Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages
Abstract
1. Introduction
2. Results
2.1. Osteoblast Proliferation
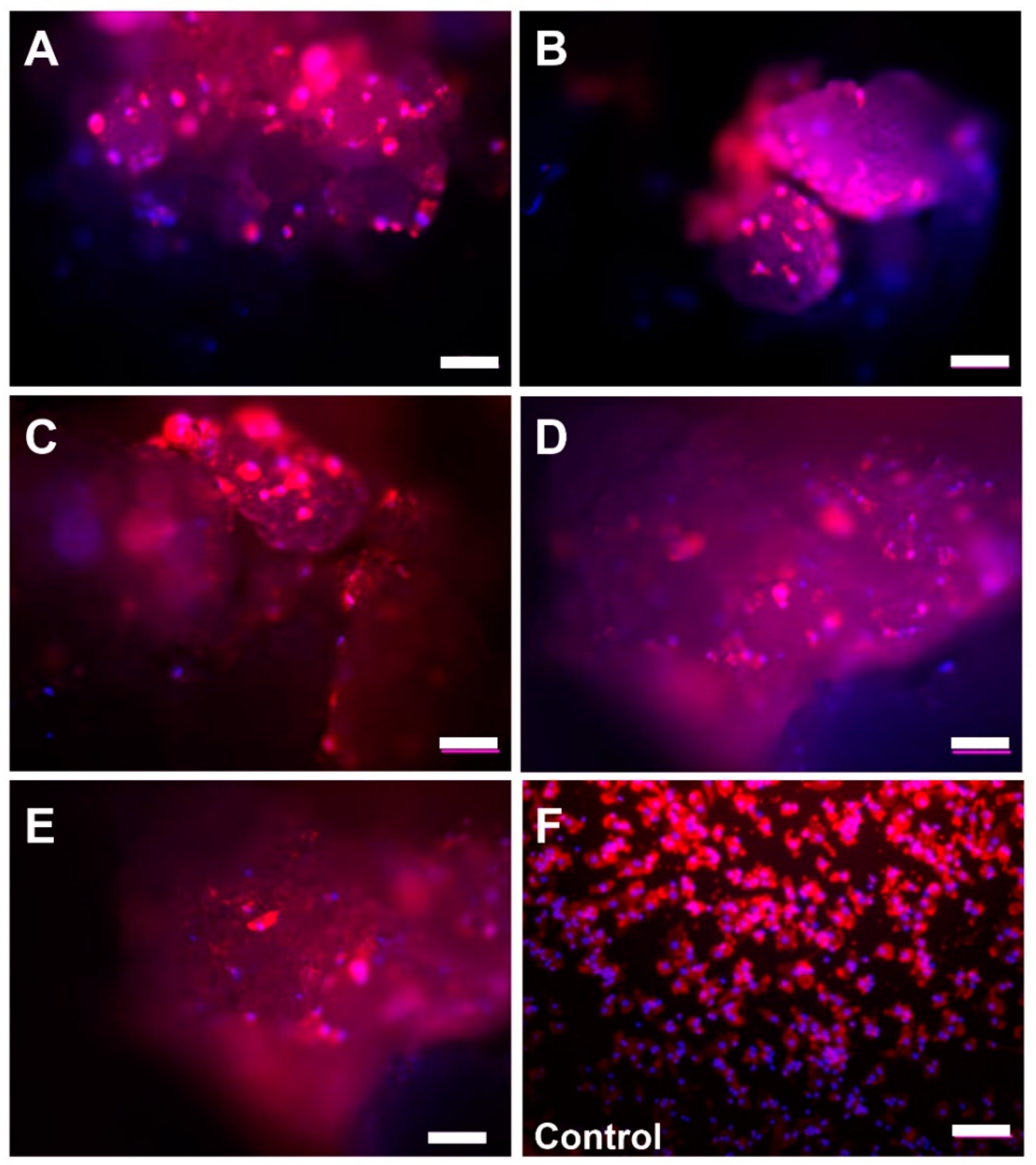
2.2. Microscopic Analysis of Monocyte Cultivation with Bone Substitutes
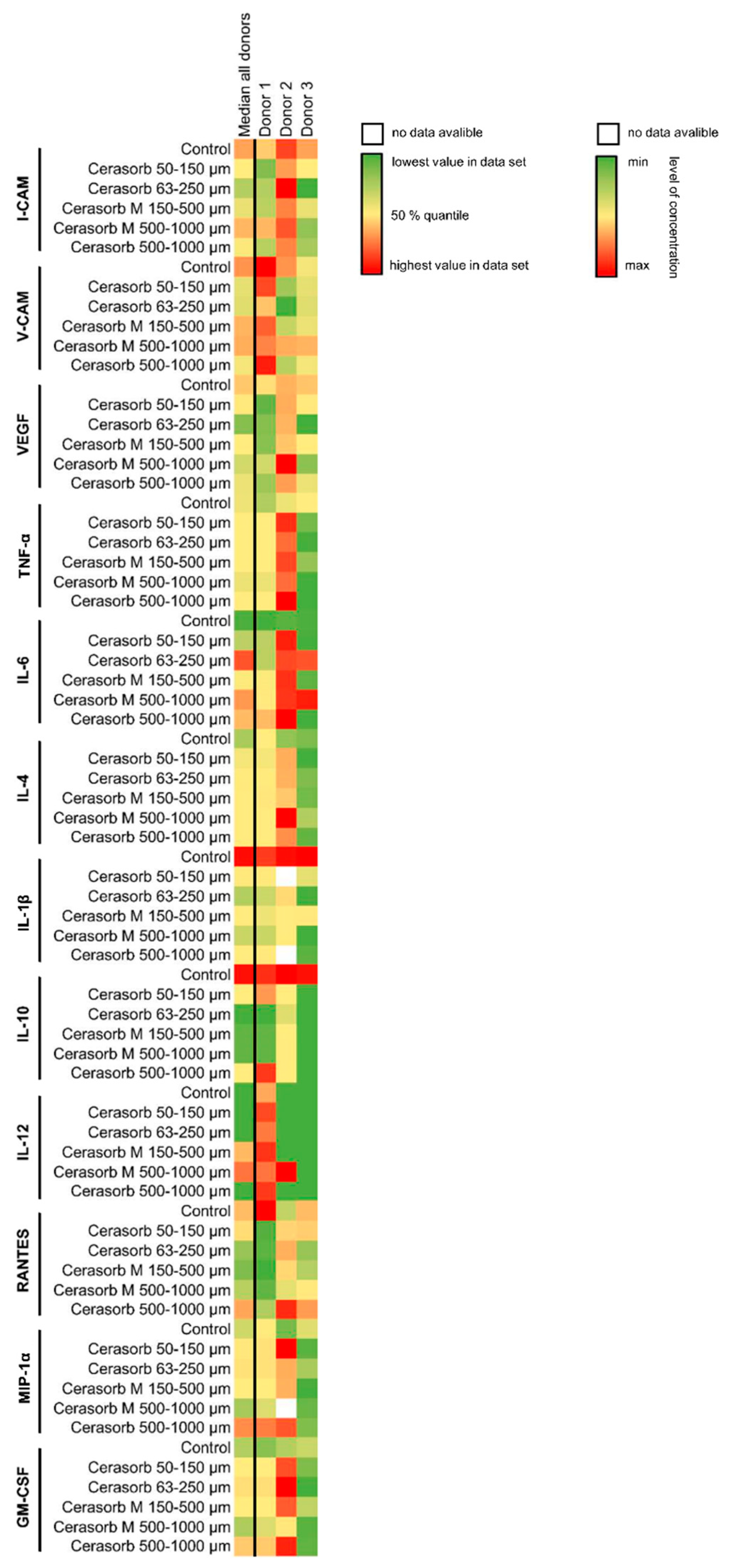
2.3. Cytokine Measurements
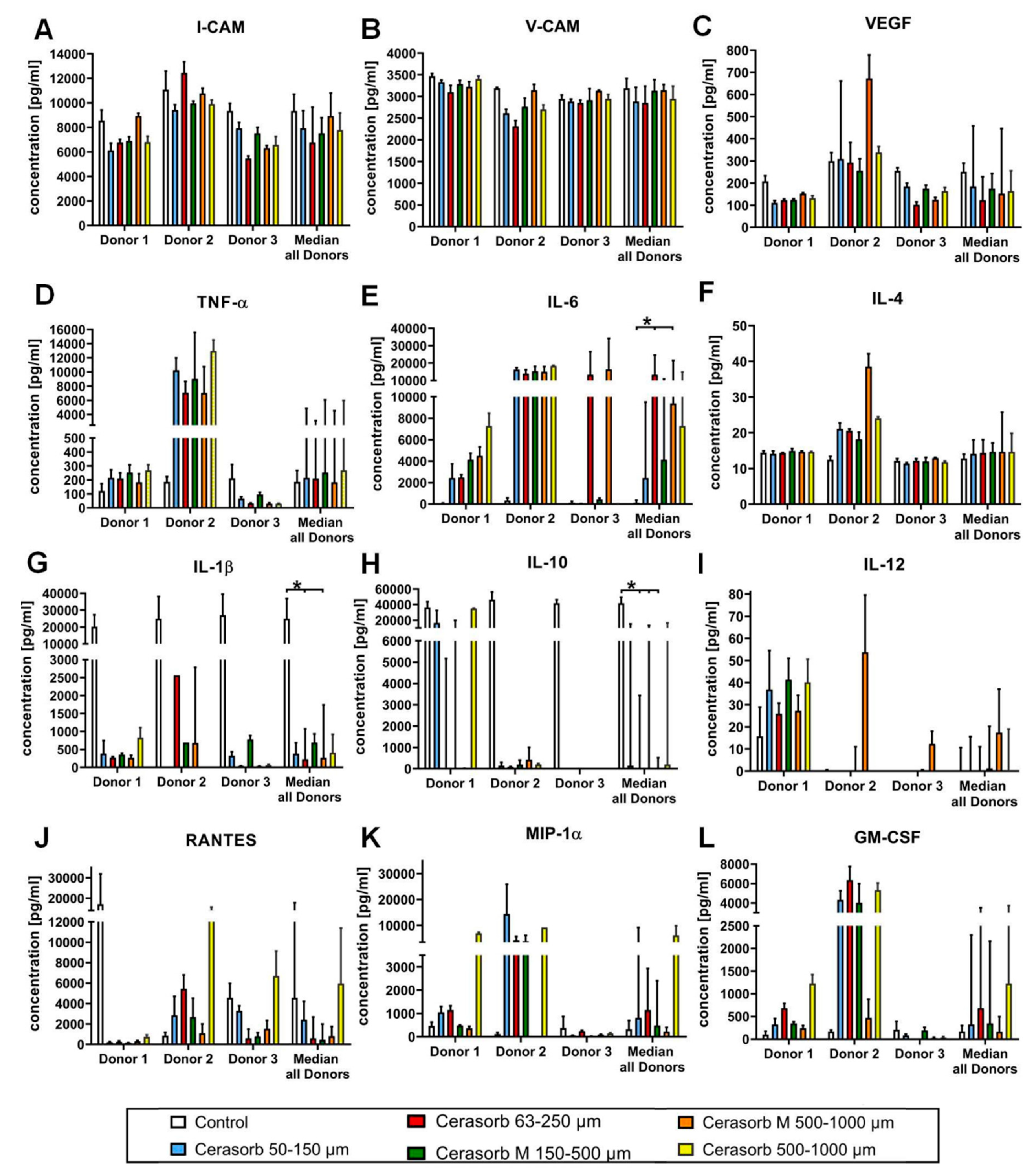
2.3.1. Intercellular Adhesion Molecule (I-CAM) Expression
2.3.2. Vascular Cell Adhesion Protein 1 (V-CAM) Expression
2.3.3. Vascular Endothelial Growth Factor (VEGF) Expression
2.3.4. Tumor Necrosis Factor α (TNF-α) Expression
2.3.5. Interleukin 6 (IL-6) Expression
2.3.6. Interleukin (IL-4) Expression
2.3.7. Interleukin 1β (IL-1β) Expression
2.3.8. Interleukin 10 (IL-10) Expression
2.3.9. Interleukin 12 (IL-12) Expression
2.3.10. CC-Chemokine Ligand 5 (CCL5, RANTES) Expression
2.3.11. Macrophage Inflammatory Protein (MIP-1α) Expression
2.3.12. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Expression
2.3.13. Interleukin 8 (IL-8)
2.3.14. Monocyte Chemoattractant Protein (MCP-1)
2.3.15. Correlation Analysis
3. Discussion
4. Materials and Methods
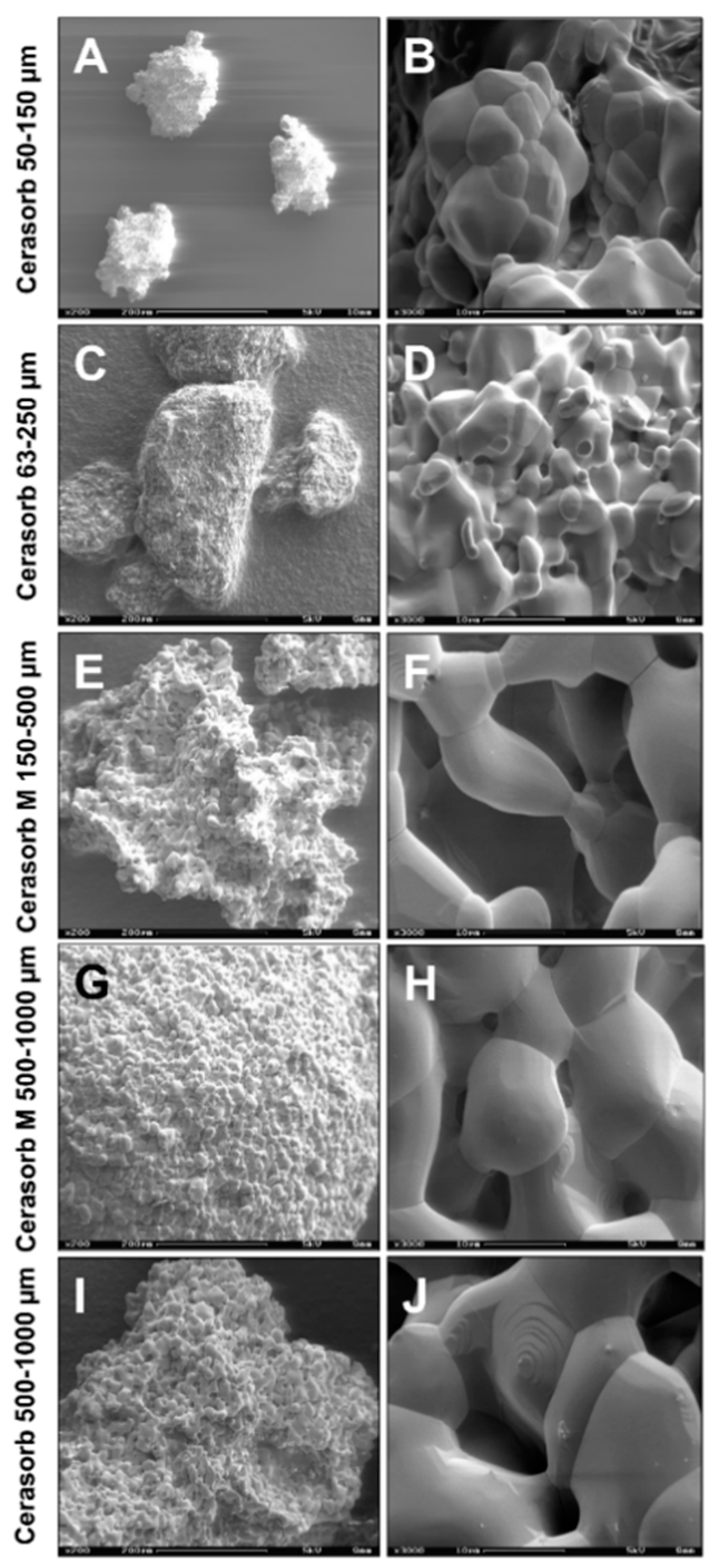
4.1. Biomaterials
4.2. Isolation of Primary Human Osteoblasts and Co-Cultivation with the Bone Substitutes
Measurements of Osteoblast Proliferation
4.3. Isolation of Primary Human Monocytes and Co-Cultivation with the Bone Substitutes
4.3.1. Visualization of the Cells and Image Acquisition
4.3.2. Measurements of Cytokine Synthesis
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. Part A 2016, 104, 413–418. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: Dependence on material surface properties. J. Biomed. Mater. Res. A 2015, 103, 1380–1390. [Google Scholar] [CrossRef]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. Part A 2007, 83, 585–596. [Google Scholar] [CrossRef]
- Tang, L.; Jennings, T.A.; Eaton, J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. USA 1998, 95, 8841–8846. [Google Scholar] [CrossRef]
- Aghbali, A.; Rafieyan, S.; Mohamed-Khosroshahi, L.; Baradaran, B.; Shanehbandi, D.; Kouhsoltani, M. IL-4 induces the formation of multinucleated giant cells and expression of β5 integrin in central giant cell lesion. Med. Oral Patol. Oral Cir. Bucal 2016, 22, e1–e6. [Google Scholar] [CrossRef][Green Version]
- DeFife, K.M.; Jenney, C.R.; McNally, A.K.; Colton, E.; Anderson, J.M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J. Immunol. 1997, 158, 3385–3390. [Google Scholar] [PubMed]
- Walch, L.; Massade, L.; Dufilho, M.; Brunet, A.; Rendu, F. Pro-atherogenic effect of interleukin-4 in endothelial cells: Modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis 2006, 187, 285–291. [Google Scholar] [CrossRef]
