Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation
Abstract
1. Introduction
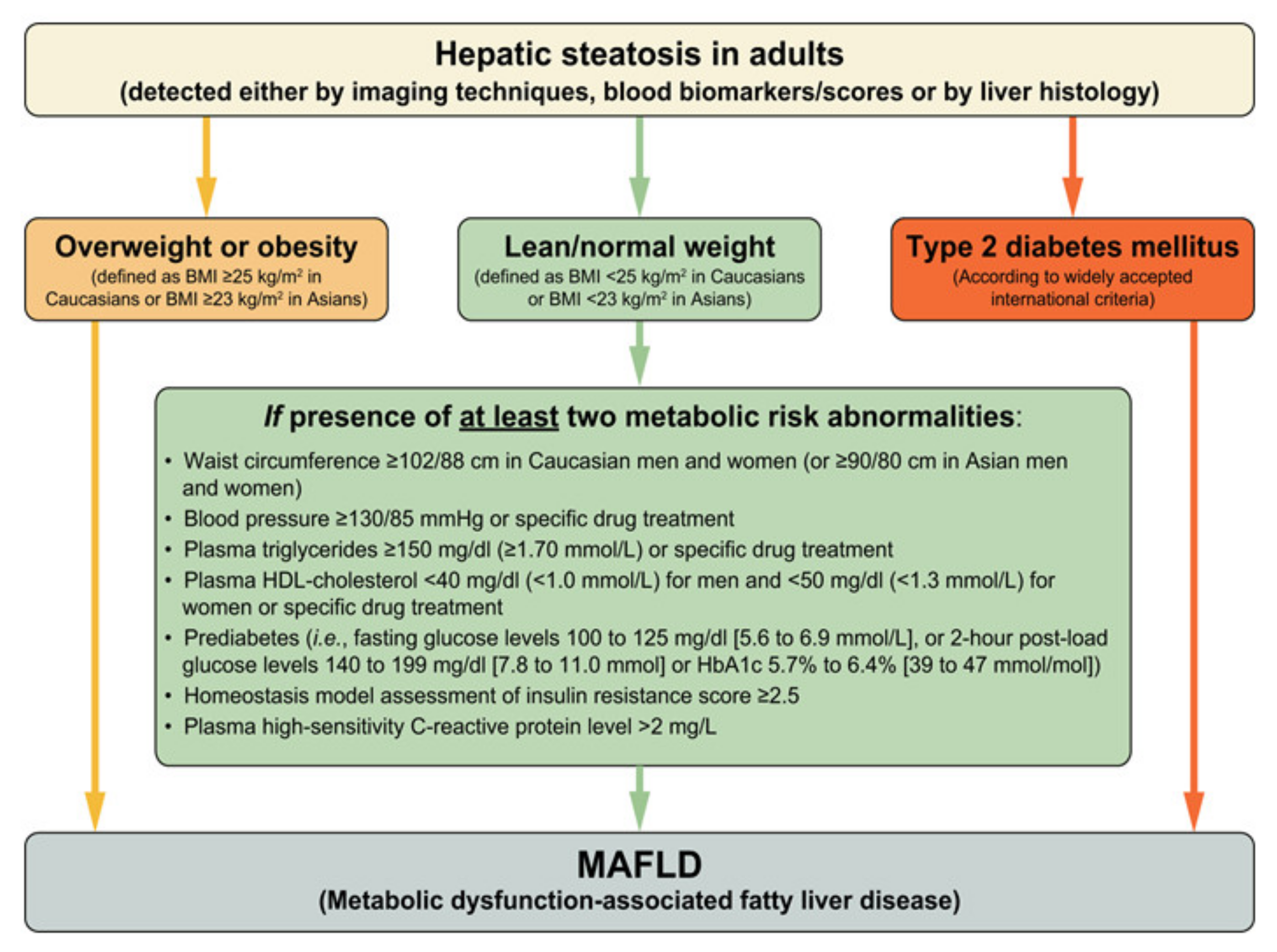
