Seminal Plasma: Relevant for Fertility?
Abstract
1. Introduction
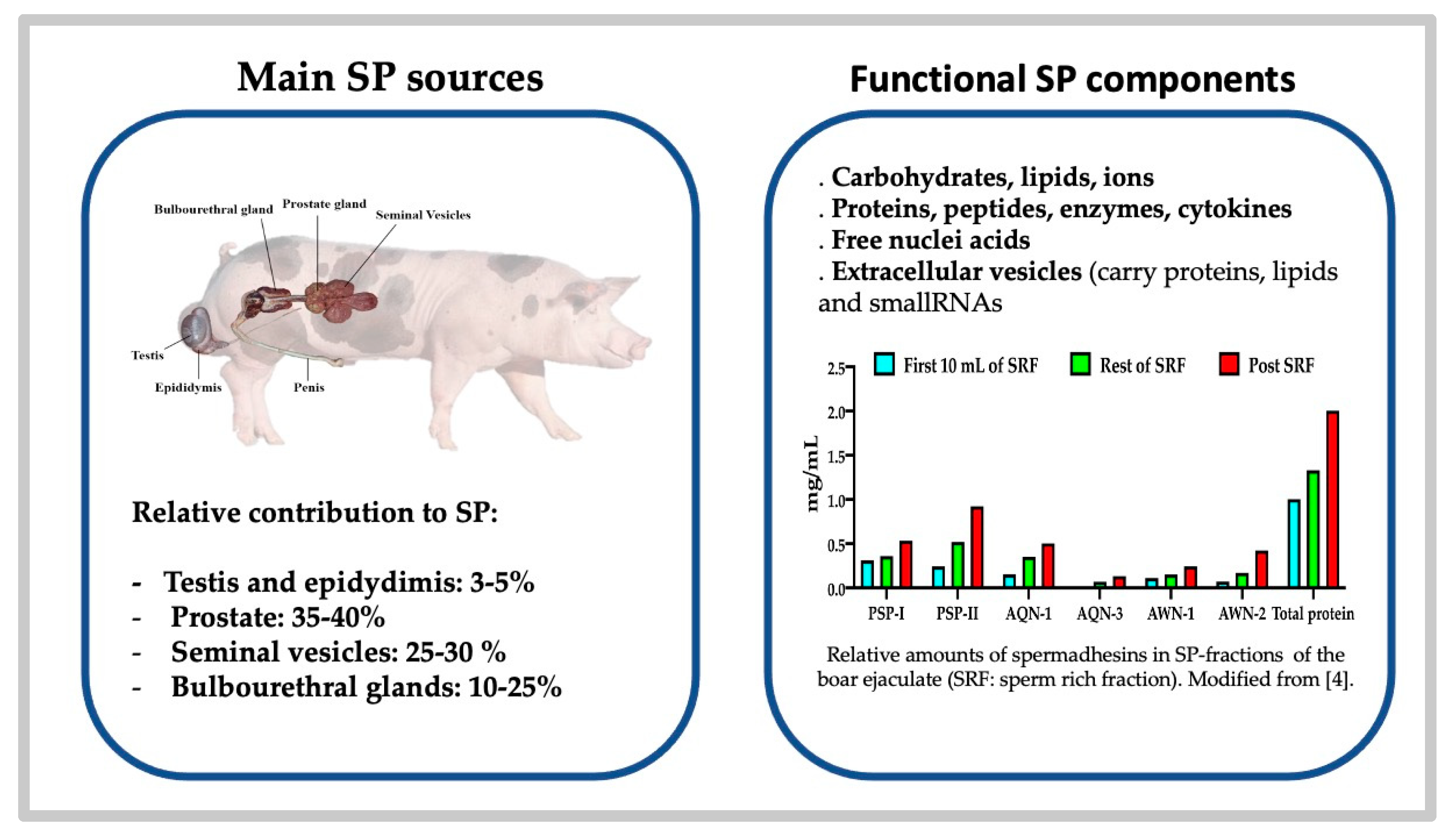
2. The Composition of the Seminal Plasma: Comparative Aspects
2.1. The Building of an Ejaculate Defines the Diversity of Its Composition
2.2. The SP Proteome, What Does It Contain and What Does It Impact?
2.3. Cytokines in the Seminal Plasma: The Effectors of Such Signaling?
2.4. Enzymes of the Seminal Plasma: Do They Play a Role in Fertility or Are They Simple Markers of Sperm Function?
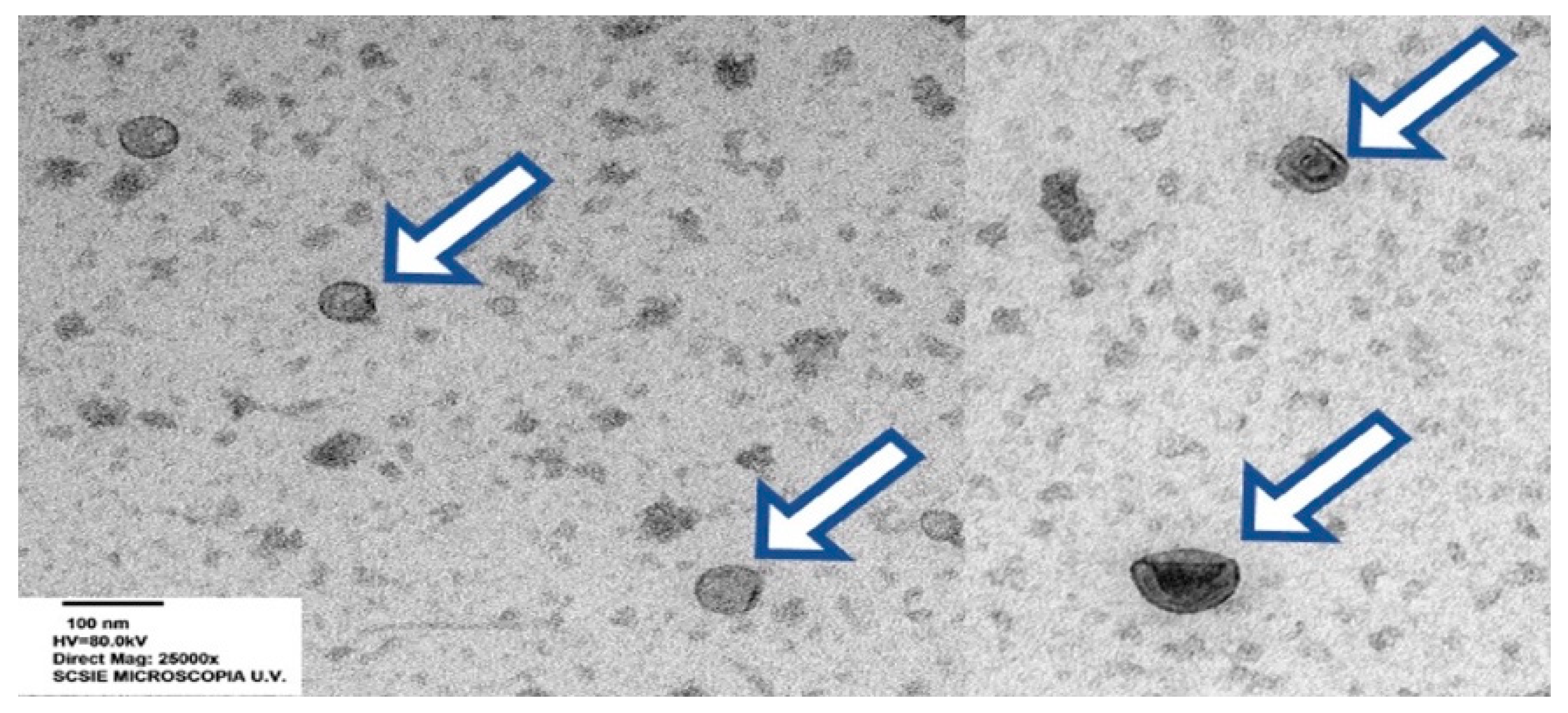
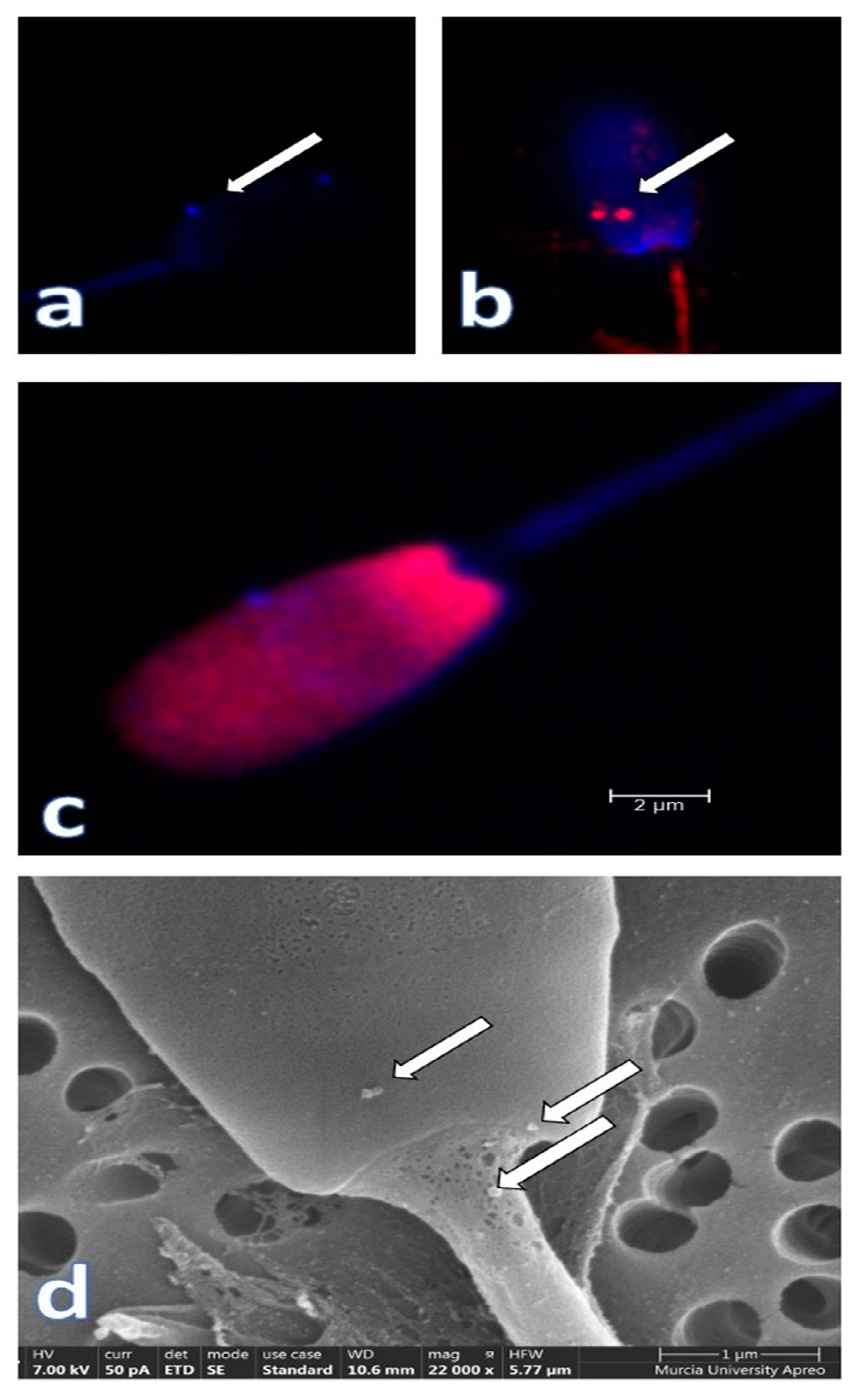
3. The Particulate Seminal Plasma: The Most Relevant Component of Seminal Plasma?
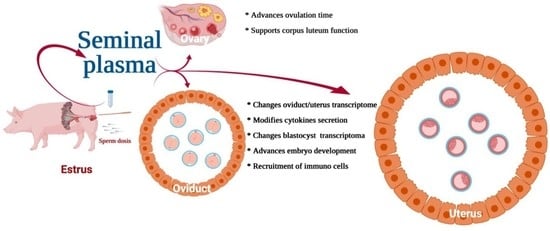
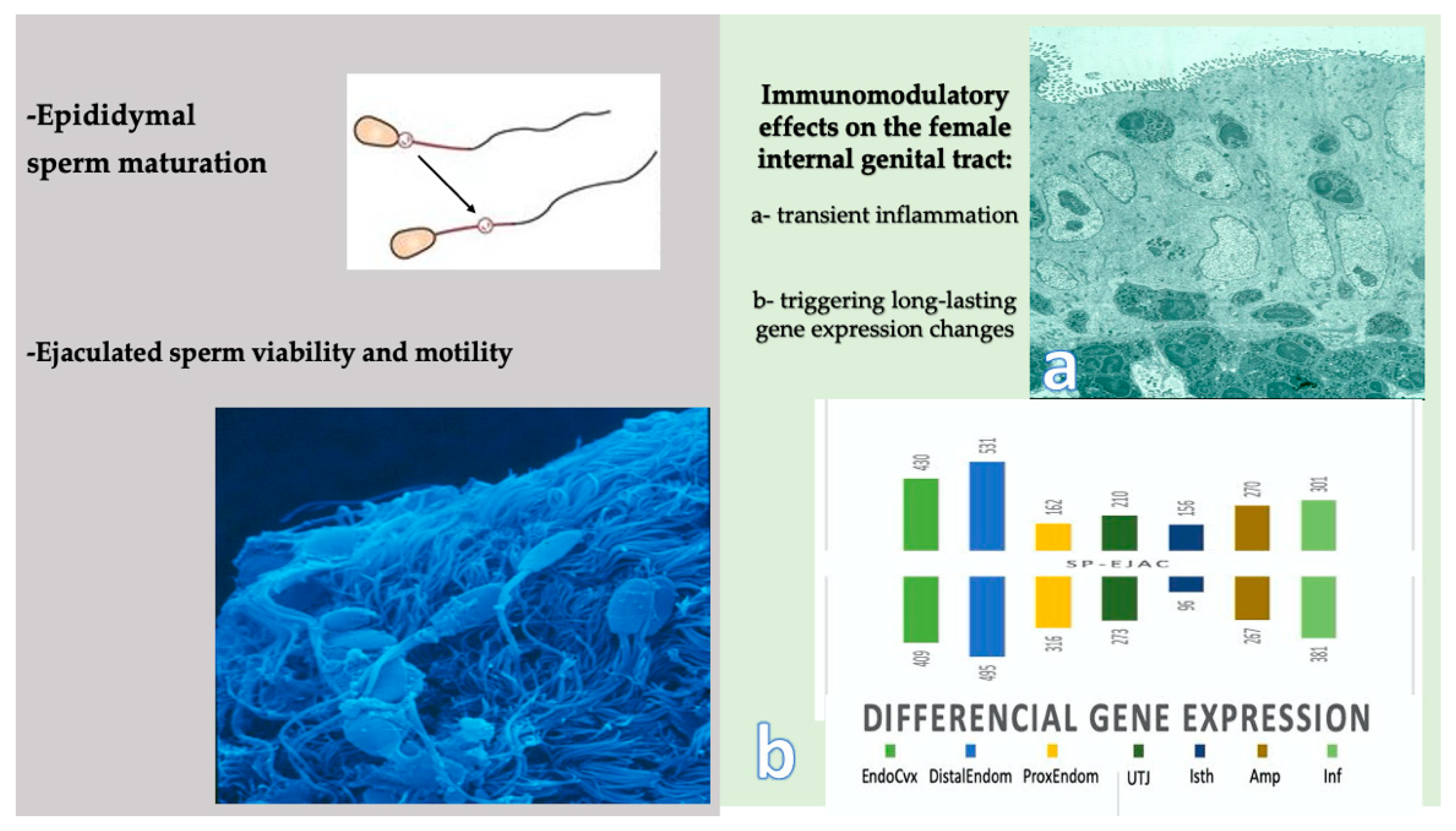
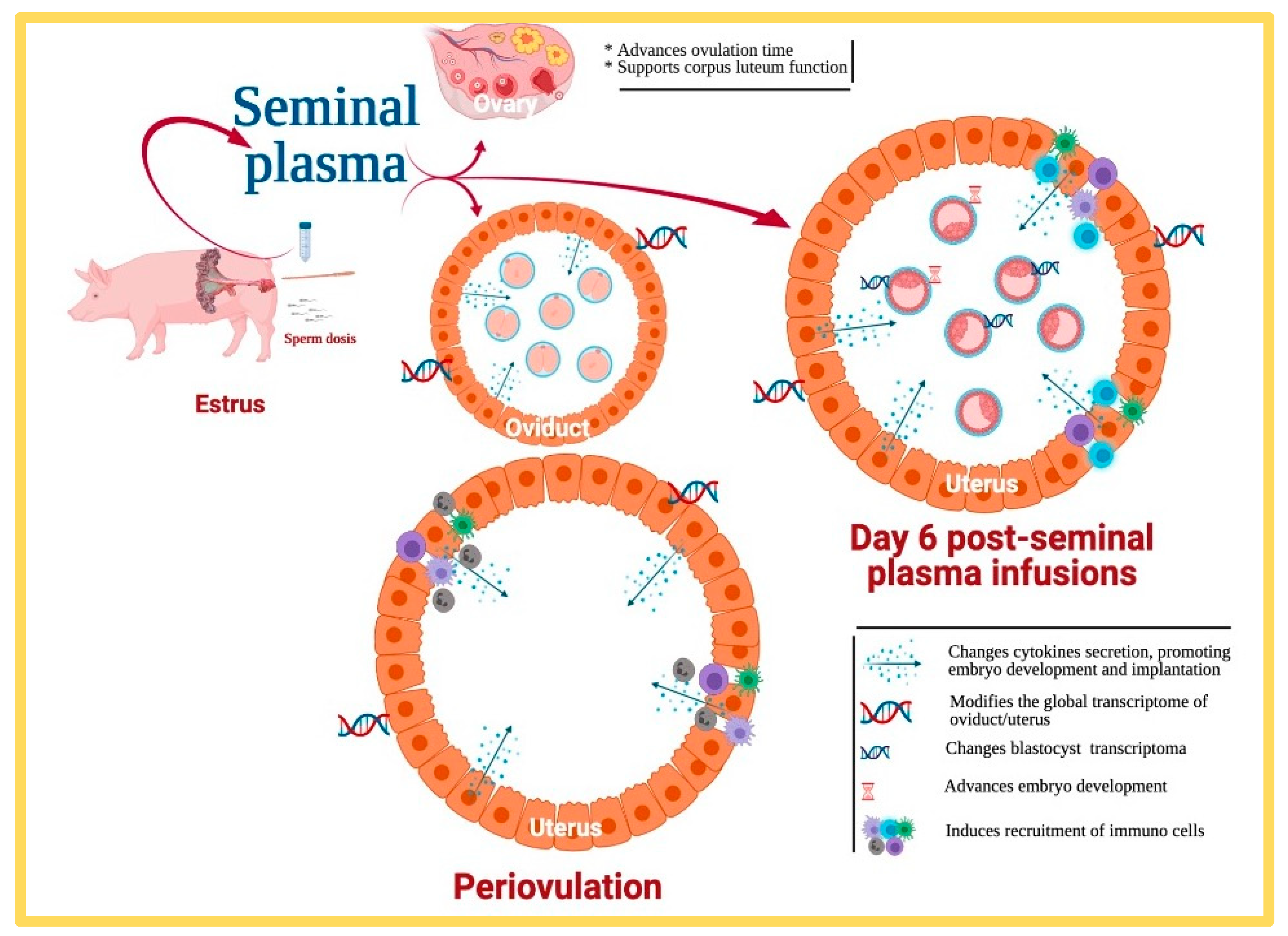
4. What Proof Do We Really Have That Seminal Plasma Really Affects Fertility?
5. What Else Can Be Done with Our Current Knowledge of SP?
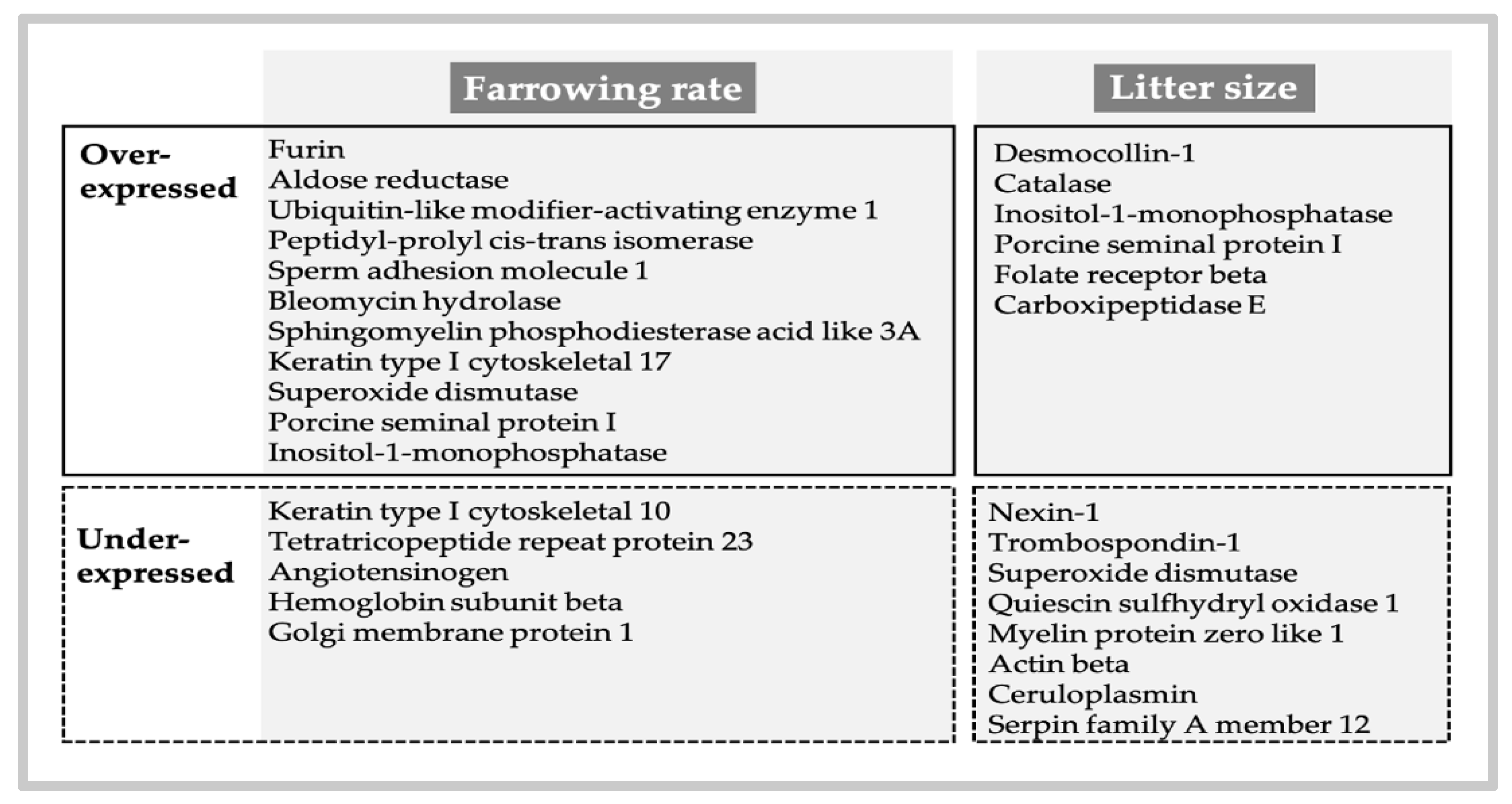
5.1. Can We Improve Andrology Diagnosis by Using Specific SP Biomarkers?
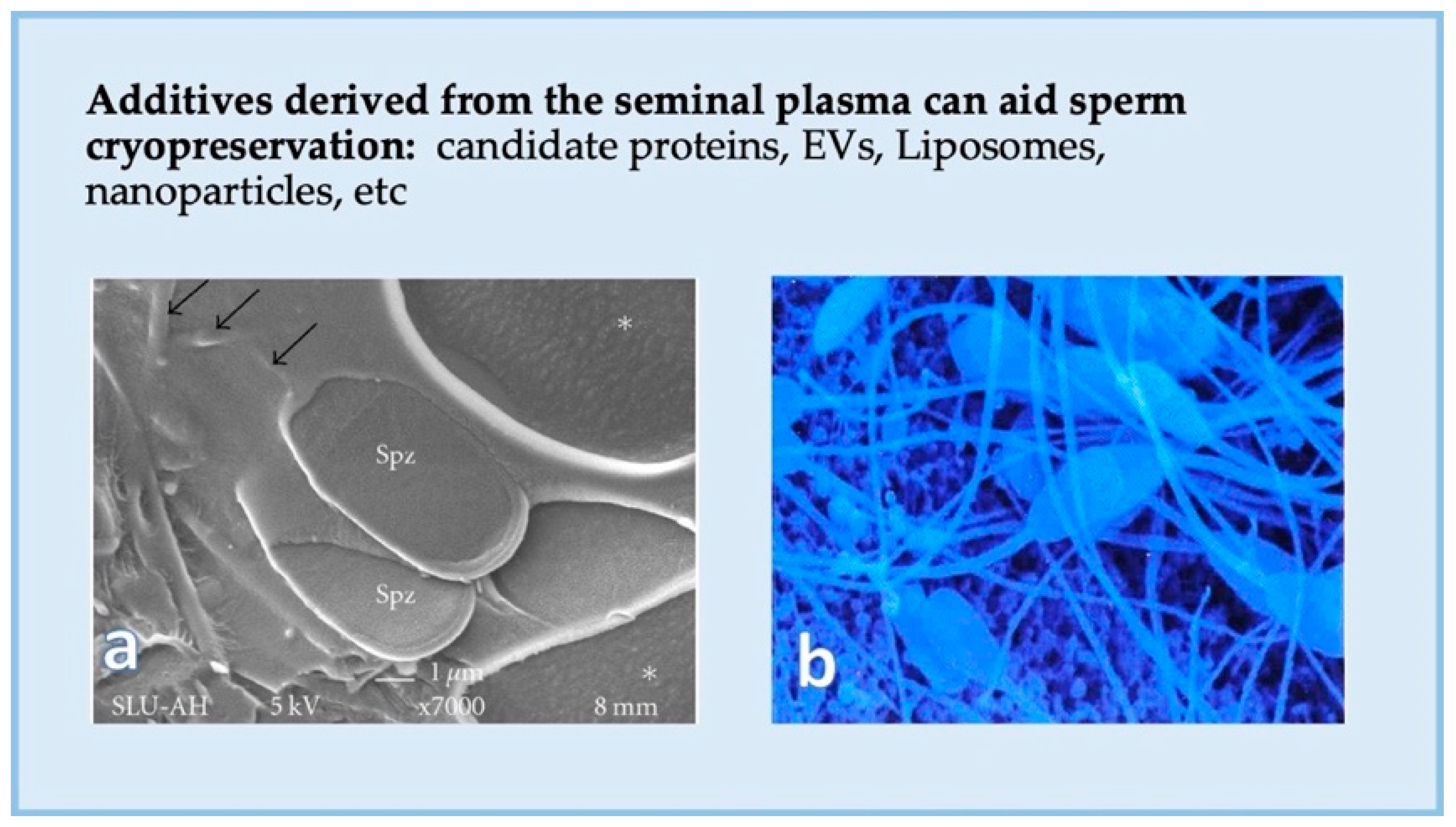
5.2. Can We Enhance Sperm Function and Cryosurvival Using Seminal Plasma?
6. The Future
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Martínez, H.; Saravia, F.; Wallgren, M.; Roca, J.; Peña, F.J. Influence of seminal plasma on the kinematics of boar spermatozoa during freezing. Theriogenology 2008, 70, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Saravia, F.; Wallgren, M.; Johannisson, A.; Calvete, J.J.; Sanz, L.; Peña, F.J.; Roca, J.; Rodríguez-Martínez, H. Exposure to the seminal plasma of different portions of the boar ejaculate modulates the survival of spermatozoa cryopreserved in MiniFlatPacks. Theriogenology 2009, 71, 662–675. [Google Scholar] [CrossRef]
- Atikuzzaman, M.; Alvarez-Rodriguez, M.; Vicente-Carrillo, A.; Johnsson, M.; Wright, D.; Rodriguez-Martinez, H. Conserved gene expression in sperm reservoirs between birds and mammals in response to mating. BMC Genomics 2017, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.; Martinez, E.; Roca, J.; Vazquez, J.; et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McGraw, L.A.; Suarez, S.S.; Wolfner, M.F. On a matter of seminal importance. Bioessays 2015, 37, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Assisted reproductive techniques for cattle breeding in developing countries: A critical appraisal of their value and limitations. Reprod. Domest. Anim. 2012, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
- CHANG, M.C. A detrimental effect of seminal plasma on the fertilizing capacity of sperm. Nature 1957, 179, 258–259. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; da Silva Passarelli, M.; de Freitas, F.V.; Ravagnani, G.M.; Torres, M.A.; Martins, S.M.M.K.; Yeste, M.; de Andrade, A.F.C. Removal of seminal plasma prior to liquid storage of boar spermatozoa: A practice that can improve their fertilizing ability. Theriogenology 2019, 125, 79–86. [Google Scholar] [CrossRef]
- Mogielnicka-Brzozowska, M.; Kordan, W. Characteristics of selected seminal plasma proteins and their application in the improvement of the reproductive processes in mammals. Pol. J. Vet. Sci. 2011, 14, 489–499. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Funahashi, H. Methods for Improving In Vitro and In Vivo Boar Sperm Fertility. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 40–47. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sharkey, D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016, 106, 511–519. [Google Scholar] [CrossRef]
- Rozeboom, K.J.; Troedsson, M.H.; Hodson, H.H.; Shurson, G.C.; Crabo, B.G. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine. J. Anim. Sci. 2000, 78, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Sprecher, D.J.; Ross, P.; Friendship, R.M.; Kirkwood, R.N. Effect of sperm cryopreservation and supplementing semen doses with seminal plasma on the establishment of a sperm reservoir in gilts. Reprod. Domest. Anim. 2007, 42, 149–152. [Google Scholar] [CrossRef]
- Abad, M.; Garcia, J.C.; Sprecher, D.J.; Cassar, G.; Friendship, R.M.; Buhr, M.M.; Kirkwood, R.N. Effect of insemination-ovulation interval and addition of seminal plasma on sow fertility to insemination of cryopreserved sperm. Reprod. Domest. Anim. 2007, 42, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Heise, A.; Kähn, W.; Volkmann, D.H.; Thompson, P.N.; Gerber, D. Influence of seminal plasma on fertility of fresh and frozen-thawed stallion epididymal spermatozoa. Anim. Reprod. Sci. 2010, 118, 48–53. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Domínguez, J.C.; Peña, F.; Alegre, B.; Gonzalez, R.; Castro, M.J.; Habing, G.; Kirkwood, R. Thawing boar semen in the presence of seminal plasma: Effects on sperm quality and fertility. Anim. Reprod. Sci. 2009, 119, 160–165. [Google Scholar] [CrossRef]
- McPherson, F.J.; Nielsen, S.G.; Chenoweth, P.J. Semen effects on insemination outcomes in sows. Anim. Reprod. Sci. 2014, 151, 28–33. [Google Scholar] [CrossRef]
- Chutia, T.; Biswas, R.K.; Tamuli, M.K.; Deka, B.C.; Sinha, S.; Goswami, J.; Banik, S.; Kayastha, R.B. Effect of holding of semen and washing of seminal plasma on quality and fertility of Hampshire boar semen preserved at liquid state. Anim. Reprod. Sci. 2014, 145, 141–149. [Google Scholar] [CrossRef]
- Tsikis, G.; Reynaud, K.; Ferchaud, S.; Druart, X. Seminal plasma differentially alters the resistance of dog, ram and boar spermatozoa to hypotonic stress. Anim. Reprod. Sci. 2018, 193, 1–8. [Google Scholar] [CrossRef]
- Recuero, S.; Fernandez-Fuertes, B.; Bonet, S.; Barranco, I.; Yeste, M. Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa. Theriogenology 2019, 137, 36–42. [Google Scholar] [CrossRef]
- Ortiz, W.G.; Rizo, J.A.; Carvalheira, L.R.; Ahmed, B.M.S.; Estrada-Cortes, E.; Harstine, B.R.; Bromfield, J.J.; Hansen, P.J. Effects of intrauterine infusion of seminal plasma at artificial insemination on fertility of lactating Holstein cows. J. Dairy Sci. 2019, 102, 6587–6594. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Lutwak-Mann, C. Male Reproductive Function and Semen; Mann, T., Lutwak-Mann, C., Eds.; Springer: London, UK, 1981; ISBN 978-1-4471-1302-7. [Google Scholar]
- Rodriguez-Martinez, H.; Saravia, F.; Wallgren, M.; Martinez, E.A.; Sanz, L.; Roca, J.; Vazquez, J.M.; Calvete, J.J. Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J. Reprod. Immunol. 2010, 84, 57–65. [Google Scholar] [CrossRef]
- Wallgren, M.; Saravia, F.; Rodriguez-Martinez, H. The vanguard sperm cohort of the boar ejaculate is overrepresented in the tubal sperm reservoir in vivo. J. Reprod. Dev. 2010, 56, 68–72. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Martinez, C.; Wright, D.; Barranco, I.; Roca, J.; Rodriguez-Martinez, H. The Transcriptome of Pig Spermatozoa, and Its Role in Fertility. Int. J. Mol. Sci. 2020, 21, 1572. [Google Scholar] [CrossRef] [PubMed]
