Polycystin-2 Is Required for Chondrocyte Mechanotransduction and Traffics to the Primary Cilium in Response to Mechanical Stimulation
Abstract
1. Introduction
2. Results
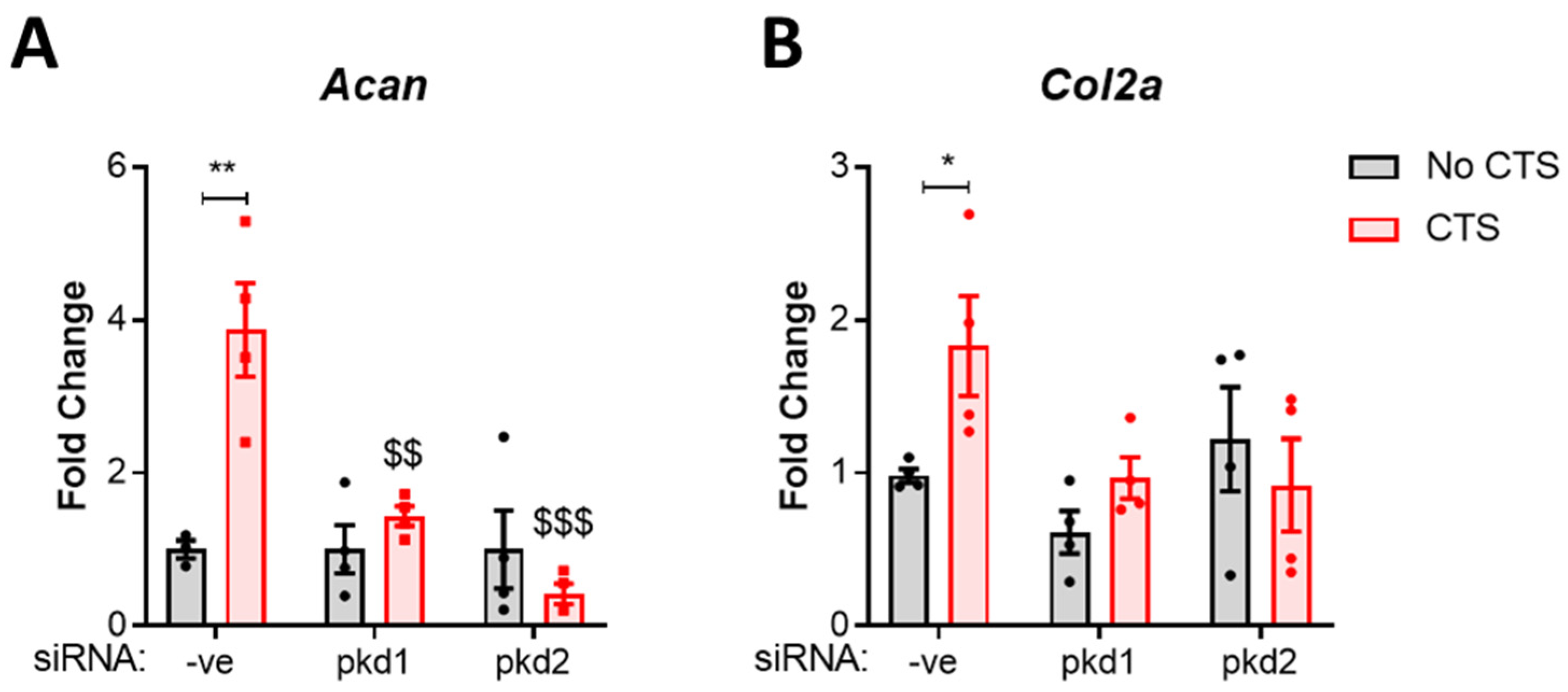
2.1. PC1 and PC2 Are Required for Anabolic Gene Expression in Response to Cyclic Tensile Strain
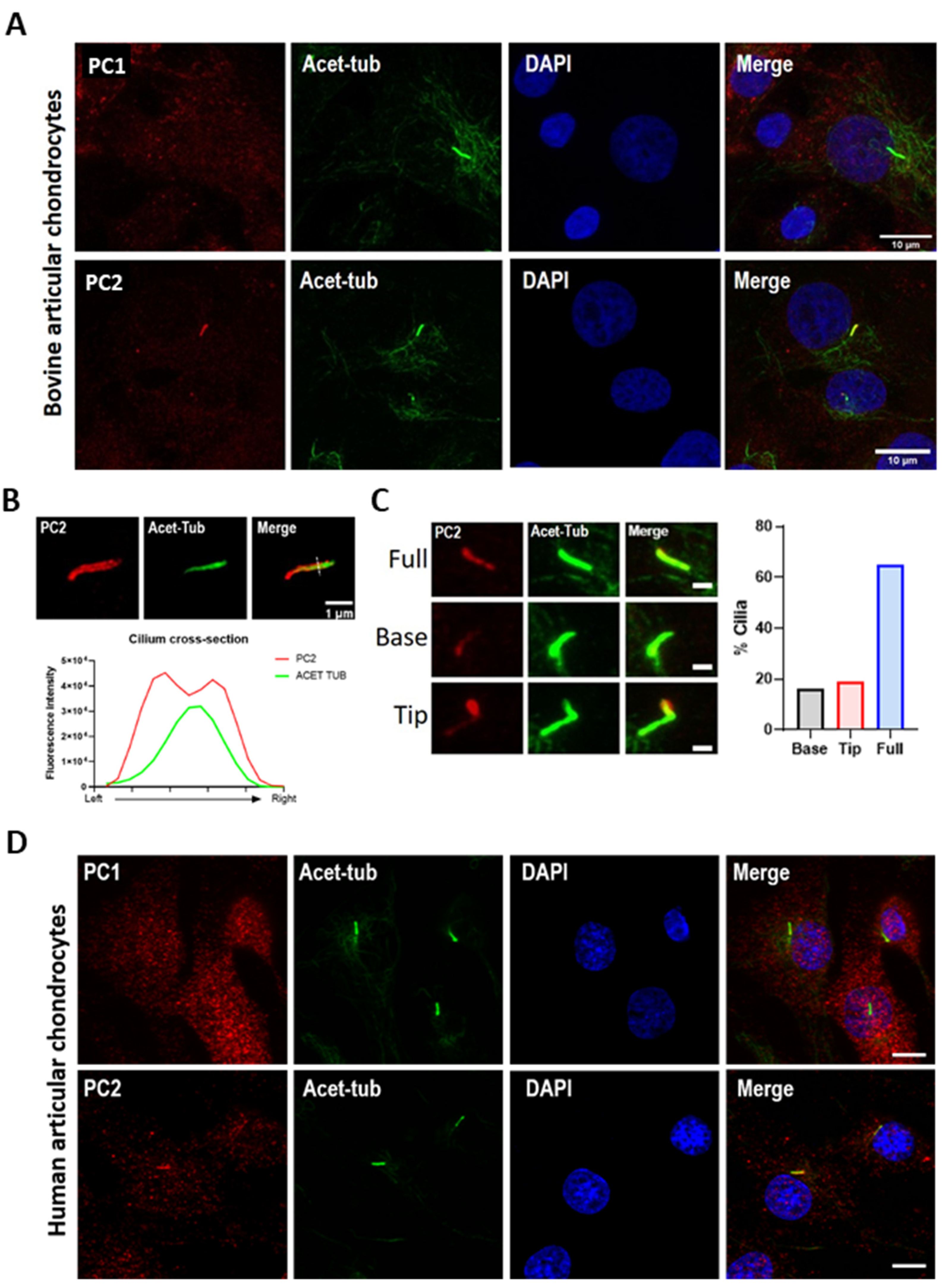
2.2. PC2 Localizes to the Chondrocyte Primary Cilium
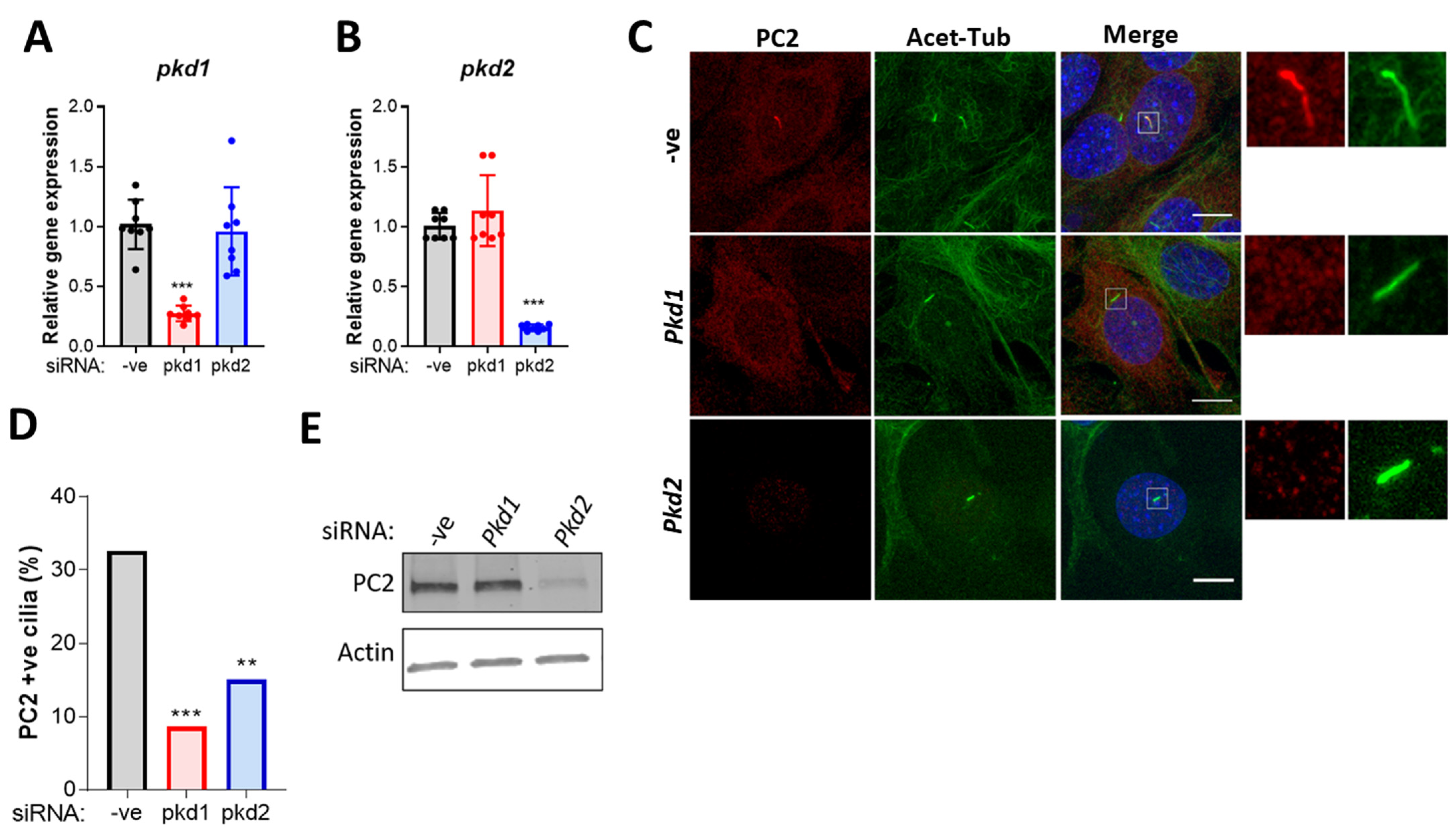
2.3. PC2 Ciliary Localization Is Dependent on PC1
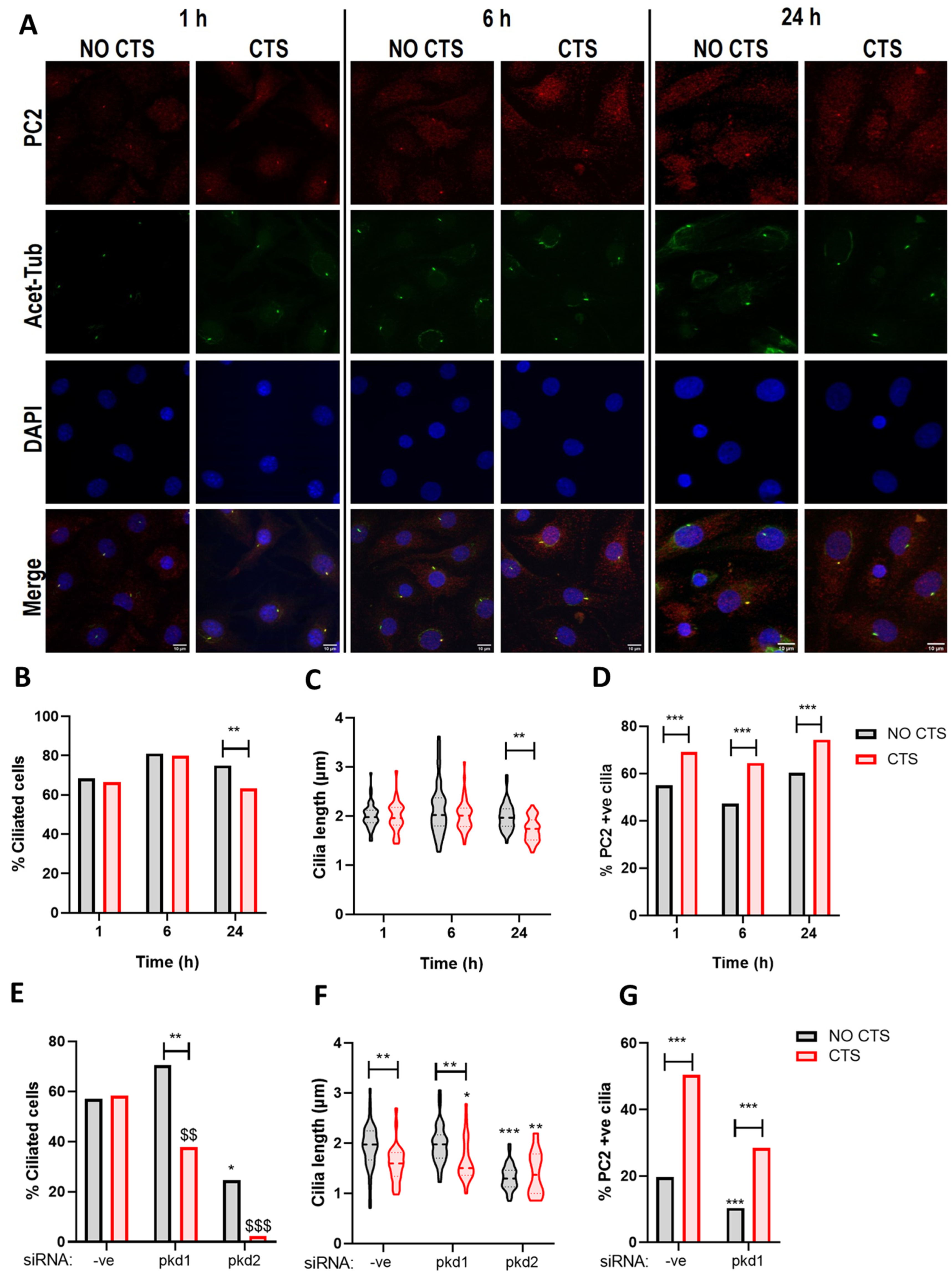
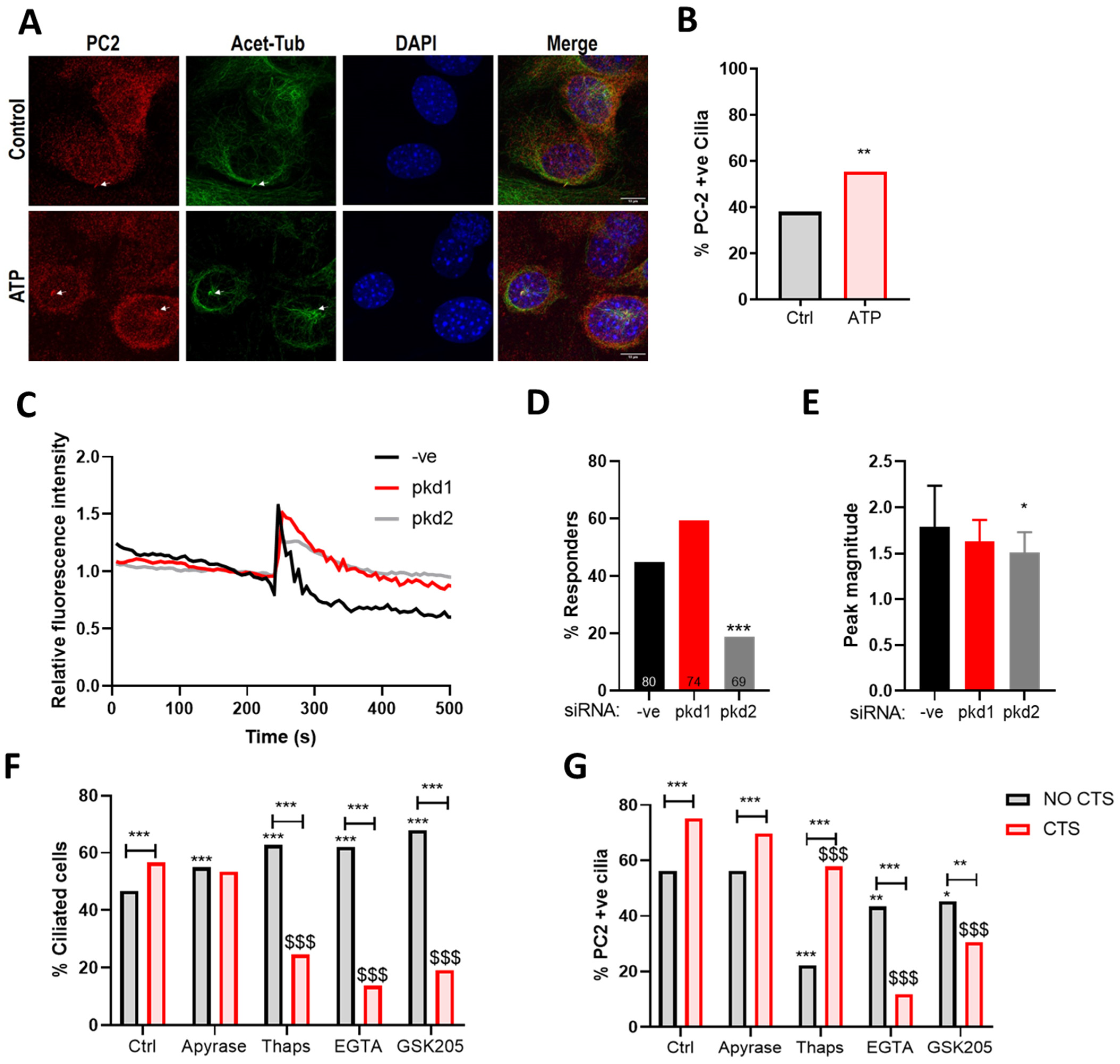
2.4. PC2 Ciliary Localization Is Increased in Response to Mechanical Stimulation and Requires PC1
2.5. The Mechanosensitive Increase in PC2 Cilia Localization Is Not Dependent upon ATP Release
2.6. The Mechanosensitive Increase in PC2 Cilia Localization Is Dependent upon TRPV4 Activation and the Influx of Extracellular Ca2+
3. Discussion
4. Materials and Methods
4.1. Cell and Tissue Culture
4.2. siRNA Knockdown
4.3. Application of Cyclic Tensile Strain
4.4. Immunocytochemistry Confocal and Structural Illumination Microscopy
4.5. Ca2+ Imaging
4.6. RNA Isolation, cDNA Synthesis, and qRT-PCR
4.7. Protein Isolation and Western Analyses
4.8. Data Presentation and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pala, R.; Alomari, N.; Nauli, S.M. Primary Cilium-Dependent Signaling Mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Salter, D.M. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann. Biomed. Eng. 2004, 32, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Millward-Sadler, S.J.; Wright, M.O.; Davies, L.W.; Nuki, G.; Salter, D.M. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000, 43, 2091–2099. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Wright, M.O.; Lee, H.; Caldwell, H.; Nuki, G.; Salter, D.M. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthr. Cartil. 2000, 8, 272–278. [Google Scholar] [CrossRef]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef]