- Pike, A.C.B.; Nirupama, K.S.; Krista, M.D.; Timothy, M.W.; Colin, D.F.; Wesley, J. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor γ1. Proc. Natl. Acad. Sci. USA 2001, 98, 2443–2448. [Google Scholar]
- Frank, P.G.; Lisanti, M.P. ICAM-1: Role in inflammation and in the regulation of vascular permeability. Am. J. Physiol. Circ. Physiol. 2008, 295, H926–H927. [Google Scholar] [CrossRef]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Ozaki, K.; Nakae, H.; Matsuo, T. Cytokines differentially regulate ICAM-1 and VCAM-1 expression on human gingival fibroblasts. Clin. Exp. Immunol. 2006, 144, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wung, B.S.; Ni, C.W.; Wang, D.L. ICAM-1 induction by TNFα and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J. Biomed. Sci. 2005, 12, 91–101. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Katagiri, T.; Tamura, T.; Wada, S.; Findlay, D.M.; Martin, T.J.; Hirota, H.; Taga, T.; Kishimoto, T.; et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 1995, 182, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.A.; Sousa, S.L.; Alander, C.; Raisz, L.G.; Dinarello, C.A. Comparison of the bone-resorbing activity in the supernatants from phytohemagglutinin-stimulated human peripheral blood mononuclear cells with that of cytokines through the use of an antiserum to interleukin 1. Endocrinology 1987, 121, 1164–1170. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The Role of IL-1beta; in the Bone Loss during Rheumatic Diseases. Mediat. Inflamm. 2015, 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.H.; Wientjens, G.J.; Fibbe, W.E.; Willemze, R.; Kluin-Nelemans, H.C. Inhibition of human macrophage colony formation by interleukin 4. J. Exp. Med. 1989, 170, 577–582. [Google Scholar] [CrossRef]
- Ozawa, H.; Aiba, S.; Nakagawa, S.; Tagami, H. Interferon-gamma and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7-1) expression. Eur. J. Immunol. 1996, 26, 648–652. [Google Scholar] [CrossRef]
- Hamilton, J. Rheumatoid arthritis: Opposing actions of haemopoietic growth factors and slow-acting anti-rheumatic drugs. Lancet 1993, 342, 536–539. [Google Scholar] [CrossRef]
- Shinohara, H.; Yano, S.; Bucana, C.D.; Fidler, I.J. Induction of Chemokine Secretion and Enhancement of Contact-Dependent Macrophage Cytotoxicity by Engineered Expression of Granulocyte-Macrophage Colony-Stimulating Factor in Human Colon Cancer Cells. J. Immunol. 2000, 164, 2728–2737. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef] [PubMed]