- Atikuzzaman, M.; Mehta Bhai, R.; Fogelholm, J.; Wright, D.; Rodriguez-Martinez, H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Reproduction 2015, 150, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Atikuzzaman, M.; Venhoranta, H.; Wright, D.; Rodriguez-Martinez, H. Expression of immune regulatory genes in the porcine internal genital tract is differentially triggered by spermatozoa and seminal plasma. Int. J. Mol. Sci. 2019, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Sánchez, J.M.; Recuero, S.; Bagés-Arnal, S.; McDonald, M.; Kenny, D.A.; Yeste, M.; Lonergan, P.; Fernandez-Fuertes, B. Effect of Exposure to Seminal Plasma Through Natural Mating in Cattle on Conceptus Length and Gene Expression. Front. Cell Dev. Biol. 2020, 8, 341. [Google Scholar] [CrossRef]
- Atikuzzaman, M.; Sanz, L.; Pla, D.; Alvarez-Rodriguez, M.; Rubér, M.; Wright, D.; Calvete, J.J.; Rodriguez-Martinez, H. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp. Biochem. Physiol. Part D Genomics Proteomics 2017, 21, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Valero, M.L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteomics 2016, 142, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Patiño, C.; Parrilla, I.; Barranco, I.; Vergara-Barberán, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Rodriguez-Martínez, H.; Martínez, E.A.; Roca, J. New In-Depth Analytical Approach of the Porcine Seminal Plasma Proteome Reveals Potential Fertility Biomarkers. J. Proteome Res. 2018, 17, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Patiño, C.; Parrilla, I.; Li, J.; Barranco, I.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. The Proteome of Pig Spermatozoa Is Remodeled During Ejaculation. Mol. Cell. Proteomics 2019, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Kang, S.; Pang, W.-K.; Ryu, D.-Y.; Song, W.-H.; Rahman, M.S.; Park, Y.-J.; Pang, M.-G. Porcine seminal protein-I and II mRNA expression in boar spermatozoa is significantly correlated with fertility. Theriogenology 2019, 138, 31–38. [Google Scholar] [CrossRef]
- Ramm, S.A.; Oliver, P.L.; Ponting, C.P.; Stockley, P.; Emes, R.D. Sexual selection and the adaptive evolution of mammalian ejaculate proteins. Mol. Biol. Evol. 2008, 25, 207–219. [Google Scholar] [CrossRef]
- Bai, R.; Latifi, Z.; Kusama, K.; Nakamura, K.; Shimada, M.; Imakawa, K. Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 2018, 495, 1094–1101. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Parrilla, I.; Álvarez-Barrientos, A.; Pérez-Patiño, C.; Peña, F.J.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 2019, 9, 11584. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Ljunggren, S.A.; Karlsson, H.; Rodriguez-Martinez, H. Exosomes in specific fractions of the boar ejaculate contain CD44: A marker for epididymosomes? Theriogenology 2019, 140, 143–152. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Perez-Patino, C.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Barranco, I.; Roca, J.; Tvarijonaviciute, A.; Rubér, M.; Vicente-Carrillo, A.; Atikuzzaman, M.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H. Measurement of activity and concentration of paraoxonase 1 (PON-1) in seminal plasma and identification of PON-2 in the sperm of boar ejaculates. Mol. Reprod. Dev. 2015, 82, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, I.; Martinez, E.A.; Gil, M.A.; Cuello, C.; Roca, J.; Rodriguez-Martinez, H.; Martinez, C.A. Boar seminal plasma: Current insights on its potential role for assisted reproductive technologies in swine. Anim. Reprod. 2020, 17, e20200022. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef]
- Beyler, S.; Zaneveld, L. The Male Accessory Sex Glands. In Biochemistry of Mammalian Reproduction; Zaneveld, L., Chatterton, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1982; pp. 65–88. [Google Scholar]
- Lombardi, J. Comparative Vertebrate Reproduction; Kluwer Academic Publishers: Boston, MA, USA, 1998; ISBN 0792383362. [Google Scholar]
- LAKE, P.E. The male reproductive tract of the fowl. J. Anat. 1957, 91, 116–129. [Google Scholar] [PubMed]
- Etches, R.J. The Male. In Reproduction in Poultry; Etches, R., Ed.; CAB International: Wallingford, Oxon, 1996; pp. 208–233, ISBN 0851987389 9780851987385. [Google Scholar]
- Lavon, U.; Boursnell, J.C. The split ejaculate of the boar: Contributions of the epididymides and seminal vesicles. J. Reprod. Fertil. 1975, 42, 541–552. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Sperm function in cattle and pigs: Morphological and functional aspects. Arch. Tierzucht 2001, 44, 102–113. [Google Scholar]
- Purvis, K.; Magnus, O.; Mørkås, L.; Abyholm, T.; Rui, H. Ejaculate composition after masturbation and coitus in the human male. Int. J. Androl. 1986, 9, 401–406. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kareskoski, A.M.; del Alamo, M.M.R.; Güvenc, K.; Reilas, T.; Calvete, J.J.; Rodriguez-Martinez, H.; Andersson, M.; Katila, T. Protein composition of seminal plasma in fractionated stallion ejaculates. Reprod. Domest. Anim. 2011, 46, e79–e84. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawano, N.; Yoshiike, M.; Yoshida, M.; Iwamoto, T.; Morisawa, M. Physiological roles of semenogelin I and zinc in sperm motility and semen coagulation on ejaculation in humans. Mol. Hum. Reprod. 2008, 14, 151–156. [Google Scholar] [CrossRef]
- Jonsson, M.; Linse, S.; Frohm, B.; Lundwall, A.; Malm, J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem. J. 2005, 387, 447–453. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H. State of the art in farm animal sperm evaluation. Reprod. Fertil. Dev. 2007, 19, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sobrero, A.J.; Macleod, J. The immediate postcoital test. Fertil. Steril. 1962, 13, 184–189. [Google Scholar] [CrossRef]
- Padilla, L.; Lucas, X.; Parrilla, I.; Perez-Patiño, C.; Rodriguez-Martinez, H.; Roca, J.; Barranco, I. Period of Boar Ejaculate Collection Contributes to the Yearly Intra-Male Variability of Seminal Plasma Cytokines. Biology 2020, 9, 105. [Google Scholar] [CrossRef]
- Claus, R. Physiological role of seminal components in the reproductive tract of the female pig. J. Reprod. Fertil. Suppl. 1990, 40, 117–131. [Google Scholar]
- Rodríguez-Martínez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vázquez, J.M.; Martínez, E.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef]
- Duncan, M.W.; Thompson, H.S. Proteomics of semen and its constituents. Proteomics. Clin. Appl. 2007, 1, 861–875. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Mactier, S.; Kohnke, P.L.; Kershaw-Young, C.M.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteomics 2013, 91, 13–22. [Google Scholar] [CrossRef]
- Marzoni, M.; Castillo, A.; Sagona, S.; Citti, L.; Rocchiccioli, S.; Romboli, I.; Felicioli, A. A proteomic approach to identify seminal plasma proteins in roosters (Gallus gallus domesticus). Anim. Reprod. Sci. 2013, 140, 216–223. [Google Scholar] [CrossRef]
- Labas, V.; Grasseau, I.; Cahier, K.; Gargaros, A.; Harichaux, G.; Teixeira-Gomes, A.-P.; Alves, S.; Bourin, M.; Gérard, N.; Blesbois, E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics 2015, 112, 313–335. [Google Scholar] [CrossRef]