- Haycraft, C.J.; Zhang, Q.; Song, B.; Jackson, W.S.; Detloff, P.J.; Serra, R.; Yoder, B.K. Intraflagellar transport is essential for endochondral bone formation. Development 2007, 134, 307–316. [Google Scholar] [CrossRef]
- Song, B.; Haycraft, C.J.; Seo, H.S.; Yoder, B.K.; Serra, R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007, 305, 202–216. [Google Scholar] [CrossRef]
- Chang, C.F.; Ramaswamy, G.; Serra, R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthr. Cartil. 2012, 20, 152–161. [Google Scholar] [CrossRef]
- Irianto, J.; Ramaswamy, G.; Serra, R.; Knight, M.M. Depletion of chondrocyte primary cilia reduces the compressive modulus of articular cartilage. J. Biomech. 2014, 47, 579–582. [Google Scholar] [CrossRef]
- Kaushik, A.P.; Martin, J.A.; Zhang, Q.; Sheffield, V.C.; Morcuende, J.A. Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J. Orthop. Res. 2009, 27, 1093–1099. [Google Scholar] [CrossRef]
- Coveney, C.; Zhu, L.; Miotla-Zarebska, J.; Stott, B.; Parisi, I.; Batchelor, V.; Duarte, C.; Chang, E.; McSorley, E.; Vincent, T.; et al. The ciliary protein IFT88 controls post-natal cartilage thickness and influences development of osteoarthritis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Spring, K.R. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 2003, 12, 517–520. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Leonard, M.L.; Bizios, R.; Bowser, S.S. Analysis and modeling of the primary cilium bending response to fluid shear. Am. J. Physiol. 1997, 272, F132–F138. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Frokiaer, J.; Nielsen, S.; Spring, K.R. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J. Membr. Biol. 2003, 191, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Walker, R.V.; Keynton, J.L.; Grimes, D.T.; Sreekumar, V.; Williams, D.J.; Esapa, C.; Wu, D.; Knight, M.M.; Norris, D.P. Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat. Commun. 2019, 10, 4072. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.L.; Beales, P.L. The nonmotile ciliopathies. Genet. Med. 2009, 11, 386–402. [Google Scholar] [CrossRef]