- Coma, G.; Peña, R.; Blanco, J.; Rosell, A.; Borras, F.E.; Este, J.A.; Clotet, B.; Ruiz, L.; Parkhouse, R.M.E.; Bofill, M. Treatment of monocytes with interleukin (IL)-12 plus IL-18 stimulates survival, differentiation and the production of CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10. Clin. Exp. Immunol. 2006, 145, 535–544. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Kotani, A.; Hori, T.; Matsumura, Y.; Uchiyama, T. Signaling of gp34 (OX40 ligand) induces vascular endothelial cells to produce a CC chemokine RANTES/CCL5. Immunol. Lett. 2002, 84, 1–7. [Google Scholar] [CrossRef]
- Locati, M.; Deuschle, U.; Massardi, M.L.; Martinez, F.O.; Sironi, M.; Sozzani, S.; Bartfai, T.; Mantovani, A. Analysis of the Gene Expression Profile Activated by the CC Chemokine Ligand 5/RANTES and by Lipopolysaccharide in Human Monocytes. J. Immunol. 2002, 168, 3557–3562. [Google Scholar] [CrossRef]
- Bonecchi, R.; Polentarutti, N.; Luini, W.; Borsatti, A.; Bernasconi, S.; Locati, M.; Power, C.; Proudfoot, A.; Wells, T.N.; Mackay, C.; et al. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J. Immunol. 1999, 162, 474–479. [Google Scholar] [PubMed]
- Nagano, M.; Kimura, K.; Yamashita, T.; Ohneda, K.; Nozawa, D.; Hamada, H.; Yoshikawa, H.; Ochiai, N.; Ohneda, O. Hypoxia Responsive Mesenchymal Stem Cells Derived from Human Umbilical Cord Blood Are Effective for Bone Repair. Stem Cells Dev. 2010, 19, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.; Lorenz, H.; Xu, W.; Szalay, K.; Kasten, P.; Claes, L.; Augat, P.; Richter, W. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone 2007, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Wernike, E.; Montjovent, M.-O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.-A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cells Mater. 2010, 19, 30–40. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Tan, Y.-Y.; Wong, R.W.K.; Wenden, A.; Zhang, L.-K.; Rabie, A.B.M. The role of vascular endothelial growth factor in ossification. Int. J. Oral Sci. 2012, 4, 64–68. [Google Scholar] [CrossRef]
- Miyanishi, K.; Trindade, M.C.; Ma, T.; Goodman, S.B.; Schurman, D.J.; Smith, R.L. Periprosthetic Osteolysis: Induction of Vascular Endothelial Growth Factor from Human Monocyte/Macrophages by Orthopaedic Biomaterial Particles. J. Bone Miner. Res. 2003, 18, 1573–1583. [Google Scholar] [CrossRef]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Van Vlasselaer, P.; Borremans, B.; Van Den Heuvel, R.; Van Gorp, U.; de Waal Malefyt, R. Interleukin-10 inhibits the osteogenic activity of mouse bone marrow. Blood 1993, 82, 2361–2370. [Google Scholar] [CrossRef]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte preseeding leads to an increased implant bed vascularization of biphasic calcium phosphate bone substitutes via vessel maturation. J. Biomed. Mater. Res. Part A 2016, 104, 2928–2935. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Hoffmann, C.; Sader, R.; Peters, F.; Hübner, W.-D.; Kirkpatrick, C.J.; Ghanaati, S. Injectable Bone Substitute Based on β-TCP Combined with a Hyaluronan-Containing Hydrogel Contributes to Regeneration of a Critical Bone Size Defect Towards Restitutio ad Integrum. J. Oral Implantol. 2016, 42, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implant. 2015, 41, e212–e222. [Google Scholar] [CrossRef]
- Ghanaati, S.; Kirkpatrick, C.J.; Kubesch, A.; Lorenz, J.; Sader, R.A.; Udeabor, S.E.; Barbeck, M.; Choukroun, J. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-OssTM) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150–157. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Miron, R.J.; Zohdi, H.; Fujioka-Kobayashi, M.; Bosshardt, D.D. Giant cells around bone biomaterials: Osteoclasts or multi-nucleated giant cells? Acta Biomater. 2016, 46, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.; et al. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-inflammatory Tissue Response to a Newly Developed Pericardium-based Barrier Membrane for Guided Bone Regeneration. In Vivo 2020, 34, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-S.; Lee, K.-S.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Boersema, G.S.; Grotenhuis, N.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages. BioResearch Open Access 2016, 5, 6–14. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, J.; Yang, F.; Zhu, J.; Tian, X.; Chen, X. In vitro degradation and cell response of calcium carbonate composite ceramic in comparison with other synthetic bone substitute materials. Mater. Sci. Eng. C 2015, 50, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Brochériou, I.; Maouche, S.; Durand, H.; Braunersreuther, V.; Le Naour, G.; Gratchev, A.; Koskas, F.; Mach, F.; Kzhyshkowska, J.; Ninio, E. Antagonistic regulation of macrophage phenotype by M-CSF and GM-CSF: Implication in atherosclerosis. Atherosclerosis 2011, 214, 316–324. [Google Scholar] [CrossRef]
- Naik, S.H.; Metcalf, D.; Van Nieuwenhuijze, A.; Wicks, I.; Wu, L.; O’Keeffe, M.; Shortman, K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006, 7, 663–671. [Google Scholar] [CrossRef]
