Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool
Abstract
1. Introduction
2. Results and Discussion
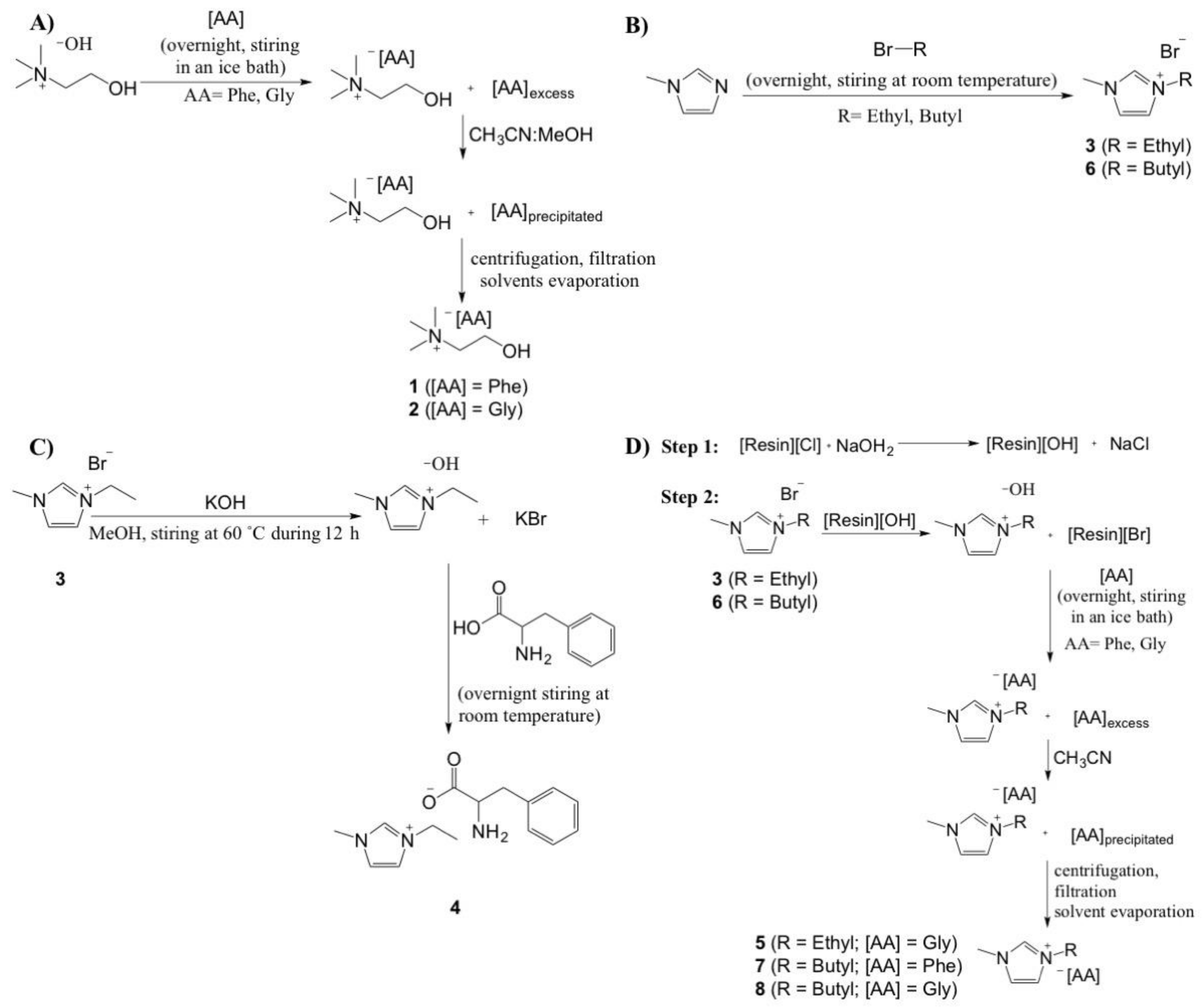
2.1. Synthesis of ILs
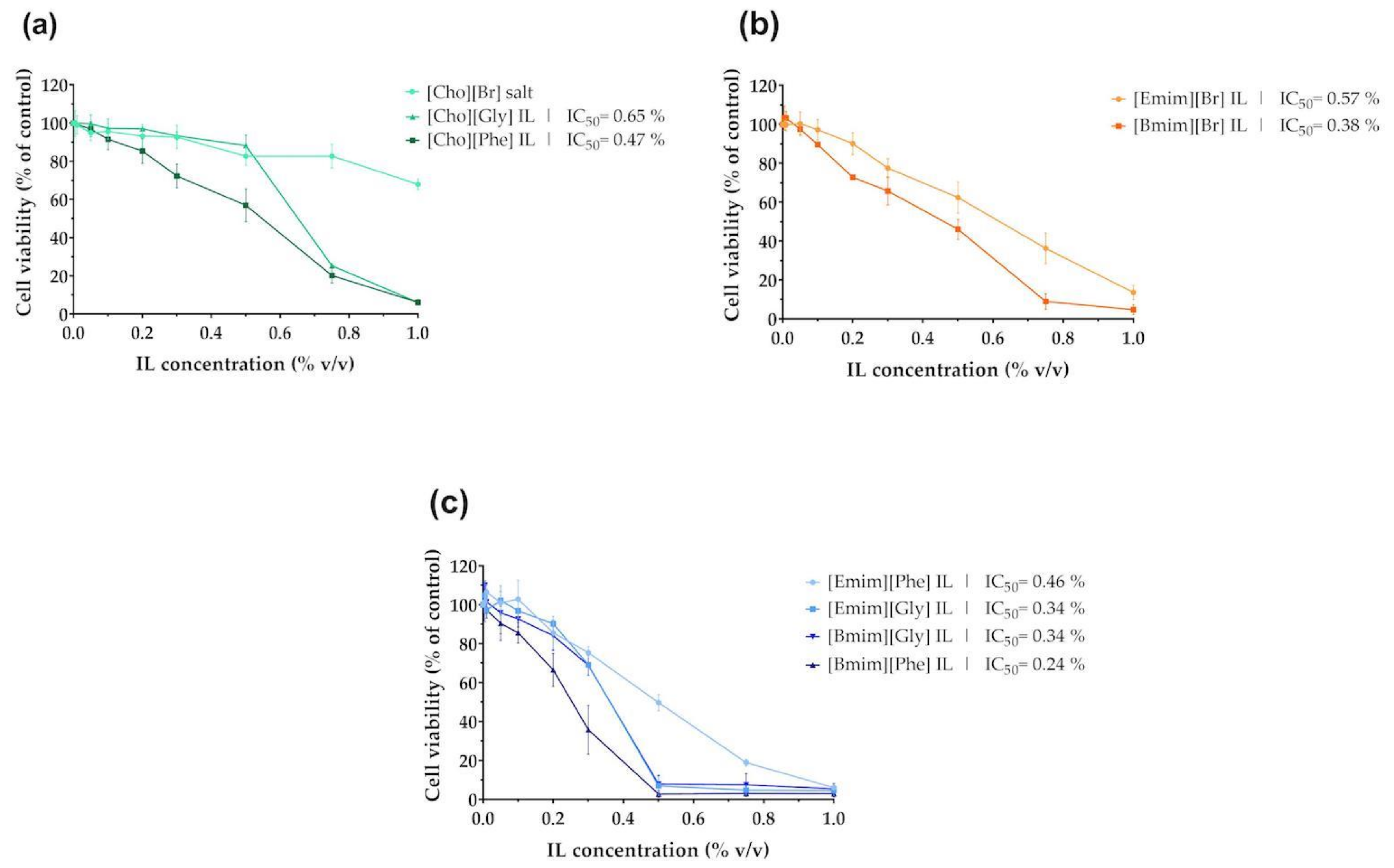
2.2. Impact of ILs on HaCaT Cells Viability
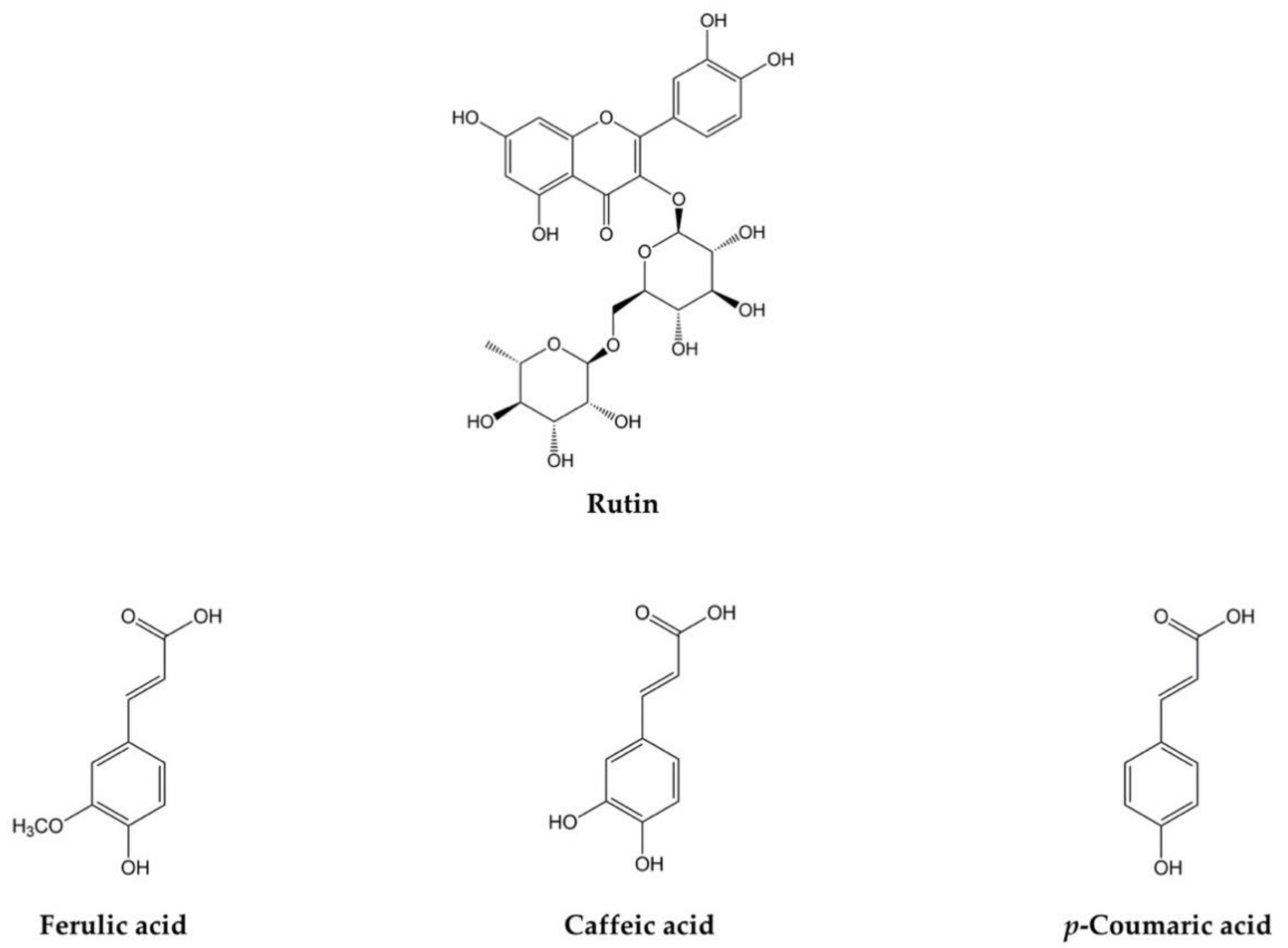
2.3. Influence of ILs on the Solubility of Poorly Soluble Phenolic Compounds (Ferulic, Caffeic, and p-Coumaric Acids, and Rutin)
2.4. Oil-in-Water (O/W) Emulsions
3. Materials and Methods
3.1. Equipment and Chemicals
3.2. Synthesis of Ionic Liquids
3.2.1. 2-hydroxyethyl-trimethylammonium-l-phenylalaninate [Cho][Phe] (1), 2-hydroxyethyl-trimethylammonium glycinate [Cho][Gly] (2).
3.2.2. 1-ethyl-3-methylimidazolium bromide [Emim][Br] (3), 1-butyl-3-methylimidazolium bromide [Bmim][Br] (6)
3.2.3. 1-ethyl-3-methylimidazolium lphenylalaninate [Emim][Phe] (4)
3.2.4. 1-ethyl-3-methylimidazolium glycinate, [Emim][Gly] (5), 1-butyl-3-methylimidazolium l-phenylalaninate [Bmim][Phe] (7) and 1-butyl-3-methylimidazolium glycinate [Bmim][Gly] (8)
3.3. Cell Culture
3.4. MTT Assay/Cell Viability Assay
3.5. Solubility Studies
3.6. Production of the Oil-in-Water (O/W) Emulsions
3.7. Stability Studies of the O/W Emulsions
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The role of ionic liquids in the pharmaceutical field: An overview of relevant applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and antibacterial activity of ionic liquids and organic salts based on penicillin g and amoxicillin hydrolysate derivatives against resistant bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Rosado, C.; Santos de Almeida, T. Applicability of ionic liquids in topical drug delivery systems: A mini review. J. Pharmacol. Clin. Res. 2018, 4, 555649–555655. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Eduarda, M.E.A.; Fernandes, A.S.; Costa, J.G.; Santos de Almeida, T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef]
- Czekanski, L.; Santos de Almeida, T.; Portugal Mota, J.; Rijo, P.; Araújo, M.E.M. Synthesis of benzoazole ionic liquids and evaluation of their antimicrobial activity. Biomed. Biopharm. Res. 2014, 11, 227–235. [Google Scholar] [CrossRef]