- de Azevedo Viana, A.G.; Ribeiro, I.M.; Carvalho, R.P.R.; Memili, E.; Moura, A.A.; Machado-Neves, M. Functional attributes of seminal proteins in bull fertility: A systematic review. Reproduction 2021, 161, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Westfalewicz, B.; Dietrich, M.A.; Mostek, A.; Partyka, A.; Bielas, W.; Niżański, W.; Ciereszko, A. Analysis of bull (Bos taurus) seminal vesicle fluid proteome in relation to seminal plasma proteome. J. Dairy Sci. 2017, 100, 2282–2298. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Leahy, T.; Rickard, J.P.; Bernecic, N.C.; Druart, X.; de Graaf, S.P. Ram seminal plasma and its functional proteomic assessment. Reproduction 2019, 157, R243–R256. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Perez-Patiño, C.; Barranco, I.; Padilla, L.C.; Martínez, E.A.; Rodriguez-Martinez, H.; Parrilla, I. Proteomics in fresh and preserved pig semen: Recent achievements and future challenges. Theriogenology 2020, 150, 41–47. [Google Scholar] [CrossRef]
- Kelly, V.; Kuy, S.; Palmer, D.; Xu, Z.; Davis, S.; Cooper, G. Characterization of bovine seminal plasma by proteomics. Proteomics 2006, 6, 5826–5833. [Google Scholar] [CrossRef]
- Viana, A.G.A.; Martins, A.M.A.; Pontes, A.H.; Fontes, W.; Castro, M.S.; Ricart, C.A.O.; Sousa, M.V.; Kaya, A.; Topper, E.; Memili, E.; et al. Proteomic landscape of seminal plasma associated with dairy bull fertility. Sci. Rep. 2018, 8, 16323. [Google Scholar] [CrossRef]
- Fu, Q.; Pan, L.; Huang, D.; Wang, Z.; Hou, Z.; Zhang, M. Proteomic profiles of buffalo spermatozoa and seminal plasma. Theriogenology 2019, 134, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Aalberts, M.; Stout, T.A.E.; Stoorvogel, W. Prostasomes: Extracellular vesicles from the prostate. Reproduction 2014, 147, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.; Sánchez-Martín, P.; Gosálvez, J.; Crespo, F. Equivalent seminal characteristics in human and stallion at first and second ejaculated fractions. Andrologia 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.K. Analysis of stallion semen and its relation to fertility. Vet. Clin. North Am. Equine Pract. 1996, 12, 119–130. [Google Scholar] [CrossRef]
- Ekhlasi-Hundrieser, M.; Schäfer, B.; Kirchhoff, C.; Hess, O.; Bellair, S.; Müller, P.; Töpfer-Petersen, E. Structural and molecular characterization of equine sperm-binding fibronectin-II module proteins. Mol. Reprod. Dev. 2005, 70, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hamann, H.; Jude, R.; Sieme, H.; Mertens, U.; Töpfer-Petersen, E.; Distl, O.; Leeb, T. A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses. Anim. Genet. 2007, 38, 259–264. [Google Scholar] [CrossRef]
- Kareskoski, A.M.; Palviainen, M.; Johannisson, A.; Katila, T. Upregulation of CRISP-3 and kallikrein in stallion seminal plasma is associated with poor tolerance of cooled storage. Reprod. Domest. Anim. 2020, 55, 496–502. [Google Scholar] [CrossRef]
- Bubenickova, F.; Postlerova, P.; Simonik, O.; Sirohi, J.; Sichtar, J. Effect of Seminal Plasma Protein Fractions on Stallion Sperm Cryopreservation. Int. J. Mol. Sci. 2020, 21, 6415. [Google Scholar] [CrossRef]
- Walters, E.M.; Agca, Y.; Ganjam, V.; Evans, T. Animal models got you puzzled?: Think pig. Ann. N. Y. Acad. Sci. 2011, 1245, 63–64. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E.; Romero, A.; Varela, P.F.; Ekhlasi-Hundrieser, M.; Dostàlovà, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia 1998, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Vázquez, J.M.; García, E.M.; Roca, J.; Martínez, E.A.; Calvete, J.J.; Sanz, L.; Ekwall, H.; Rodríguez-Martínez, H. Immunolocalization and possible functional role of PSP-I/PSP-II heterodimer in highly extended boar spermatozoa. J. Androl. 2006, 27, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Parrilla, I.; Almiñana, C.; del Olmo, D.; Roca, J.; Martínez, E.A.; Vázquez, J.M. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod. Domest. Anim. 2012, 47 (Suppl. 3), 12–21. [Google Scholar] [CrossRef]
- Bromfield, J.J. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction 2016, 152, R223–R232. [Google Scholar] [CrossRef]
- Calvete, J.J.; Ensslin, M.; Mburu, J.; Iborra, A.; Martínez, P.; Adermann, K.; Waberski, D.; Sanz, L.; Töpfer-Petersen, E.; Weitze, K.F.; et al. Monoclonal antibodies against boar sperm zona pellucida-binding protein AWN-1. Characterization of a continuous antigenic determinant and immunolocalization of AWN epitopes in inseminated sows. Biol. Reprod. 1997, 57, 735–742. [Google Scholar] [CrossRef]
- Rodríguez-Martinez, H.; Iborra, A.; Martínez, P.; Calvete, J.J. Immunoelectronmicroscopic imaging of spermadhesin AWN epitopes on boar spermatozoa bound in vivo to the zona pellucida. Reprod. Fertil. Dev. 1998, 10, 491–497. [Google Scholar] [CrossRef]
- Calvete, J.; Sanz, L.; Garcia, E.M.; Caballero, I.; Parrilla, I.; Martinez, E.; Roca, J.; Vazquez, J.; Saravia, F.; Wallgren, M.; et al. On the biological function of boar spermadhesin PSP-I/PSP-II. Reprod Domest Anim 2005, 40, 331. [Google Scholar]
- Waberski, D.; Claassen, R.; Hahn, T.; Jungblut, P.W.; Parvizi, N.; Kallweit, E.; Weitze, K.F. LH profile and advancement of ovulation after transcervical infusion of seminal plasma at different stages of oestrus in gilts. J. Reprod. Fertil. 1997, 109, 29–34. [Google Scholar] [CrossRef]
- Parrilla, I.; Martinez, C.A.; Cambra, J.M.; Lucas, X.; Ferreira-Dias, G.; Rodriguez-Martinez, H.; Cuello, C.; Gil, M.A.; Martinez, E.A. Blastocyst-Bearing Sows Display a Dominant Anti-Inflammatory Cytokine Profile Compared to Cyclic Sows at Day 6 of the Cycle. Animals 2020, 10, 2028. [Google Scholar] [CrossRef]
- Novak, S.; Ruiz-Sánchez, A.; Dixon, W.T.; Foxcroft, G.R.; Dyck, M.K. Seminal plasma proteins as potential markers of relative fertility in boars. J. Androl. 2010, 31, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Alkmin, D.V.; Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Boar sperm cryosurvival is better after exposure to seminal plasma from selected fractions than to those from entire ejaculate. Cryobiology 2014, 69, 203–210. [Google Scholar] [CrossRef]
- Mills, K.M.; Aryal, U.K.; Sobreira, T.; Minton, A.M.; Casey, T.; Stewart, K.R. Shotgun proteome analysis of seminal plasma differentiate boars by reproductive performance. Theriogenology 2020, 157, 130–139. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, Y.; Guo, C.; Li, C.; Wei, H.; Li, L.; Meng, L.; Zhang, S. Analysis of differentially abundant proteins related to boar fertility in seminal plasma using iTRAQ-based quantitative proteomics. J. Proteomics 2021, 236, 104120. [Google Scholar] [CrossRef] [PubMed]
- De Lazari, F.L.; Sontag, E.R.; Schneider, A.; Araripe Moura, A.A.; Vasconcelos, F.R.; Nagano, C.S.; Dalberto, P.F.; Bizarro, C.V.; Mattos, R.C.; Mascarenhas Jobim, M.I.; et al. Proteomic identification of boar seminal plasma proteins related to sperm resistance to cooling at 17 °C. Theriogenology 2020, 147, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Höfner, L.; Luther, A.-M.; Palladini, A.; Fröhlich, T.; Waberski, D. Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach. Int. J. Mol. Sci. 2020, 21, 6474. [Google Scholar] [CrossRef]