- Schmid, M.A.; Kingston, D.; Boddupalli, S.; Manz, M.G. Instructive cytokine signals in dendritic cell lineage commitment. Immunol. Rev. 2010, 234, 32–44. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Cook, A.D.; Hamilton, J.A. Functions of Granulocyte-Macrophage Colony-Stimulating Factor. Crit. Rev. Immunol. 2005, 25, 405–428. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Shriver, L.P.; Maresz, K.; Pedras-Vasconcelos, J.; Verthelyi, D.; Dittel, B.N. GM-CSF Production by Autoreactive T Cells Is Required for the Activation of Microglial Cells and the Onset of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007, 178, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ottenhoff, F.A.W.V.M.; de Tjitske, B.; Dennis, M.L.L.; Marieke, A.H.; Matthijs, K.; Elena, V.; Robert, K.; Arend, K.; de René, W.-M.; Tom, H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar]
- Lari, R.; Fleetwood, A.J.; Kitchener, P.D.; Cook, A.D.; Pavasovic, D.; Hertzog, P.J.; Hamilton, J.A. Macrophage lineage phenotypes and osteoclastogenesis--complexity in the control by GM-CSF and TGF-beta. Bone 2007, 40, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Rifas, L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J. Cell. Biochem. 2006, 98, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of Osteogenesis in Mesenchymal Stem Cells by Activated Monocytes/Macrophages Depends on Oncostatin M Signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Junior, C.M.; Silva, M.J.B.; Corrêa, J.D.; Madeira, M.F.M.; Garlet, T.P.; Garlet, G.P.; Cunha, F.Q.; Teixeira, M.M.; Da Silva, T.A. A Controversial Role for IL-12 in Immune Response and Bone Resorption at Apical Periodontal Sites. Clin. Dev. Immunol. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. IL-12 Alone and in Synergy with IL-18 Inhibits Osteoclast Formation In Vitro. J. Immunol. 2001, 166, 4915–4921. [Google Scholar] [CrossRef]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. Th1/Th2 Paradigm Extended: Macrophage Polarization as an Unappreciated Pathogen-Driven Escape Mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, O.; Bouler, J.-M.; Weiss, P.; Bosco, J.; Aguado, E.; Daculsi, G. Short-term effects of mineral particle sizes on cellular degradation activity after implantation of injectable calcium phosphate biomaterials and the consequences for bone substitution. Bone 1999, 25, 71S–74S. [Google Scholar] [CrossRef]
- Lange, T.; Schilling, A.F.; Peters, F.; Haag, F.; Morlock, M.M.; Rueger, J.M.; Amling, M. Proinflammatory and osteoclastogenic effects of beta-tricalciumphosphate and hydroxyapatite particles on human mononuclear cells in vitro. Biomaterials 2009, 30, 5312–5318. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, Z.; Jiang, H.; Cao, Z.; Liu, J.; Zhang, X. Gene Modification of Transforming Growth Factor beta (TGF-beta) and Interleukin 10 (IL-10) in Suppressing Mt Sonicate Induced Osteoclast Formation and Bone Absorption. Med. Sci. Monit. 2018, 24, 5200–5207. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.; Liu, Y.; Moore, K.W.; Mui, A.L. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: Evidence for Stat3-dependent and -independent pathways. EMBO J. 1998, 17, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Van Vlasselaer, P. IL-10 and Bone Formation/Hematopoiesis, Interleukin-10; Springer: Berlin/Heidelberg, Germany, 1995; pp. 59–67. [Google Scholar]