- Dias, A.R.; Costa-Rodrigues, J.; Fernandes, M.H.; Ferraz, R.; Prudêncio, C. The Anticancer Potential of Ionic Liquids. ChemMedChem 2017, 12, 11–18. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Medchemcomm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, L.; Wang, C.; Yang, Y.; Zhou, W.; Li, F.; Tan, Z. Assessment of the cytotoxicity of ionic liquids on Spodoptera frugiperda 9 (Sf-9) cell lines via in vitro assays. J. Hazard. Mater. 2018, 348, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, B.V.; Brasil Romão, G.; Andrade, R.S.; Barretto Cicarelli, R.M.; Trovatti, E.; Chiari-Andrèo, B.G.; Iglesias, M. Cytotoxic effect of protic ionic liquids in HepG2 and HaCat human cells:: In vitro and in silico studies. Toxicol Res. 2019, 8, 447–458. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.; Zanatta, M.; Ferreira, A.S.; Corvo, M.C.; Cabrita, E.J. Revisiting ionic liquid structure-property relationship: A critical analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; de Almeida, T.S.; Costa, J.G. In vitro cytotoxicity assessment of ferulic, caffeic and p-coumaric acids on human renal cancer cells. Biomed. Biopharm. Res. 2020, 17. [Google Scholar] [CrossRef]
- Costa, J.G.; Keser, V.; Jackson, C.; Saraiva, N.; Guerreiro, Í.; Almeida, N.; Camões, S.P.; Manguinhas, R.; Castro, M.; Miranda, J.P.; et al. A multiple endpoint approach reveals potential in vitro anticancer properties of thymoquinone in human renal carcinoma cells. Food Chem. Toxicol. 2020, 136, 111076. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Costa, J.G.; Vidovic, B.; Saraiva, N.; do Céu Costa, M.; Del Favero, G.; Marko, D.; Oliveira, N.G.; Fernandes, A.S. Contaminants: A dark side of food supplements? Free Radic Res. 2019, 53, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Pêgo, C.; Vidovic, B.B.; Oliveira, N.G.; Fernandes, A.S.; Costa, J.G. Fruit and vegetable juices and breast cancer. Cancer Oxidative Stress Diet. Antioxid. 2021, 235–244. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-balcerek, A.; Stuper-szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. Eur. J. Med. Technol. 2019, 4, 24–32. [Google Scholar]
- Santos de Almeida, T.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Kaur, R.; Kaur, R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustain. Chem. Eng. 2020, 8, 6263–6272. [Google Scholar] [CrossRef]
- Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; De Almeida, T.S.; Fonte, P. Ionic liquid-polymer nanoparticle hybrid systems as new tools to deliver poorly soluble drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Goto, M. Ionic liquids: Future solvents and reagents for pharmaceuticals. J. Chem. Eng. Japan 2011, 44, 370–381. [Google Scholar] [CrossRef]