- Luongo, C.; Abril-Sánchez, S.; Hernández, J.G.; García-Vázquez, F.A. Seminal plasma mitigates the adverse effect of uterine fluid on boar spermatozoa. Theriogenology 2019, 136, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Robertson, S.A. Seminal fluid and immune adaptation for pregnancy—Comparative biology in mammalian species. Reprod. Domest. Anim. 2014, 49, 27–36. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- Ben Chehida, Y.; Denis, B.; Claisse, G.; Joly, D. [What the study of seminal fluid proteins in Drosophila tells us about the evolution of reproduction]. Med. Sci. (Paris) 2014, 30, 651–657. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Martinez, H.; Tienthai, P.; Atikuzzaman, M.; Vicente-Carrillo, A.; Rubér, M.; Alvarez-Rodriguez, M. The ubiquitous hyaluronan: Functionally implicated in the oviduct? Theriogenology 2016, 86, 182–186. [Google Scholar] [CrossRef]
- Barranco, I.; Ruber, M.; Perez-Patino, C.; Atikuzzaman, M.; Martinez, E.A.; Roca, J.; Rodriguez-Martinez, H. The Seminal Plasma of the Boar is Rich in Cytokines, with Significant Individual and Intra-Ejaculate Variation. Am. J. Reprod. Immunol. 2015, 74, 523–532. [Google Scholar] [CrossRef]
- O’Leary, S.; Armstrong, D.T.; Robertson, S.A. Transforming growth factor-β (TGFβ) in porcine seminal plasma. Reprod. Fertil. Dev. 2011, 23, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Padilla, L.; Martínez-Hernández, J.; Barranco, I.; Lucas, X.; Pastor, L.M.; Rodriguez-Martínez, H.; Roca, J.; Parrilla, I. Granulocyte-macrophage colony stimulating factor (GM-CSF) is fully expressed in the genital tract, seminal plasma and spermatozoa of male pigs. Sci. Rep. 2020, 10, 13360. [Google Scholar] [CrossRef] [PubMed]
- Padilla, L.; Barranco, I.; Parrilla, I.; Lucas, X.; Rodriguez-Martinez, H.; Roca, J. Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage. Biology 2020, 9, 276. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015, 108, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 2007, 13, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Ingman, W.V.; O’Leary, S.; Sharkey, D.J.; Tremellen, K.P. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J. Reprod. Immunol. 2002, 57, 109–128. [Google Scholar] [CrossRef]
- Robertson, S.A. Seminal fluid signaling in the female reproductive tract: Lessons from rodents and pigs. J. Anim. Sci. 2007, 85, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jalali, B.M.; Kitewska, A.; Wasielak, M.; Bodek, G.; Bogacki, M. Effects of seminal plasma and the presence of a conceptus on regulation of lymphocyte-cytokine network in porcine endometrium. Mol. Reprod. Dev. 2014, 81, 270–281. [Google Scholar] [CrossRef]
- Waberski, D.; Schäfer, J.; Bölling, A.; Scheld, M.; Henning, H.; Hambruch, N.; Schuberth, H.J.; Pfarrer, C.; Wrenzycki, C.; Hunter, R.H.F. Seminal plasma modulates the immune-cytokine network in the porcine uterine tissue and pre-ovulatory follicles. PLoS ONE 2018, 13, 1–21. [Google Scholar] [CrossRef]
- Martinez, C.A.; Ruber, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules 2020, 10, 554. [Google Scholar] [CrossRef]
- Nongbua, T.; Guo, Y.; Ntallaris, T.; Rubèr, M.; Rodriguez-Martinez, H.; Humblot, P.; Morrell, J. Bull seminal plasma stimulates in vitro production of TGF-β, IL-6 and IL-8 from bovine endometrial epithelial cells, depending on dose and bull fertility. J. Reprod. Immunol. 2020, 142, 103179. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Sharkey, D.J. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001, 13, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Immune regulation of conception and embryo implantation-all about quality control? J. Reprod. Immunol. 2010, 85, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Cambra, J.M.; Lucas, X.; Ferreira-Dias, G.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A.; Cuello, C.; Parrilla, I. Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development. Biology 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.-H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Estienne, A.; Brossaud, A.; Reverchon, M.; Ramé, C.; Froment, P.; Dupont, J. Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. Int. J. Mol. Sci. 2020, 21, 3581. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, Y.; Bastard, J.-P.; McAvoy, C.; Fellahi, S.; Dupont, J.; Levy, R. Adipokines in Semen: Physiopathology and Effects on Spermatozoas. Int. J. Endocrinol. 2018, 2018, 3906490. [Google Scholar] [CrossRef]
- Thomas, S.; Kratzsch, D.; Schaab, M.; Scholz, M.; Grunewald, S.; Thiery, J.; Paasch, U.; Kratzsch, J. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil. Steril. 2013, 99, 1256–1263. [Google Scholar] [CrossRef]
- Lackey, B.R.; Gray, S.L.; Henricks, D.M. Measurement of leptin and insulin-like growth factor-I in seminal plasma from different species. Physiol. Res. 2002, 51, 309–311. [Google Scholar]
- Jope, T.; Lammert, A.; Kratzsch, J.; Paasch, U.; Glander, H.-J. Leptin and leptin receptor in human seminal plasma and in human spermatozoa. Int. J. Androl. 2003, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Bongrani, A.; Elfassy, Y.; Brun, J.S.; Ramé, C.; Mellouk, N.; Fellahi, S.; Bastard, J.P.; Levy, R.; Vasseur, C.; Froment, P.; et al. Expression of adipokines in seminal fluid of men of normal weight. Asian J. Androl. 2019, 21, 528–530. [Google Scholar] [PubMed]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015, 5, 18538. [Google Scholar] [CrossRef] [PubMed]
- Barrier-Battut, I.; Dacheux, J.L.; Gatti, J.L.; Rouviere, P.; Stanciu, C.; Dacheux, F.; Vidament, M. Seminal plasma proteins and semen characteristics in relation with fertility in the stallion. Anim. Reprod. Sci. 2005, 89, 255–258. [Google Scholar]
- Novak, S.; Smith, T.A.; Paradis, F.; Burwash, L.; Dyck, M.K.; Foxcroft, G.R.; Dixon, W.T. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology 2010, 74, 956–967. [Google Scholar] [CrossRef]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Alkmin, D.V.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. The activity of paraoxonase type 1 (PON-1) in boar seminal plasma and its relationship with sperm quality, functionality, and in vivo fertility. Andrology 2015, 3, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sopková, D.; Andrejcakova, Z.; Vlckova, R.; Danišová, O.; Supuka, P.; Ondrasovicova, S.; Petrilla, V. Lactate dehydrogenase as a possible indicator of reproductive capacity of boars. Indian J. Anim. Sci. 2015, 85, 143–147. [Google Scholar]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef]
- Tamessar, C.T.; Trigg, N.A.; Nixon, B.; Skerrett-Byrne, D.A.; Sharkey, D.J.; Robertson, S.A.; Bromfield, E.G.; Schjenken, J.E. Roles of male reproductive tract extracellular vesicles in reproduction. Am. J. Reprod. Immunol. 2021, 85, e13338. [Google Scholar] [CrossRef]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef]