- Ghanaati, S.; Barbeck, M.; Hilbig, U.; Hoffmann, C.; Unger, R.; Sader, R.; Peters, F.; Kirkpatrick, C. An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011, 7, 4018–4028. [Google Scholar] [CrossRef]
- Haberstroh, U.; Pocock, J.; Gómez-Guerrero, C.; Helmchen, U.; Hamann, A.; Gutierrez-Ramos, J.C.; Stahl, R.A.K.; Thaiss, F. Expression of the chemokines MCP-1/CCL2 and RANTES/CCL5 is differentially regulated by infiltrating inflammatory cells. Kidney Int. 2002, 62, 1264–1276. [Google Scholar] [CrossRef]
- Suffee, N.; Richard, B.; Hlawaty, H.; Oudar, O.; Charnaux, N.; Sutton, A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem. Soc. Trans. 2011, 39, 1649–1653. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Kao, Y.-T.; Huang, W.-K.; Lin, K.-Y.; Wu, S.-C.; Hsu, S.-C.; Schuyler, S.C.; Li, L.-Y.; Lu, F.L.; Lu, J. CCL5/RANTES is important for inducing osteogenesis of human mesenchymal stem cells and is regulated by dexamethasone. Biosci. Trends 2014, 8, 138–143. [Google Scholar] [CrossRef]
- Hirohata, S. Human Th1 responses driven by IL-12 are associated with enhanced expression of CD40 ligand. Clin. Exp. Immunol. 1999, 115, 78–85. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; Friedrich, R.E.; Precht, C.; Hanken, H.; Gröbe, A.; et al. PEO-generated Surfaces Support Attachment and Growth of Cells In Vitro with No Additional Benefit for Micro-Roughness in Sa (0.2–4 mum). In Vivo 2016, 30, 27–33. [Google Scholar]
- Zimmermann, C.E.; Gierloff, M.; Hedderich, J.; Açil, Y.; Wiltfang, J.; Terheyden, H. Biocompatibility of bone graft substitutes: Effects on survival and proliferation of porcine multilineage stem cells in vitro. Folia Morphol. 2011, 70, 154–160. [Google Scholar]
- Piccinini, M.; Prosperi, S.; Preve, E.; Rebaudi, A.; Bucciotti, F. In Vitro Biocompatibility Assessment and In Vivo Behavior of a New Osteoconductive betaTCP Bone Substitute. Implant Dent. 2016, 25, 456–463. [Google Scholar] [CrossRef]