- Silva, E.D.O.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compreensive Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, C.C.N.; Liao, W.; Lan, Y.; Hung, C. Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-Glycoprotein. Molecules 2020, 25, 247. [Google Scholar] [CrossRef]
- Júlio, A.; Antunes, C.; Mineiro, R.; Raposo, M.; Caparica, R.; Araújo, M.E.M.; Rosado, C.; Fonte, P.; de Almeida, T.S. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018, 15, 96–102. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Zeng, L.; Wang, C.; Yang, Y.; Tan, Z. Assessment of the toxicity and biodegradation of amino acid-based ionic liquids. RSC Adv. 2019, 9, 10100–10108. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huang, K.; Fan, J.-P.; Tao, D.-J. Chemical solvent in chemical solvent: A class of hybrid materials for effective capture of CO2. Sep. Mater. Devices Process. 2012, 59, 215–228. [Google Scholar] [CrossRef]
- Rajkumar, T.; Ranga Rao, G. Synthesis and characterization of hybrid molecular material prepared by ionic liquid and silicotungstic acid. Mater. Chem Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—benefits and threats. Int J. Mol. Sci 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Pubchem: Open chemistry database Caffeic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Caffeic-acid#section=Solubility (accessed on 3 March 2021).
- Fontecha-Cámara, M.A.; Álvarez, M.A.; López-Ramón, V.; Moreno-Castilla, C. Fenton oxidation of gallic and p-coumaric acids in water assisted by an activated carbon cloth. Water Sci. Technol. 2015, 71, 789–794. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 25, 901–908. [Google Scholar] [CrossRef] [PubMed]
- ANVISA—National Health Surveillance Agency. Cosmetic Products Stability Guide; ANVISA Publising House: Brasilia, Brazil, 2004; Volume 1, ISBN 8588233150. [Google Scholar]
- Du Plessis, J.L.; Stefaniak, A.B.; Wilhelm, K.P. Measurement of Skin Surface pH. Curr. Probl. Dermatol. 2018, 54, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tian, H.; Xiang, D. Stabilizing the oil-in-water emulsions using the mixtures of Dendrobium officinale polysaccharides and gum arabic or propylene glycol alginate. Molecules 2020, 25, 759. [Google Scholar] [CrossRef]
- Forsyth, S.A.; Pringle, J.M.; Macfarlane, D.R. Ionic liquids-an overview. Aust J. Chem 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Behrend, O.; Ax, K.; Schubert, H. Influence of continuous phase viscosity on emulsification by ultrasound. Ultrason. Sonochem 2000, 7, 77–85. [Google Scholar] [CrossRef]