- Delling, M.; DeCaen, P.G.; Doerner, J.F.; Febvay, S.; Clapham, D.E. Primary cilia are specialized calcium signalling organelles. Nature 2013, 504, 311–314. [Google Scholar] [CrossRef]
- Rich, D.R.; Clark, A.L. Chondrocyte primary cilia shorten in response to osmotic challenge and are sites for endocytosis. Osteoarthr. Cartil. 2012, 20, 923–930. [Google Scholar] [CrossRef]
- Farnum, C.E.; Wilsman, N.J. Orientation of primary cilia of articular chondrocytes in three-dimensional space. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.K.; Zuo, N.; Haycraft, C.J.; Jensen, C.G.; Poole, C.A.; McGlashan, S.R.; Knight, M.M. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012, 26, 1663–1671. [Google Scholar] [CrossRef]
- Aguiari, G.; Campanella, M.; Manzati, E.; Pinton, P.; Banzi, M.; Moretti, S.; Piva, R.; Rizzuto, R.; del Senno, L. Expression of polycystin-1 C-terminal fragment enhances the ATP-induced Ca2+ release in human kidney cells. Biochem. Biophys. Res. Commun. 2003, 301, 657–664. [Google Scholar] [CrossRef]
- Chowdhury, T.T.; Knight, M.M. Purinergic pathway suppresses the release of.NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J. Cell Physiol. 2006, 209, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Rais, Y.; Reich, A.; Simsa-Maziel, S.; Moshe, M.; Idelevich, A.; Kfir, T.; Miosge, N.; Monsonego-Ornan, E. The growth plate’s response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell. Mol. Life Sci. 2015, 72, 597–615. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Leong, D.J.; Zhuo, Z.; Majeska, R.J.; Cardoso, L.; Spray, D.C.; Goldring, M.B.; Cobelli, N.J.; Sun, H.B. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthr. Cartil. 2016, 24, 892–901. [Google Scholar] [CrossRef]