- Belleannée, C.; Calvo, É.; Caballero, J.; Sullivan, R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 2013, 89, 30. [Google Scholar] [CrossRef]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832–58847. [Google Scholar] [CrossRef] [PubMed]
- Skalnikova, H.K.; Bohuslavova, B.; Turnovcova, K.; Juhasova, J.; Juhas, S.; Rodinova, M.; Vodicka, P. Isolation and Characterization of Small Extracellular Vesicles from Porcine Blood Plasma, Cerebrospinal Fluid, and Seminal Plasma. Proteomes 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G.; et al. Expression Pattern of Seminal Plasma Extracellular Vesicle Small RNAs in Boar Semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef] [PubMed]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Piehl, L.L.; Fischman, M.L.; Hellman, U.; Cisale, H.; Miranda, P.V. Boar seminal plasma exosomes: Effect on sperm function and protein identification by sequencing. Theriogenology 2013, 79, 1071–1082. [Google Scholar] [CrossRef]
- Piehl, L.L.; Cisale, H.; Torres, N.; Capani, F.; Sterin-Speziale, N.; Hager, A. Biochemical characterization and membrane fluidity of membranous vesicles isolated from boar seminal plasma. Anim. Reprod. Sci. 2006, 92, 401–410. [Google Scholar] [CrossRef]
- Siciliano, L.; Marcianò, V.; Carpino, A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Q.; Zhang, L.; Xiang, W. Extracellular Vesicles: Recent Developments in Aging and Reproductive Diseases. Front. Cell Dev. Biol. 2020, 8, 577084. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H. Aspects of the electrolytic composition of boar epididymal fluid with reference to sperm maturation and storage. Reprod. Domest. Anim. 1991, 13–27. [Google Scholar]
- Amann, R.P.; Hammerstedt, R.H.; Veeramachaneni, D.N. The epididymis and sperm maturation: A perspective. Reprod. Fertil. Dev. 1993, 5, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; De Iuliis, G.N.; Dun, M.D.; Nixon, B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. (Lausanne) 2018, 9, 59. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Ekstedt, E.; Einarsson, S. Acidification of epididymal fluid in the boar. Int. J. Androl. 1990, 13, 238–243. [Google Scholar] [CrossRef]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and prostasomes: Their roles in posttesticular maturation of the sperm cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B.; et al. Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation. Mol. Cell. Proteomics 2019, 18, S91–S108. [Google Scholar] [CrossRef]
- Légaré, C.; Akintayo, A.; Blondin, P.; Calvo, E.; Sullivan, R. Impact of male fertility status on the transcriptome of the bovine epididymis. Mol. Hum. Reprod. 2017, 23, 355–369. [Google Scholar] [CrossRef]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Vicente-Carrillo, A.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione Peroxidase 5 Is Expressed by the Entire Pig Male Genital Tract and Once in the Seminal Plasma Contributes to Sperm Survival and In Vivo Fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef]
- Barranco, I.; Perez-Patiño, C.; Tvarijonaviciute, A.; Parrilla, I.; Vicente-Carrillo, A.; Alvarez-Rodriguez, M.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Active paraoxonase 1 is synthesised throughout the internal boar genital organs. Reproduction 2017, 154, 237–243. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef]
- Ramos Angrimani, D.S.; Nichi, M.; Losano, J.D.A.; Lucio, C.F.; Lima Veiga, G.A.; Franco, M.V.M.J.; Vannucchi, C.I. Fatty acid content in epididymal fluid and spermatozoa during sperm maturation in dogs. J. Anim. Sci. Biotechnol. 2017, 8, 18. [Google Scholar] [CrossRef]
- Zhou, W.; Stanger, S.J.; Anderson, A.L.; Bernstein, I.R.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Dun, M.D.; Nixon, B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019, 17, 35. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell 2018, 46, 481–494. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Martin-DeLeon, P.A. Epididymosomes: Transfer of fertility-modulating proteins to the sperm surface. Asian J. Androl. 2015, 17, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Saewu, A.; Kadunganattil, S.; Raghupathy, R.; Kongmanas, K.; Diaz-Astudillo, P.; Hermo, L.; Tanphaichitr, N. Clusterin in the mouse epididymis: Possible roles in sperm maturation and capacitation. Reproduction 2017, 154, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.S.; Hu, J.; Chapple, D.G.; O’Bryan, M.K. The functions of CAP superfamily proteins in mammalian fertility and disease. Hum. Reprod. Update 2020, 26, 689–723. [Google Scholar] [CrossRef]
- Sutovsky, P.; Moreno, R.; Ramalho-Santos, J.; Dominko, T.; Thompson, W.E.; Schatten, G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001, 114, 1665–1675. [Google Scholar] [PubMed]
- D’Amours, O.; Frenette, G.; Bordeleau, L.-J.; Allard, N.; Leclerc, P.; Blondin, P.; Sullivan, R. Epididymosomes Transfer Epididymal Sperm Binding Protein 1 (ELSPBP1) to Dead Spermatozoa During Epididymal Transit in Bovine1. Biol. Reprod. 2012, 87. [Google Scholar] [CrossRef] [PubMed]
- Girouard, J.; Frenette, G.; Sullivan, R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int. J. Androl. 2011, 34, e475–e486. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Ntzouni, M.; Wright, D.; Khan, K.I.; López-Béjar, M.; Martinez, C.A.; Rodriguez-Martinez, H. Chicken seminal fluid lacks CD9- and CD44-bearing extracellular vesicles. Reprod. Domest. Anim. 2020, 55, 293–300. [Google Scholar] [CrossRef]
- Cordeiro, L.; Lin, H.-L.H.; Vitorino Carvalho, A.; Grasseau, I.; Uzbekov, R.; Besbois, E. First insights on seminal extracellular vesicles in chickens of contrasted fertility. Reproduction 2021. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Martinez, C.A.; Wright, D.; Rodríguez-Martinez, H. The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract. Sci. Rep. 2020, 10, 5061. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cambra, J.M.; Gil, M.A.; Parrilla, I.; Alvarez-Rodriguez, M.; Rodriguez-Martinez, H.; Cuello, C.; Martinez, E.A. Seminal Plasma Induces Overexpression of Genes Associated with Embryo Development and Implantation in Day-6 Porcine Blastocysts. Int. J. Mol. Sci. 2020, 21, 3662. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Lipshultz, L.I. Rapid progression in our understanding of extracellular vesicles and male infertility. Fertil. Steril. 2019, 111, 881–882. [Google Scholar] [CrossRef]
- Leahy, T.; Rickard, J.P.; Pini, T.; Gadella, B.M.; de Graaf, S.P. Quantitative Proteomic Analysis of Seminal Plasma, Sperm Membrane Proteins, and Seminal Extracellular Vesicles Suggests Vesicular Mechanisms Aid in the Removal and Addition of Proteins to the Ram Sperm Membrane. Proteomics 2020, 20, e1900289. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; Sostaric, E.; Wubbolts, R.; Wauben, M.W.M.; Nolte-’t Hoen, E.N.M.; Gadella, B.M.; Stout, T.A.E.; Stoorvogel, W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta 2013, 1834, 2326–2335. [Google Scholar] [CrossRef]