- Calixto, L.S.; Infante, V.H.P.; Maia Campos, P.M.B.G. Design and Characterization of Topical Formulations: Correlations Between Instrumental and Sensorial Measurements. AAPS PharmSciTech 2018, 19, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberle, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The manganese(III) porphyrin MnTnHex-2-PyP 5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.S.; Fernandes, A.S.; Costa, J.G.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Differential effects of methoxyamine on doxorubicin cytotoxicity and genotoxicity in MDA-MB-231 human breast cancer cells. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 757, 140–147. [Google Scholar] [CrossRef]
- Pereira, M.; Pereira, N.; Rosado, C.; Areias de Oliveira, C.; D’Almeida Peres, D.; Araújo, M.E.; Robles Velasco, M.V.; Rolim Baby, A.; Portugal Mota, J.; Santos de Almeida, T. Photostabilization of sunscreens by incorporation of tea as the external phase. J. Biomed. Biopharm. Res. 2015, 12, 107–116. [Google Scholar] [CrossRef]
| IL | Color | Physical State at RT | Melting Point (°C) | Reaction Yield (%) | ||
|---|---|---|---|---|---|---|
| Acronym | Cation | Anion | ||||
| [Cho][Phe] (1) | 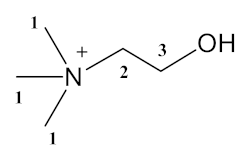 | 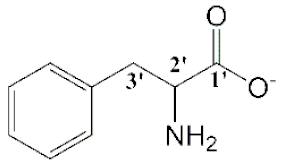 | Orange | Viscous liquid | - | 83 |
| [Cho][Gly] (2) | 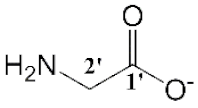 | Yellow | Viscous Liquid | - | 92 | |
| [Emim][Br] (3) |  | Br− | White | Viscous Solid | 63-65 | 99 |
| [Emim][Phe] (4) | 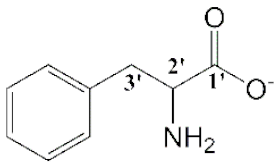 | Yellow | Viscous Liquid | - | 67 | |
| [Emim][Gly] (5) | 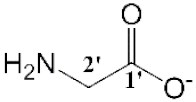 | Light yellow | Viscous Liquid | - | 65 | |
| [Bmim][Br] (6) | 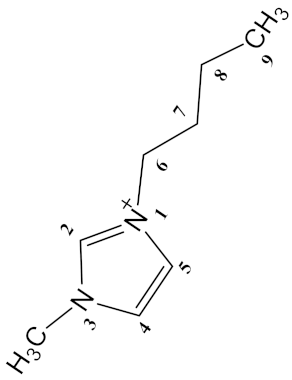 | Br− | White | Viscous Solid | 64–66 | 72 |
| [Bmim][Phe] (7) | 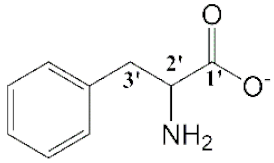 | Yellow | Viscous Liquid | - | 62 | |
| [Bmim][Gly] (8) | 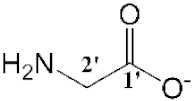 | Light yellow | Viscous Liquid | - | 65 | |
| Solubility (mg/mL) | Solvent (% w/w) | ||||
|---|---|---|---|---|---|
| Ferulic Acid | Caffeic Acid | p-Coumaric Acid | Rutin | ||
| 0.63 ± 0.04 a | 0.45 ± 0.04 a | 0.70 ± 0.04 a–d | 0.20 ± 0.01 a | 100 | Water |
| 0.63 ± 0.02 a,b | 0.45 ± 0.04 a | 0.65 ± 0.01 a–c | 0.19 ± 0.01 a | 99.99:0.01 | Water:[Cho][Br] |
| 0.67 ± 0.01 a,b | 0.45 ± 0.02 a | 0.65 ± 0.01 a–c | 0.20 ± 0.01 a | 99.9:0.1 | |