- Vandorpe, D.H.; Chernova, M.N.; Jiang, L.; Sellin, L.K.; Wilhelm, S.; Stuart-Tilley, A.K.; Walz, G.; Alper, S.L. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J. Biol. Chem. 2001, 276, 4093–4101. [Google Scholar] [CrossRef]
- Ćelić, A.S.; Petri, E.T.; Benbow, J.; Hodsdon, M.E.; Ehrlich, B.E.; Boggon, T.J. Calcium-induced conformational changes in C-terminal tail of polycystin-2 are necessary for channel gating. J. Biol. Chem. 2012, 287, 17232–17240. [Google Scholar] [CrossRef]
- Qian, F.; Germino, F.J.; Cai, Y.; Zhang, X.; Somlo, S.; Germino, G.G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997, 16, 179–183. [Google Scholar] [CrossRef]
- Coveney, C.R.; Collins, I.; Mc Fie, M.; Chanalaris, A.; Yamamoto, K.; Wann, A.K.T. Cilia protein IFT88 regulates extracellular protease activity by optimizing LRP-1–mediated endocytosis. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jerman, S.; Ward, H.H.; Lee, R.; Lopes, C.A.M.; Fry, A.M.; MacDougall, M.; Wandinger-Ness, A. OFD1 and Flotillins Are Integral Components of a Ciliary Signaling Protein Complex Organized by Polycystins in Renal Epithelia and Odontoblasts. PLoS ONE 2014, 9, e106330. [Google Scholar] [CrossRef] [PubMed]
- Chapin, H.C.; Rajendran, V.; Caplan, M.J. Polycystin-1 Surface Localization Is Stimulated by Polycystin-2 and Cleavage at the G Protein-coupled Receptor Proteolytic Site. Mol. Biol. Cell 2010, 21, 4338–4348. [Google Scholar] [CrossRef]
- Freedman, B.S.; Lam, A.Q.; Sundsbak, J.L.; Iatrino, R.; Su, X.; Koon, S.J.; Wu, M.; Daheron, L.; Harris, P.C.; Zhou, J.; et al. Reduced Ciliary Polycystin-2 in Induced Pluripotent Stem Cells from Polycystic Kidney Disease Patients with PKD1 Mutations. J. Am. Soc. Nephrol. 2013, 24, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Gainullin, V.G.; Hopp, K.; Ward, C.J.; Hommerding, C.J.; Harris, P.C. Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J. Clin. Investig. 2015, 125, 607–620. [Google Scholar] [CrossRef]
