, N.; Hernandez-Matas, C.; Zabulis, X.; Kociok, N.; Brockmann, C.; Joussen, A.M.; Strauss, O. In vivo analysis of the time and spatial activation pattern of microglia in the retina following laser-induced choroidal neovascularization. Exp. Eye Res. 2015, 139, 13–21. [Google Scholar] [CrossRef] [PubMed]
| Weeks after STZ | Week 0 | Week 2 | Week 4 | Week 8 | |||||
|---|---|---|---|---|---|---|---|---|---|
| NTN4WT | Control | 15.1 ± 0.9 | 14.7 ± 0.8 | 15.8 ± 0.4 | 17.4 ± 0.6 | ||||
| Diabetic | 18.1 ± 0.3 | ns | 32.1 ± 0.8 | *** | 32.7 ± 0.7 | *** | 39.0 ± 0.6 | *** | |
| NTN4KO | Control | 16.6 ± 0.9 | 15.2 ± 0.5 | 14.9 ± 0.7 | 17.8 ± 1.8 | ||||
| Diabetic | 17.2 ± 0.4 | ns | 31.6 ± 0.8 | ** | 35.5 ± 1.9 | *** | 36.8 ± 0.8 | *** | |
| Weeks after STZ | Week 0 | Week 2 | Week 4 | Week 8 | |
|---|---|---|---|---|---|
| NTN4WT | Control | 23.8 ± 0.0 | 23.8 ± 0.0 | 26.0 ± 0.0 | 28.2 ± 0.0 |
| Diabetic | 24.8 ± 0.0 | 24.5 ± 0.0 | 24.9 ± 0.0 | 25.8 ± 0.0 | |
| NTN4KO | Control | 24.6 ± 0.0 | 25.6 ± 0.0 | 27.2 ± 0.1 | 29.2 ± 0.1 |
| Diabetic | 24.6 ± 0.0 | 23.8 ± 0.0 | 24.4 ± 0.0 | 25.1 ± 0.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Garcia, S.; Reichhart, N.; Kociok, N.; Skosyrski, S.; Joussen, A.M. Anti-Inflammatory Role of Netrin-4 in Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 4481. https://doi.org/10.3390/ijms22094481
Crespo-Garcia S, Reichhart N, Kociok N, Skosyrski S, Joussen AM. Anti-Inflammatory Role of Netrin-4 in Diabetic Retinopathy. International Journal of Molecular Sciences. 2021; 22(9):4481. https://doi.org/10.3390/ijms22094481
Chicago/Turabian StyleCrespo-Garcia, Sergio, Nadine Reichhart, Norbert Kociok, Sergej Skosyrski, and Antonia M. Joussen. 2021. "Anti-Inflammatory Role of Netrin-4 in Diabetic Retinopathy" International Journal of Molecular Sciences 22, no. 9: 4481. https://doi.org/10.3390/ijms22094481