| 0.65 ± 0.01 a,b | 0.45 ± 0.02 a | 0.61 ± 0.01 a,b | 0.18 ± 0.01 a | 99.8:0.2 | |
| 0.63 ± 0.02 a,b | 0.49 ± 0.02 a,b | 0.52 ± 0.05 a | 0.18 ± 0.01 a | 99.7:0.3 | |
| 0.63 ± 0.01 a | 0.55 ± 0.01 a–d | 0.52 ± 0.05 a | 0.18 ± 0.01 a | 99.5:0.5 | |
| 0.63 ± 0.04 a | 0.54 ± 0.06 a–d | 0.73 ± 0.01 b–d | 0.26 ± 0.01 a | 99.99:0.01 | Water:[Cho][Phe] |
| 0.85 ± 0.07 a–f | 1.02 ± 0.03 h | 0.88 ± 0.04 d–g | 0.78 ± 0.08 b | 99.9:0.1 | |
| 1.06 ± 0.01 e–h | 1.42 ± 0.08 i | 1.39 ± 0.02 i–k | 1.44 ± 0.10 e | 99.8:0.2 | |
| 1.52 ± 0.13 i,j | 2.00 ± 0.12 k,l | 1.58 ± 0.02 l,m | 2.49 ± 0.12 i | 99.7:0.3 | |
| 2.12 ± 0.19 m–o | 2.35 ± 0.06 m | 1.74 ± 0.02 m,n | 2.78 ± 0.15 j | 99.5:0.5 | |
| 0.65 ± 0.08 a,b | 0.52 ± 0.03 a–c | 0.73 ± 0.05 b–d | 0.24 ± 0.01 a | 99.99:0.01 | Water:[Cho][Gly] |
| 0.94 ± 0.06 c–f | 0.88 ± 0.04 e–h | 0.96 ± 0.12 f,g | 0.82 ± 0.05 b,c | 99.9:0.1 | |
| 1.23 ± 0.29 g,h | 1.39 ± 0.07 i | 1.54 ± 0.07 k,l | 1.73 ± 0.03 f–h | 99.8:0.2 | |
| 1.66 ± 0.21 j,k | 1.83 ± 0.03 j,k | 1.86 ± 0.13 n,o | 2.63 ± 0.06 i,j | 99.7:0.3 | |
| 2.25 ± 0.10 n,o | 2.35 ± 0.03 m | 2.09 ± 0.11 p | 2.83 ± 0.09 j | 99.5:0.5 | |
| 0.61 ± 0.01 a | 0.46 ± 0.02 a | 0.69 ± 0.02 a–c | 0.18 ± 0.02 a | 99.99:0.01 | Water:[Emim][Br] |
| 0.62 ± 0.01 a | 0.47 ± 0.01 a,b | 0.73 ± 0.04 b–d | 0.21 ± 0.01 a | 99.9:0.1 | |
| 0.64 ± 0.03 a,b | 0.49 ± 0.03 a,b | 0.75 ± 0.06 b–e | 0.21 ± 0.02 a | 99.8:0.2 | |
| 0.64 ± 0.06 a,b | 0.51 ± 0.05 a,b | 0.74 ± 0.04 b–d | 0.24 ± 0.01 a | 99.7:0.3 | |
| 0.70 ± 0.01 a–c | 0.54 ± 0.01 a–d | 0.80 ± 0.02 c–f | 0.26 ± 0.02 a | 99.5:0.5 | |
| 0.66 ± 0.04 a,b | 0.47 ± 0.02 a,b | 0.70 ± 0.07 a–d | 0.20 ± 0.01 a | 99.99:0.01 | Water:[Emim][Phe] |
| 0.89 ± 0.05 b–f | 0.65 ± 0.01 a–d | 0.96 ± 0.03 f,g | 0.76 ± 0.05 b | 99.9:0.1 | |
| 1.00 ± 0.07 d–g | 0.74 ± 0.01 d–g | 0.99 ± 0.04 g | 1.03 ± 0.07 c,d | 99.8:0.2 | |
| 1.09 ± 0.04 f–h | 0.99 ± 0.03 h | 1.21 ± 0.06 h,i | 1.33 ± 0.07 e | 99.7:0.3 | |
| 1.80 ± 0.18 k,l | 1.44 ± 0.04 i | 1.95 ± 0.05 o,p | 1.58 ± 0.04 e–g | 99.5:0.5 | |
| 0.65 ± 0.08 a,b | 0.50 ± 0.05 a,b | 0.70 ± 0.07 a–d | 0.25 ± 0.01 a | 99.99:0.01 | Water:[Emim][Gly] |
| 1.03 ± 0.06 e–h | 0.94 ± 0.05 g,h | 0.92 ± 0.02 e–g | 0.96 ± 0.14 b–d | 99.9:0.1 | |
| 1.62 ± 0.05 j,k | 1.08 ± 0.09 h | 1.43 ± 0.13 k,l | 1.52 ± 0.16 e,f | 99.8:0.2 | |
| 2.02 ± 0.08 l–n | 1.77 ± 0.11 j | 1.60 ± 0.09 l,m | 2.61 ± 0.01 i,j | 99.7:0.3 | |
| 2.65 ± 0.21 p | 2.12 ± 0.11 l | 2.11 ± 0.03 p | 2.82 ± 0.19 j | 99.5:0.5 | |