- Miyakawa, A.; Ibarra, C.; Malmersjö, S.; Aperia, A.; Wiklund, P.; Uhlén, P. Intracellular calcium release modulates polycystin-2 trafficking. BMC Nephrol. 2013, 14, 34. [Google Scholar] [CrossRef]
- Liu, X.; Vien, T.; Duan, J.; Sheu, S.H.; DeCaen, P.G.; Clapham, D.E. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Elife 2018, 7, e33183. [Google Scholar] [CrossRef]
- McGlashan, S.R.; Knight, M.M.; Chowdhury, T.T.; Joshi, P.; Jensen, C.G.; Kennedy, S.; Poole, C.A. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol. Int. 2010, 34, 441–446. [Google Scholar] [CrossRef]
- Thompson, C.; Chapple, J.; Knight, M. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: A feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthr. Cartil. 2014, 22, 490–498. [Google Scholar] [CrossRef]
- Iomini, C.; Tejada, K.; Mo, W.; Vaananen, H.; Piperno, G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 2004, 164, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Millward-Sadler, S.J.; Wright, M.O.; Flatman, P.W.; Salter, D.M. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 2004, 41, 567–575. [Google Scholar] [PubMed]
- Doerr, N.; Wang, Y.; Kipp, K.R.; Liu, G.; Benza, J.J.; Pletnev, V.; Pavlov, T.S.; Staruschenko, A.; Mohieldin, A.M.; Takahashi, M.; et al. Regulation of Polycystin-1 Function by Calmodulin Binding. PLoS ONE 2016, 11, e0161525. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P. The chondrocyte: A cell under pressure. Br. J. Rheumatol. 1994, 33, 901–908. [Google Scholar] [CrossRef]
- Guilak, F.; Ratcliffe, A.; Mow, V.C. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal microscopy study. J. Orthop. Res. 1995, 13, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.O.; Nishida, K.; Bavington, C.; Godolphin, J.L.; Dunne, E.; Walmsley, S.; Jobanputra, P.; Nuki, G.; Salter, D.M. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: Evidence of a role for alpha 5 beta 1 integrin as a chondrocyte mechanoreceptor. J. Orthop. Res. 1997, 15, 742–747. [Google Scholar] [CrossRef]