- Ignatius, A.A.; Schmidt, C.; Kaspar, D.; Claes, L.E. In vitro biocompatibility of resorbable experimental glass ceramics for bone substitutes. J. Biomed. Mater. Res. 2001, 55, 285–294. [Google Scholar] [CrossRef]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Hartjen, P.; Hoffmann, A.; Henningsen, A.; Barbeck, M.; Kopp, A.; Kluwe, L.; Precht, C.; Quatela, O.; Gaudin, R.; Heiland, M.; et al. Plasma Electrolytic Oxidation of Titanium Implant Surfaces: Microgroove-Structures Improve Cellular Adhesion and Viability. In Vivo 2018, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G. Primary Cell Cultures and Immortal Cell Lines; eLS.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Riddy, D.M.; Goy, E.; Delerive, P.; Summers, R.J.; Sexton, P.M.; Langmead, C.J. Comparative genotypic and phenotypic analysis of human peripheral blood monocytes and surrogate monocyte-like cell lines commonly used in metabolic disease research. PLoS ONE 2018, 13, e0197177. [Google Scholar] [CrossRef]
- Peters, F.; Reif, D. Functional Materials for Bone Regeneration from Beta-Tricalcium Phosphate. Mater. Werkst. 2004, 35, 203–207. [Google Scholar] [CrossRef]
- Unger, R.E.; Halstenberg, S.; Sartoris, A.; Kirkpatrick, C.J. Human endothelial and osteoblast co-cultures on 3D biomaterials. Methods Mol. Biol. 2001, 695, 229–241. [Google Scholar]


| Time (h) | Control (1) | Cerasorb 50–150 µm (2) | Cerasorb 63–250 µm (3) | Cerasorb M 150–500 µm (4) | Cerasorb M 500–1000 µm (5) | Cerasorb 500–1000 µm (6) |
|---|---|---|---|---|---|---|
| 0 | 1 ± 0.06 (b-f) | 1.15 ± 0.05 | 1.14 ± 0.03 | 1.15 ± 0.05 | 1.22 ± 0.06 | 1.13 ± 0.06 |
| 1 | 0.95 ± 0.03 (b-f) | 1.09 ± 0.06 | 1.14 ± 0.05 | 1.14 ± 0.03 | 1.16 ± 0.04 | 1.09 ± 0.04 |
| 2 | 0.92 ± 0.06 (b-f) | 1.08 ± 0.07 | 1.14 ± 0.03 | 1.11 ± 0.02 | 1.09 ± 0.04 | 1.06 ± 0.05 |
| 3 | 0.98 ± 0.03 (b-f) | 1.10 ± 0.07 | 1.17 ± 0.04 (f) | 1.13 ± 0.05 | 1.11 ± 0.05 | 1.08 ± 0.03 |
| 4 | 1.21 ± 0.05 (c,e) | 1.26 ± 0.06 | 1.38 ± 0.05 (f) | 1.30 ± 0.1 | 1.35 ± 0.05 (f) | 1.23 ± 0.07 |
| 5 | 2.87 ± 0.22 | 2.58 ± 0.08 (c,e) | 2.96 ± 0.2 (f) | 2.78 ± 0.26 | 2.96 ± 0.13 (f) | 2.49 ± 0.18 |
| 6 | 5.23 ± 0.41 (b,f) | 4.23 ± 0.07 (c,e) | 4.93 ± 0.33 (f) | 4.76 ± 0.44 | 5.03 ± 0.19 (f) | 4.08 ± 0.27 |
| 24 | 6.05 ± 0.46 (b,f) | 4.73 ± 0.12 (c,e) | 5.53 ± 0.31 (f) | 5.40 ± 0.51 | 5.73 ± 0.2 (f) | 4.60 ± 0.27 |
| 48 | 11.32 ± 0.76 (b-e) | 8.06 ± 0.33 (c,e) | 9.08 ± 0.47 (f) | 9.41 ± 0.96 (f) | 9.90 ± 0.44 (f) | 7.46 ± 0.44 |
| Bone Substitute Characteristic | p-Value |
|---|---|
| Granular shape | 0.001 |
| Granular size | 0.540 |
| Pore size | 0.310 |
| Parameter | Bone Substitute Characteristic | p-Value | 95% CI |
|---|---|---|---|
| GM-CSF | Granular shape | 0.482 | −1464.59–703.62 |
| Granular size | 0.430 | −543.09–235.76 | |
| Pore size | 0.006 | 537.91–2969.85 | |
| I-CAM | Granular shape | 0.849 | −979.35–809.44 |
| Granular size | 0.978 | −316.78–325.77 | |
| Pore size | 0.444 | −1386.75–619.62 | |
| IL-10 | Granular shape | 0.045 | 201.86–16,847.43 |
| Granular size | 0.744 | −3475.30–2503.96 | |