- Vilanova-Perez, T.; Jones, C.; Balint, S.; Dragovic, R.; L Dustin, M.; Yeste, M.; Coward, K. Exosomes derived from HEK293T cells interact in an efficient and noninvasive manner with mammalian sperm in vitro. Nanomedicine (Lond) 2020, 15, 1965–1980. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Tremellen, K.P.; Jasper, M.J.; Gemzell-Danielsson, K.; Robertson, S.A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J. Immunol. 2012, 188, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Martinez, C.A.; Alvarez-Rodriguez, M.; Parrilla, I.; Lucas, X.; Ferreira-Dias, G.; Yeste, M.; Rodriguez-Martinez, H.; Roca, J. Seminal Plasma Modulates miRNA Expression by Sow Genital Tract Lining Explants. Biomolecules 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G.; Yeste, M. The role of miRNAs in male human reproduction: A systematic review. Andrology 2020, 8, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Jodar, M. Sperm and seminal plasma RNAs: What roles do they play beyond fertilization? Reproduction 2019, 158, R113–R123. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Lei, A.; Zhang, H.; Niu, H.; Li, X.; Zhang, P.; Liao, M.; Lv, Y.; Zhu, Z.; et al. Early cleavage of preimplantation embryos is regulated by tRNA(Gln-TTG)-derived small RNAs present in mature spermatozoa. J. Biol. Chem. 2020, 295, 10885–10900. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Q.; Zheng, Y.; Liu, Z.; Meng, X.; Zeng, W.; Lu, H. Human sperm tsRNA as potential biomarker and therapy target for male fertility. Reproduction 2021, 161, 111–122. [Google Scholar] [CrossRef]
- Berezikov, E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. 2011, 12, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hua, J.; Wang, L.; Xu, B.; Zhang, H.; Ye, N.; Zhang, Z.; Yu, D.; Cooke, H.J.; Zhang, Y.; et al. MicroRNA and piRNA profiles in normal human testis detected by next generation sequencing. PLoS ONE 2013, 8, e66809. [Google Scholar] [CrossRef] [PubMed]
- Chioccarelli, T.; Manfrevola, F.; Ferraro, B.; Sellitto, C.; Cobellis, G.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Expression Patterns of Circular RNAs in High Quality and Poor Quality Human Spermatozoa. Front. Endocrinol. (Lausanne) 2019, 10, 435. [Google Scholar] [CrossRef]
- Gòdia, M.; Castelló, A.; Rocco, M.; Cabrera, B.; Rodríguez-Gil, J.E.; Balasch, S.; Lewis, C.; Sánchez, A.; Clop, A. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility. Sci. Rep. 2020, 10, 7985. [Google Scholar] [CrossRef]
- Gòdia, M.; Estill, M.; Castelló, A.; Balasch, S.; Rodríguez-Gil, J.E.; Krawetz, S.A.; Sánchez, A.; Clop, A. A RNA-Seq Analysis to Describe the Boar Sperm Transcriptome and Its Seasonal Changes. Front. Genet. 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Pértille, F.; Alvarez-Rodriguez, M.; da Silva, A.N.; Barranco, I.; Roca, J.; Guerrero-Bosagna, C.; Rodriguez-Martinez, H. Sperm Methylome Profiling Can Discern Fertility Levels in the Porcine Biomedical Model. Int. J. Mol. Sci. 2021, 22, 2679. [Google Scholar] [CrossRef]
- Krausz, C.; Escamilla, A.R.; Chianese, C. Genetics of male infertility: From research to clinic. Reproduction 2015, 150, R159–R174. [Google Scholar] [CrossRef]
- Fagerlind, M.; Stålhammar, H.; Olsson, B.; Klinga-Levan, K. Expression of miRNAs in Bull Spermatozoa Correlates with Fertility Rates. Reprod. Domest. Anim. 2015, 50, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Swanson, G.; Krawetz, S.A. A history of why fathers’ RNA matters. Biol. Reprod. 2018, 99, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodriguez, J.M.; Ortega-Ferrusola, C.; Gil, M.C.; Martín-Cano, F.E.; Gaitskell-Phillips, G.; Rodríguez-Martínez, H.; Hinrichs, K.; Álvarez-Barrientos, A.; Román, Á.; Peña, F.J. Transcriptome analysis reveals that fertilization with cryopreserved sperm downregulates genes relevant for early embryo development in the horse. PLoS ONE 2019, 14, e0213420. [Google Scholar] [CrossRef]
- Roa-Espitia, A.L.; Hernández-Rendón, E.R.; Baltiérrez-Hoyos, R.; Muñoz-Gotera, R.J.; Cote-Vélez, A.; Jiménez, I.; González-Márquez, H.; Hernández-González, E.O. Focal adhesion kinase is required for actin polymerization and remodeling of the cytoskeleton during sperm capacitation. Biol. Open 2016, 5, 1189–1199. [Google Scholar] [CrossRef]
- Gòdia, M.; Reverter, A.; González-Prendes, R.; Ramayo-Caldas, Y.; Castelló, A.; Rodríguez-Gil, J.-E.; Sánchez, A.; Clop, A. A systems biology framework integrating GWAS and RNA-seq to shed light on the molecular basis of sperm quality in swine. Genet. Sel. Evol. 2020, 52, 72. [Google Scholar] [CrossRef]
- Roca, J.; Broekhuijse, M.L.W.J.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar Differences In Artificial Insemination Outcomes: Can They Be Minimized? Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 48–55. [Google Scholar] [CrossRef]
- Recuero, S.; Sánchez, J.M.; Mateo-Otero, Y.; Bagés-Arnal, S.; McDonald, M.; Behura, S.K.; Spencer, T.E.; Kenny, D.A.; Yeste, M.; Lonergan, P.; et al. Mating to Intact, but Not Vasectomized, Males Elicits Changes in the Endometrial Transcriptome: Insights From the Bovine Model. Front. Cell Dev. Biol. 2020, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Martinez, C.A.; Wright, D.; Rodriguez-Martinez, H. Does the Act of Copulation per se, without Considering Seminal Deposition, Change the Expression of Genes in the Porcine Female Genital Tract? Int. J. Mol. Sci. 2020, 21, 5477. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J.; Palsson-McDermott, E.; Hennessy, E.J.; Martin, C.; O’Leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’Neill, L.A.J. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Liu, W.; Wang, X.; Chen, Y.; Du, Q.; Zhao, X.; Zhang, H.; Liu, S.-L.; Tong, D.; Huang, Y. Brucella Omp25 Upregulates miR-155, miR-21-5p, and miR-23b to Inhibit Interleukin-12 Production via Modulation of Programmed Death-1 Signaling in Human Monocyte/Macrophages. Front. Immunol. 2017, 8, 708. [Google Scholar] [CrossRef]
- Marzano, G.; Chiriacò, M.S.; Primiceri, E.; Dell’Aquila, M.E.; Ramalho-Santos, J.; Zara, V.; Ferramosca, A.; Maruccio, G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2020, 40, 107498. [Google Scholar] [CrossRef]
- Morgan, H.; Eid, N.; Khoshkerdar, A.; Watkins, A. Defining the male contribution to embryo quality and offspring health in assisted reproduction in farm animals. Anim. Reprod. 2020, 17. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. ART in Europe, 2015: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoz038. [Google Scholar] [CrossRef]
- Crawford, G.; Ray, A.; Gudi, A.; Shah, A.; Homburg, R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: Review of the literature and meta-analysis. Hum. Reprod. Update 2015, 21, 275–284. [Google Scholar] [CrossRef]
- Ata, B.; Abou-Setta, A.M.; Seyhan, A.; Buckett, W. Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer). Cochrane database Syst. Rev. 2018, 2, CD011809. [Google Scholar] [CrossRef] [PubMed]