- Fu, S.; Thompson, C.; Ali, A.; Wang, W.; Chapple, J.; Mitchison, H.; Beales, P.; Wann, A.; Knight, M. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthr. Cartil. 2019, 27, 1064–1074. [Google Scholar] [CrossRef]
- Huang, J.; Ballou, L.R.; Hasty, K.A. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene 2007, 404, 101–109. [Google Scholar] [CrossRef]
- Chowdhury, T.T.; Akanji, O.O.; Salter, D.M.; Bader, D.L.; Lee, D.A. Dynamic compression influences interleukin-1beta-induced nitric oxide and prostaglandin E2 release by articular chondrocytes via alterations in iNOS and COX-2 expression. Biorheology 2008, 45, 257–274. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Griffin, T.M.; Liedtke, W.; Guilak, F. Increased susceptibility ofTrpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann. Rheum. Dis. 2013, 72, 300–304. [Google Scholar] [CrossRef]
- Wen, J.; Chen, Z.; Zhao, M.; Zu, S.; Zhao, S.; Wang, S.; Zhang, X. Cell Deformation at the Air-Liquid Interface Evokes Intracellular Ca2+ Increase and ATP Release in Cultured Rat Urothelial Cells. Front. Physiol. 2021, 12, 631022. [Google Scholar] [CrossRef]
- Pizzoni, A.; Bazzi, Z.; Di Giusto, G.; Alvarez, C.L.; Rivarola, V.; Capurro, C.; Schwarzbaum, P.J.; Ford, P. Release of ATP by TRPV4 activation is dependent upon the expression of AQP2 in renal cells. J. Cell. Physiol. 2021, 236, 2559–2571. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Gohr, C.M.; Mitton-Fitzgerald, E.; Lutz, M.K.; Dubyak, G.R.; Ryan, L.M. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res. Ther. 2013, 15, R154. [Google Scholar] [CrossRef]
- Boulter, C.; Mulroy, S.; Webb, S.; Fleming, S.; Brindle, K.; Sandford, R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc. Natl. Acad. Sci. USA 2001, 98, 12174–12179. [Google Scholar] [CrossRef]