| 0.63 ± 0.03 a,b | 0.45 ± 0.02 a | 0.69 ± 0.02 a–c | 0.19 ± 0.01 a | 99.99:0.01 | Water:[Bmim][Br] |
| 0.63 ± 0.02 a,b | 0.52 ± 0.01 a–c | 0.66 ± 0.03 a–c | 0.20 ± 0.01 a | 99.9:0.1 | |
| 0.64 ± 0.02 a,b | 0.55 ± 0.01 a–d | 0.67 ± 0.03 a–c | 0.21 ± 0.01 a | 99.8:0.2 | |
| 0.65 ± 0.03 a,b | 0.56 ± 0.01 a–d | 0.68 ± 0.03 a–c | 0.21 ± 0.01 a | 99.7:0.3 | |
| 0.67 ± 0.01 a,b | 0.58 ± 0.02 a–d | 0.69 ± 0.04 a–c | 0.21 ± 0.01 a | 99.5:0.5 | |
| 0.66 ± 0.04 a,b | 0.50 ± 0.01 a,b | 0.73 ± 0.04 b–d | 0.21 ± 0.01 a | 99.99:0.01 | Water:[Bmim][Phe] |
| 0.81 ± 0.01 a–e | 0.67 ± 0.02 b–e | 0.99 ± 0.01 g | 0.78 ± 0.06 b | 99.9:0.1 | |
| 1.02 ± 0.01 e–h | 0.72 ± 0.05 c–f | 1.00 ± 0.01 g | 1.07 ± 0.01 d | 99.8:0.2 | |
| 1.10 ± 0.01 f–h | 0.89 ± 0.01 f–h | 1.21 ± 0.02 h | 1.46 ± 0.03 e | 99.7:0.3 | |
| 1.81 ± 0.02 k,l | 1.02 ± 0.01 h | 1.40 ± 0.14 j,k | 1.78 ± 0.13 g,h | 99.5:0.5 | |
| 0.75 ± 0.08 a–d | 0.52 ± 0.02 a–c | 0.79 ± 0.06 b–f | 0.28 ± 0.04 a | 99.99:0.01 | Water:[Bmim][Gly] |
| 1.27 ± 0.09 h,i | 0.95 ± 0.04 h | 1.22 ± 0.01 h–j | 0.96 ± 0.11 b–d | 99.9:0.1 | |
| 1.95 ± 0.10 l,m | 1.50 ± 0.34 i | 1.75 ± 0.11 m,n | 1.85 ± 0.20 h | 99.8:0.2 | |
| 2.30 ± 0.07 o | 2.11 ± 0.15 l | 1.97 ± 0.03 o,p | 3.24 ± 0.18 k | 99.7:0.3 | |
| 3.57 ± 0.07 q | 2.52 ± 0.06 m | 2.30 ± 0.07 q | 4.67 ± 0.35 l | 99.5:0.5 | |
| O/W Emulsions | ||||
|---|---|---|---|---|
| Composition | Without IL and Drug | With IL and without Drug | With Drug and without IL | With Drug and IL |
| Crodafos® CES | 3.5 | 3.5 | 3.5 | 3.5 |
| Isopropyl myristate | 2.0 | 2.0 | 2.0 | 2.0 |
| BHT | 0.1 | 0.1 | 0.1 | 0.1 |
| EDTA Na2 | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylene glycol | 5.0 | 5.0 | 5.0 | 5.0 |
| Polyethylene glycol 400 | 5.0 | 5.0 | 5.0 | 5.0 |
| Parabens solution | 1.0 | 1.0 | 1.0 | 1.0 |
| Drug | – | - | Maximum solubility 1 | Maximum solubility 1 |
| IL | – | 0.2 or 0.5 | - | 0.2 or 0.5 |
| Triethanolamine | q.s. to pH = 5 | |||
| Deionized water | q.s. to 100 | |||
| IL | % IL | After Formulation | Stability Studies | |||
|---|---|---|---|---|---|---|
| Visual Analysis | Viscosity (mPas) | After Centrifugation | After Gradual Heating | Viscosity (mPas) after 6 Temperature Cycles | ||
| Control 1 | - | Stable | 5170 ± 90 | Unstable | Unstable | - |
| [Cho][Br] | 0.2 | Stable | 9000 ± 100 | Stable | Stable | 10,320 ± 80 |
| [Cho][Phe] | 0.2 | Stable | 12,700 ± 102 | 15,400 ± 100 | ||
| [Cho][Gly] | 0.2 | Stable | 11,800 ± 52 | 13,100 ± 105 | ||