- Kanannejad, Z.; Namavar Jahromi, B.; Gharesi-Fard, B. T Cell Subsets Profiling in Unexplained Infertile Women with Successful and Unsuccessful in Vitro Fertilization Outcome: Focus on the Effect of Seminal Plasma. Iran. J. Allergy. Asthma. Immunol. 2019, 18, 163–172. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Roca, J.; Ferreira-Dias, G.; Pallares, F.J.; Lucas, X.; Vazquez, J.M.; Martinez, E.A.; Gil, M.A.; et al. Seminal Plasma Modifies the Transcriptional Pattern of the Endometrium and Advances Embryo Development in Pigs. Front. Vet. Sci. 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Mahé, C.; Reynaud, K.; Tsikis, G.; Mermillod, P.; Druart, X. Sperm interactions with the female reproductive tract: A key for successful fertilization in mammals. Mol. Cell. Endocrinol. 2020, 516, 110956. [Google Scholar] [CrossRef]
- Hernández-Silva, G.; Chirinos, M. Proteins from male and female reproductive tracts involved in sperm function regulation. Zygote 2019, 27, 5–16. [Google Scholar] [CrossRef] [PubMed]
- De Lazari, F.L.; Sontag, E.R.; Schneider, A.; Moura, A.A.A.; Vasconcelos, F.R.; Nagano, C.S.; Mattos, R.C.; Jobim, M.I.M.; Bustamante-Filho, I.C. Seminal plasma proteins and their relationship with sperm motility and morphology in boars. Andrologia 2019, 51, e13222. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; Recuero, S.; Chaves, B.R.; Garcia-Bonavila, E.; Llavanera, M.; Pinart, E.; Bonet, S.; De Andrade, A.F.C.; Yeste, M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. Int. J. Mol. Sci. 2020, 21, 4520. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Jasper, M.J.; Warnes, G.M.; Armstrong, D.T.; Robertson, S.A. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction 2004, 128, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Robertson, S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef]
- Rhodes, M.; Brendemuhl, J.H.; Hansen, P.J. Litter characteristics of gilts artificially inseminated with transforming growth factor-beta. Am. J. Reprod. Immunol. 2006, 56, 153–156. [Google Scholar] [CrossRef]
- Foxcroft, G.R.; Dyck, M.K.; Ruiz-Sanchez, A.; Novak, S.; Dixon, W.T. Identifying useable semen. Theriogenology 2008, 70, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Memili, E. Functional aspects of seminal plasma and sperm proteins and their potential as molecular markers of fertility. Anim. Reprod. 2016, 13, 191–199. [Google Scholar] [CrossRef]
- Gross, N.; Peñagaricano, F.; Khatib, H. Integration of whole-genome DNA methylation data with RNA sequencing data to identify markers for bull fertility. Anim. Genet. 2020, 51, 502–510. [Google Scholar] [CrossRef]
- Dyck, M.K.; Foxcroft, G.R.; Novak, S.; Ruiz-Sanchez, A.; Patterson, J.; Dixon, W.T. Biological markers of boar fertility. Reprod. Domest. Anim. 2011, 46 (Suppl. 2), 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, W.; Ouyang, S.; Yuan, S. The Vehicle Determines the Destination: The Significance of Seminal Plasma Factors for Male Fertility. Int. J. Mol. Sci. 2020, 21, 8499. [Google Scholar] [CrossRef]
- Otasevic, V.; Kalezic, A.; Macanovic, B.; Jankovic, A.; Stancic, A.; Garalejic, E.; Korac, A.; Korac, B. Evaluation of the antioxidative enzymes in the seminal plasma of infertile men: Contribution to classic semen quality analysis. Syst. Biol. Reprod. Med. 2019, 65, 343–349. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, N.K. Emerging role of Novel Seminal Plasma Bio-markers in Male Infertility: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 170–179. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, N.K. An Insight into Novel Sperm Cell Proteins as Bio-markers for Male Infertility: A Review. Curr. Mol. Med. 2021. [Google Scholar] [CrossRef]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arangasamy, A.; Krawetz, S.A. Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 2019, 86, 1485–1504. [Google Scholar] [CrossRef]
- Hashemi, M.-S.; Mozdarani, H.; Ghaedi, K.; Nasr-Esfahani, M.H. Could analysis of testis-specific genes, as biomarkers in seminal plasma, predict presence of focal spermatogenesis in non-obstructive azoospermia? Andrologia 2020, 52, e13483. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Fernandez-Fuertes, B.; Padilla, L.; Delgado-Bermúdez, A.; Tvarijonaviciute, A.; Yeste, M. Seminal Plasma Anti-Müllerian Hormone: A Potential AI-Boar Fertility Biomarker? Biology 2020, 9, 78. [Google Scholar] [CrossRef]
- Thelie, A.; Réhault-Godbert, S.; Poirier, J.-C.; Fouchécourt, S.; Blesbois, E. The seminal acrosin-inhibitor ClTI1/SPINK2 is a fertility-associated marker in the chicken. Mol. Reprod. Dev. 2019, 86. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kratz, E.M. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker? Mol. Reprod. Dev. 2020, 87, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Cunha Bustamante-Filho, I.; Renato Menegassi, S.; Ribas Pereira, G.; Dias Salton, G.; Mosena Munari, F.; Roberto Schneider, M.; Costa Mattos, R.; Otávio Jardim Barcellos, J.; Pereira Laurino, J.; Obino Cirne-Lima, E.; et al. Bovine seminal plasma osteopontin: Structural modelling, recombinant expression and its relationship with semen quality. Andrologia 2021, 53, e13905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, F.; Dai, L. Cyclooxygenase 1 (COX1) as an indicator of sperm quality in humans. Andrologia 2020, 52, e13537. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Padilla, L.; Romeu, X.; Roca, J.; Barranco, I.; Yeste, M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs-Relationship with Sperm Morphology. Antioxidants 2020, 9, 741. [Google Scholar] [CrossRef]
- Mehrparvar, B.; Chashmniam, S.; Nobakht, F.; Amini, M.; Javidi, A.; Minai-Tehrani, A.; Arjmand, B.; Gilany, K. Metabolic profiling of seminal plasma from teratozoospermia patients. J. Pharm. Biomed. Anal. 2020, 178, 112903. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Fernández-López, P.; Gil-Caballero, S.; Fernandez-Fuertes, B.; Bonet, S.; Barranco, I.; Yeste, M. (1)H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites. Biomolecules 2020, 10, 906. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Liu, Y.; Liang, H.-L.; Xu, Q.-Q.; Liu, Z.-H.; Weng, X.-G. Metabolomic differences of seminal plasma between boars with high and low average conception rates after artificial insemination. Reprod. Domest. Anim. 2021, 56, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Sharma, R.; Samanta, L.; Gupta, S.; Dias, T.R.; Martins, A.D. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. World J. Mens. Health 2019. [Google Scholar] [CrossRef]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Corral-Vazquez, C.; Salas-Huetos, A.; Blanco, J.; Vidal, F.; Sarrate, Z.; Anton, E. Sperm microRNA pairs: New perspectives in the search for male fertility biomarkers. Fertil. Steril. 2019, 112, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Yang, Y.; Liu, X.; Chen, Y. Seminal plasma miR-210-3p is a biomarker for screening dyszoospermia caused by varicocele. Andrologia 2019, 51, e13244. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Dieckmann, K.-P.; Grobelny, F.; Salzbrunn, A.; Oing, C.; Schulze, W.; Belge, G. Expression of miRNA-371a-3p in seminal plasma and ejaculate is associated with sperm concentration. Andrology 2019, 7, 469–474. [Google Scholar] [CrossRef]