- Xiao, Z.; Baudry, J.; Cao, L.; Huang, J.; Chen, H.; Yates, C.R.; Li, W.; Dong, B.; Waters, C.M.; Smith, J.C.; et al. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis. J. Clin. Investig. 2018, 128, 157–174. [Google Scholar] [CrossRef]
- Merrick, D.; Mistry, K.; Wu, J.; Gresko, N.; E Baggs, J.; HogenEsch, J.B.; Sun, Z.; Caplan, M.J. Polycystin-1 regulates bone development through an interaction with the transcriptional coactivator TAZ. Hum. Mol. Genet. 2019, 28, 16–30. [Google Scholar] [CrossRef]
- Dalagiorgou, G.; Piperi, C.; Georgopoulou, U.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell. Mol. Life Sci. 2013, 70, 167–180. [Google Scholar] [CrossRef]
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730. [Google Scholar]
- Dalagiorgou, G.; Piperi, C.; Adamopoulos, C.; Georgopoulou, U.; Gargalionis, A.N.; Spyropoulou, A.; Zoi, I.; Nokhbehsaim, M.; Damanaki, A.; Deschner, J.; et al. Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis. Cell. Mol. Life Sci. 2017, 74, 921–936. [Google Scholar] [CrossRef]
- Wu, G.; Markowitz, G.S.; Li, L.; D’Agati, V.D.; Factor, S.M.; Geng, L.; Tibara, S.; Tuchman, J.; Cai, Y.; Park, J.H.; et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat. Genet. 2000, 24, 75–78. [Google Scholar] [CrossRef]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Lee, S.H.; Liedtke, W.; Guilak, F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef]
- Xiao, Z.; Cao, L.; Liang, Y.; Huang, J.; Stern, A.R.; Dallas, M.; Johnson, M.; Quarles, L.D. Osteoblast-Specific Deletion of Pkd2 Leads to Low-Turnover Osteopenia and Reduced Bone Marrow Adiposity. PLoS ONE 2014, 9, e114198. [Google Scholar] [CrossRef]
- Su, Q.; Hu, F.; Ge, X.; Lei, J.; Yu, S.; Wang, T.; Zhou, Q.; Mei, C.; Shi, Y. Structure of the human PKD1-PKD2 complex. Science 2018, 361, eaat9819. [Google Scholar] [CrossRef]