| [Emim][Br] | 0.2 | Stable | 9100 ± 97 | 11,000 ± 101 | ||
| [Emim][Phe] | 0.2 | Stable | 10,000 ± 132 | 12,400 ± 129 | ||
| [Emim][Gly] | 0.2 | Stable | 10,400 ± 188 | 13,100 ± 77 | ||
| [Bmim][Br] | 0.2 | Stable | 8750 ± 65 | 9500 ± 90 | ||
| [Bmim][Phe] | 0.2 | Stable | 9100 ± 80 | 11,000 ± 85 | ||
| [Bmim][Gly] | 0.2 | Stable | 9200 ± 120 | 11,400 ± 112 | ||
| Drug | IL | % IL | After Formulation | Stability Studies | |||
|---|---|---|---|---|---|---|---|
| Visual Analysis | Viscosity (mPas) | After Centrifuge | After Gradual Heating | Viscosity (mPas) after 6 Temperature Cycles | |||
| Ferulic Acid | Control 2a | - | Stable | 8000 ± 80 | Unstable | Unstable | - |
| [Cho][Gly] | 0.2 | Stable | 12,000 ± 75 | Stable | Stable | 12,500 ± 100 | |
| 0.5 | Stable | 13,700 ± 110 | 15,000 ± 90 | ||||
| [Emim][Gly] | 0.2 | Stable | 11,300 ± 100 | 12,000 ± 100 | |||
| [Bmim][Gly] | 0.2 | Stable | 10,000 ± 130 | 12,600 ± 100 | |||
| Caffeic Acid | Control 2b | - | Stable | 8500 ± 100 | Unstable | Unstable | - |
| [Cho][Gly] | 0.2 | Stable | 11,000 ± 95 | Stable | Stable | 12,000 ± 90 | |
| 0.5 | Stable | 12,000 ± 100 | 15,500 ± 95 | ||||
| [Emim][Gly] | 0.2 | Stable | 11,200 ± 90 | 14,100 ± 80 | |||
| [Bmim][Gly] | 0.2 | Stable | 11,000 ± 80 | 14,500 ± 90 | |||
| p-Coumaric Acid | Control 2c | - | Stable | 8200 ± 100 | Unstable | Unstable | - |
| [Cho][Gly] | 0.2 | Stable | 12,000 ± 100 | Stable | Stable | 15,500 ± 100 | |
| 0.5 | Stable | 13,500 ± 100 | 17,000 ± 90 | ||||
| [Emim][Gly] | 0.2 | Stable | 10,300 ± 90 | 14,600 ± 100 | |||
| [Bmim][Gly] | 0.2 | Stable | 10,200 ± 100 | 14,000 ± 100 | |||
| Rutin | Control 2d | - | Stable | 7500 ± 150 | Unstable | Unstable | - |
| [Cho][Gly] | 0.2 | Stable | 12,800 ± 100 | Stable | Stable | 13,100 ± 105 | |
| 0.5 | Stable | 13,400 ± 90 | 16,000 ± 100 | ||||
| [Emim][Gly] | 0.2 | Stable | 10,400 ± 188 | 13,100 ± 77 | |||
| [Bmim][Gly] | 0.2 | Stable | 9220 ± 50 | 11,140 ± 52 | |||
| IL | % IL | Accelerated Stability | Shelf Test | |
|---|---|---|---|---|
| Heating at Oven | Cooling at Refrigerator | Viscosity (mPas) | ||
| [Cho][Br] | 0.2 | 12,100 ± 100 | 12215 ± 120 | 13,400 ± 50 |
| [Cho][Phe] | 0.2 | 15,500 ± 85 | 14950 ± 80 | 16,200 ± 65 |
| [Cho][Gly] | 0.2 | 15,220 ± 50 | 15000 ± 80 | 16,050 ± 100 |
| [Emim][Br] | 0.2 | 11,900 ± 100 | 11500 ± 50 | 13,100 ± 90 |
| [Emim][Phe] | 0.2 | 12,200 ± 100 | 11950 ± 50 | 13,450 ± 70 |
| [Emim][Gly] | 0.2 | 12,450 ± 100 | 12320 ± 100 | 14,120 ± 90 |