- Oliphant, G.; Reynolds, A.B.; Thomas, T.S. Sperm surface components involved in the control of the acrosome reaction. Am. J. Anat. 1985, 174, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R. The “switching on” of mammalian spermatozoa: Molecular events involved in promotion and regulation of capacitation. Mol. Reprod. Dev. 2010, 77, 197–208. [Google Scholar] [CrossRef]
- Way, A.L.; Griel, L.C.J.; Killian, G.J. Effects of accessory sex gland fluid on viability, capacitation, and the acrosome reaction of cauda epididymal bull spermatozoa. J. Androl. 2000, 21, 213–219. [Google Scholar]
- Manjunath, P.; Thérien, I. Role of seminal plasma phospholipid-binding proteins in sperm membrane lipid modification that occurs during capacitation. J. Reprod. Immunol. 2002, 53, 109–119. [Google Scholar] [CrossRef]
- Bergeron, A.; Crête, M.-H.; Brindle, Y.; Manjunath, P. Low-density lipoprotein fraction from hen’s egg yolk decreases the binding of the major proteins of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biol. Reprod. 2004, 70, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Brinsko, S.P.; Crockett, E.C.; Squires, E.L. Effect of centrifugation and partial removal of seminal plasma on equine spermatozoal motility after cooling and storage. Theriogenology 2000, 54, 129–136. [Google Scholar] [CrossRef]
- Maxwell, W.M.; Welch, G.R.; Johnson, L.A. Viability and membrane integrity of spermatozoa after dilution and flow cytometric sorting in the presence or absence of seminal plasma. Reprod. Fertil. Dev. 1996, 8, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Semen plasma proteins prevent cold-shock membrane damage to ram spermatozoa. Theriogenology 2001, 56, 425–434. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; Mayor, G.M.; Almiñana, C.; Calvete, J.J.; Sanz, L.; Roca, J.; Martinez, E.A. PSP-I/PSP-II spermadhesin exert a decapacitation effect on highly extended boar spermatozoa. Int. J. Androl. 2009, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Thomas, C.A.; Gravance, C.G.; Marshall, C.E.; DeJarnette, J.M.; Allen, C.H. Seminal plasma addition attenuates the dilution effect in bovine sperm. Theriogenology 2001, 56, 31–40. [Google Scholar] [CrossRef]
- Höfner, L.; Luther, A.-M.; Waberski, D. The role of seminal plasma in the liquid storage of spermatozoa. Anim. Reprod. Sci. 2020, 220, 106290. [Google Scholar] [CrossRef]
- Gaitskell-Phillips, G.; Martín-Cano, F.E.; Ortiz-Rodríguez, J.M.; Silva-Rodríguez, A.; Rodríguez-Martínez, H.; Gil, M.C.; Ortega-Ferrusola, C.; Peña, F.J. Seminal plasma AnnexinA2 protein is a relevant biomarker for stallions which require removal of seminal plasma for sperm survival upon refrigeration. Biol. Reprod. 2020, 103, 1275–1288. [Google Scholar] [CrossRef]
- Parrilla, I.; Vazquez, J.M.; Caballero, I.; Gil, M.A.; Hernandez, M.; Roca, J.; Lucas, X.; Martinez, E.A. Optimal characteristics of spermatozoa for semen technologies in pigs. Soc. Reprod. Fertil. Suppl. 2009, 66, 37–50. [Google Scholar]
- Vadnais, M.L.; Roberts, K.P. Effects of seminal plasma on cooling-induced capacitative changes in boar sperm. J. Androl. 2007, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Asano, A.; Eriksson, B.; Niwa, K.; Nagai, T.; Rodriguez-Martinez, H. Capacitation status and in vitro fertility of boar spermatozoa: Effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int. J. Androl. 2002, 25, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Essawe, E.M.; Wallgren, M.; Wulf, M.; Aurich, C.; Macías-García, B.; Sjunnesson, Y.; Morrell, J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 2018, 115, 99–107. [Google Scholar] [CrossRef] [PubMed]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Roca, J.; Calvete, J.J.; Sanz, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Vázquez, J.M.; Martínez, E.A. Cryosurvival and in vitro fertilizing capacity postthaw is improved when boar spermatozoa are frozen in the presence of seminal plasma from good freezer boars. J. Androl. 2007, 28, 689–697. [Google Scholar] [CrossRef]
- Eriksson, B.M.; Vazquez, J.M.; Martinez, E.A.; Roca, J.; Lucas, X.; Rodriguez-Martinez, H. Effects of holding time during cooling and of type of package on plasma membrane integrity, motility and in vitro oocyte penetration ability of frozen-thawed boar spermatozoa. Theriogenology 2001, 55, 1593–1605. [Google Scholar] [CrossRef]
- Okazaki, T.; Abe, S.; Yoshida, S.; Shimada, M. Seminal plasma damages sperm during cryopreservation, but its presence during thawing improves semen quality and conception rates in boars with poor post-thaw semen quality. Theriogenology 2008, 71, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Shimada, M. New strategies of boar sperm cryopreservation: Development of novel freezing and thawing methods with a focus on the roles of seminal plasma. Anim. Sci. J. 2012, 83, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gago, R.; Álvarez-Rodríguez, M.; Alonso, M.E.; González, J.R.; Alegre, B.; Domínguez, J.C.; Martínez-Pastor, F. Thawing boar semen in the presence of seminal plasma improves motility, modifies subpopulation patterns and reduces chromatin alterations. Reprod. Fertil. Dev. 2017, 29, 1576–1584. [Google Scholar] [CrossRef]
- Kaeoket, K.; Chanapiwat, P.; Tummaruk, P.; Techakumphu, M.; Kunavongkrit, A. A preliminary study on using autologous and heterologous boar sperm supernatant from freezing processes as post-thawing solution: Its effect on sperm motility. Trop. Anim. Health Prod. 2011, 43, 1049–1055. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Soler, A.J.; González-Fernández, L.; Alves, M.G.; Oliveira, P.F.; Martín-Hidalgo, D. Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals 2020, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Khalil, W.A.; Alharbi, M.G.; Lee, S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animal 2020, 10, 2281. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. https://doi.org/10.3390/ijms22094368
Rodriguez-Martinez H, Martinez EA, Calvete JJ, Peña Vega FJ, Roca J. Seminal Plasma: Relevant for Fertility? International Journal of Molecular Sciences. 2021; 22(9):4368. https://doi.org/10.3390/ijms22094368
Chicago/Turabian StyleRodriguez-Martinez, Heriberto, Emilio A. Martinez, Juan J. Calvete, Fernando J. Peña Vega, and Jordi Roca. 2021. "Seminal Plasma: Relevant for Fertility?" International Journal of Molecular Sciences 22, no. 9: 4368. https://doi.org/10.3390/ijms22094368
APA StyleRodriguez-Martinez, H., Martinez, E. A., Calvete, J. J., Peña Vega, F. J., & Roca, J. (2021). Seminal Plasma: Relevant for Fertility? International Journal of Molecular Sciences, 22(9), 4368. https://doi.org/10.3390/ijms22094368