- Kottgen, M.; Buchholz, B.; Garcia-Gonzalez, M.A.; Kotsis, F.; Fu, X.; Doerken, M.; Boehlke, C.; Steffl, D.; Tauber, R.; Wegierski, T.; et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 2008, 182, 437–447. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.-N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Johnson, G.P.; Stavenschi, E.; Eichholz, K.F.; Corrigan, M.A.; Fair, S.; Hoey, D.A. Mesenchymal stem cell mechanotransduction is cAMP dependent and regulated by adenylyl cyclase 6 and the primary cilium. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Wann, A.K.T.; Knight, M.M. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell. Mol. Life Sci. 2012, 69, 2967–2977. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, C.L.; McFie, M.; Chapple, J.P.; Beales, P.; Knight, M.M. Polycystin-2 Is Required for Chondrocyte Mechanotransduction and Traffics to the Primary Cilium in Response to Mechanical Stimulation. Int. J. Mol. Sci. 2021, 22, 4313. https://doi.org/10.3390/ijms22094313
Thompson CL, McFie M, Chapple JP, Beales P, Knight MM. Polycystin-2 Is Required for Chondrocyte Mechanotransduction and Traffics to the Primary Cilium in Response to Mechanical Stimulation. International Journal of Molecular Sciences. 2021; 22(9):4313. https://doi.org/10.3390/ijms22094313
Chicago/Turabian StyleThompson, Clare L., Megan McFie, J. Paul Chapple, Philip Beales, and Martin M. Knight. 2021. "Polycystin-2 Is Required for Chondrocyte Mechanotransduction and Traffics to the Primary Cilium in Response to Mechanical Stimulation" International Journal of Molecular Sciences 22, no. 9: 4313. https://doi.org/10.3390/ijms22094313
APA StyleThompson, C. L., McFie, M., Chapple, J. P., Beales, P., & Knight, M. M. (2021). Polycystin-2 Is Required for Chondrocyte Mechanotransduction and Traffics to the Primary Cilium in Response to Mechanical Stimulation. International Journal of Molecular Sciences, 22(9), 4313. https://doi.org/10.3390/ijms22094313