| Pore size | 0.906 | −9881.50–8788.75 | |
| IL-12 | Granular shape | 0.207 | −255.20–1141.16 |
| Granular size | 0.004 | −630.90–−129.31 | |
| Pore size | 0.158 | −225.25–1340.96 | |
| IL-1β | Granular shape | 0.535 | −548.29–1034.63 |
| Granular size | 0.920 | −276.50–305.15 | |
| Pore size | 0.885 | −924.09–800.54 | |
| IL-4 | Granular shape | 0.898 | −3.72–3.27 |
| Granular size | 0.097 | −2.31–0.20 | |
| Pore size | 0.702 | −4.67–3.17 | |
| IL-6 | Granular shape | 0.128 | −13,127.60–1715.92 |
| Granular size | 0.374 | −3851.93–1480.02 | |
| Pore size | 0.263 | −3649.27–12,999.74 | |
| MIP-1β | Granular shape | 0.066 | −217.96–6310.53 |
| Granular size | 0.557 | −1629.13–894.62 | |
| Pore size | 0.868 | −3417.73–4030.69 | |
| RANTES | Granular shape | 0.048 | 21.25–3826.12 |
| Granular size | 0.000 | −2099.05–−699.89 | |
| Pore size | 0.009 | 771.52–5046.04 | |
| TNF-α | Granular shape | 0.138 | −519.91–3605.44 |
| Granular size | 0.609 | −929.70–552.17 | |
| Pore size | 0.570 | −2969.07–1658.06 | |
| V-CAM | Granular shape | 0.036 | 11.42–318.39 |
| Granular size | 0.365 | −80.10–30.17 | |
| Pore size | 0.002 | −447.26–−102.95 | |
| VEGF | Granular shape | 0.286 | −59.23–195.20 |
| Granular size | 0.830 | −40.81–50.58 | |
| Pore size | 0.191 | −236.50–48.87 |
| Material Name | Granule Shape | Granule Size (µm) | Pore Size (µm) | Intergranular Porosity (%) | Overall Porosity (%) |
|---|---|---|---|---|---|
| Cerasorb® M | polygonal | 500–1000 | 0.1–500 | 65 | 80 |
| Cerasorb® M | polygonal | 150–500 | 0.1–500 | 65 | 76 |
| Cerasorb® PARO/PERIO | polygonal | 63–250 | 0.1–50 | 25 | 40 |
| Cerasorb® | round | 500–1000 | 0.1–50 | 35 | 58 |
| Cerasorb® | round | 50–150 | 0.1–50 | 35 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbeck, M.; Schröder, M.-L.; Alkildani, S.; Jung, O.; Unger, R.E. Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages. Int. J. Mol. Sci. 2021, 22, 4442. https://doi.org/10.3390/ijms22094442
Barbeck M, Schröder M-L, Alkildani S, Jung O, Unger RE. Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages. International Journal of Molecular Sciences. 2021; 22(9):4442. https://doi.org/10.3390/ijms22094442
Chicago/Turabian StyleBarbeck, Mike, Marie-Luise Schröder, Said Alkildani, Ole Jung, and Ronald E. Unger. 2021. "Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages" International Journal of Molecular Sciences 22, no. 9: 4442. https://doi.org/10.3390/ijms22094442
APA StyleBarbeck, M., Schröder, M.-L., Alkildani, S., Jung, O., & Unger, R. E. (2021). Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages. International Journal of Molecular Sciences, 22(9), 4442. https://doi.org/10.3390/ijms22094442