| [Bmim][Br] | 0.2 | 9500 ± 100 | 9900 ± 100 | 9900 ± 50 |
| [Bmim][Phe] | 0.2 | 10,300 ± 50 | 10220 ± 100 | 11,000 ± 100 |
| [Bmim][Gly] | 0.2 | 10,175 ± 50 | 10300 ± 70 | 11,110 ± 50 |
| Drug | IL | % IL | Accelerated Stability | Shelf Test | |
|---|---|---|---|---|---|
| Heating at Oven | Cooling at Refrigerator | Viscosity (mPas) | |||
| Ferulic Acid | [Cho][Gly] | 0.2 | 15,500 ± 100 | 15,410 ± 50 | 16,250 ± 100 |
| 0.5 | 16,300 ± 50 | 16,570 ± 110 | 17,120 ± 120 | ||
| [Emim][Gly] | 0.2 | 12,320 ± 80 | 12,200 ± 50 | 14,200 ± 110 | |
| [Bmim][Gly] | 0.2 | 11,000 ± 50 | 10,900 ± 50 | 12,100 ± 100 | |
| Caffeic Acid | [Cho][Gly] | 0.2 | 13,100 ± 50 | 13,310 ± 80 | 14,850 ± 50 |
| 0.5 | 13,300 ± 60 | 13,140 ± 100 | 15,680 ± 50 | ||
| [Emim][Gly] | 0.2 | 12,520 ± 100 | 12,600 ± 100 | 14,200 ± 150 | |
| [Bmim][Gly] | 0.2 | 12,100 ± 100 | 11,990 ± 100 | 14,620 ± 80 | |
| p-Coumaric Acid | [Cho][Gly] | 0.2 | 13,250 ± 50 | 13,500 ± 100 | 16,000 ± 100 |
| 0.5 | 13,900 ± 50 | 14,540 ± 100 | 17,120 ± 150 | ||
| [Emim][Gly] | 0.2 | 11,950 ± 50 | 12,000 ± 100 | 14,850 ± 100 | |
| [Bmim][Gly] | 0.2 | 11,400 ± 50 | 11,355 ± 100 | 14,700 ± 100 | |
| Rutin | [Cho][Gly] | 0.2 | 15,800 ± 50 | 16,000 ± 100 | 16,550 ± 120 |
| 0.5 | 16,750 ± 60 | 17,010 ± 100 | 18,225 ± 115 | ||
| [Emim][Gly] | 0.2 | 12,450 ± 50 | 12,800 ± 100 | 14,780 ± 100 | |
| [Bmim][Gly] | 0.2 | 11,500 ± 50 | 11,650 ± 100 | 12,380 ± 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caparica, R.; Júlio, A.; Fernandes, F.; Araújo, M.E.M.; Costa, J.G.; Santos de Almeida, T. Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool. Int. J. Mol. Sci. 2021, 22, 4338. https://doi.org/10.3390/ijms22094338
Caparica R, Júlio A, Fernandes F, Araújo MEM, Costa JG, Santos de Almeida T. Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool. International Journal of Molecular Sciences. 2021; 22(9):4338. https://doi.org/10.3390/ijms22094338
Chicago/Turabian StyleCaparica, Rita, Ana Júlio, Filipe Fernandes, Maria Eduarda M. Araújo, João Guilherme Costa, and Tânia Santos de Almeida. 2021. "Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool" International Journal of Molecular Sciences 22, no. 9: 4338. https://doi.org/10.3390/ijms22094338
APA StyleCaparica, R., Júlio, A., Fernandes, F., Araújo, M. E. M., Costa, J. G., & Santos de Almeida, T. (2021). Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool. International Journal of Molecular Sciences, 22(9), 4338. https://doi.org/10.3390/ijms22094338