2. Metabolic Dysfunction Associated Fatty Liver Disease (MAFLD)
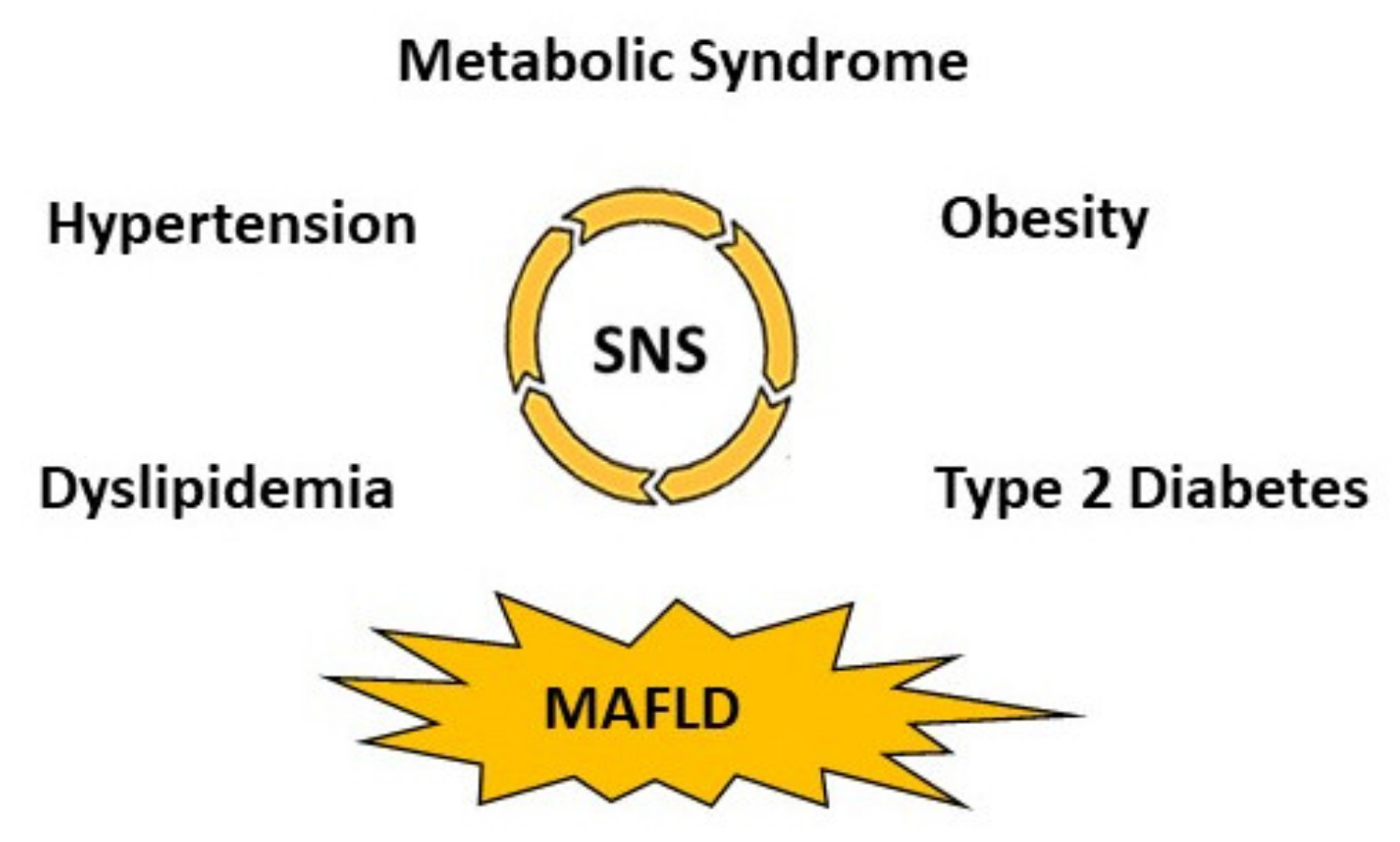
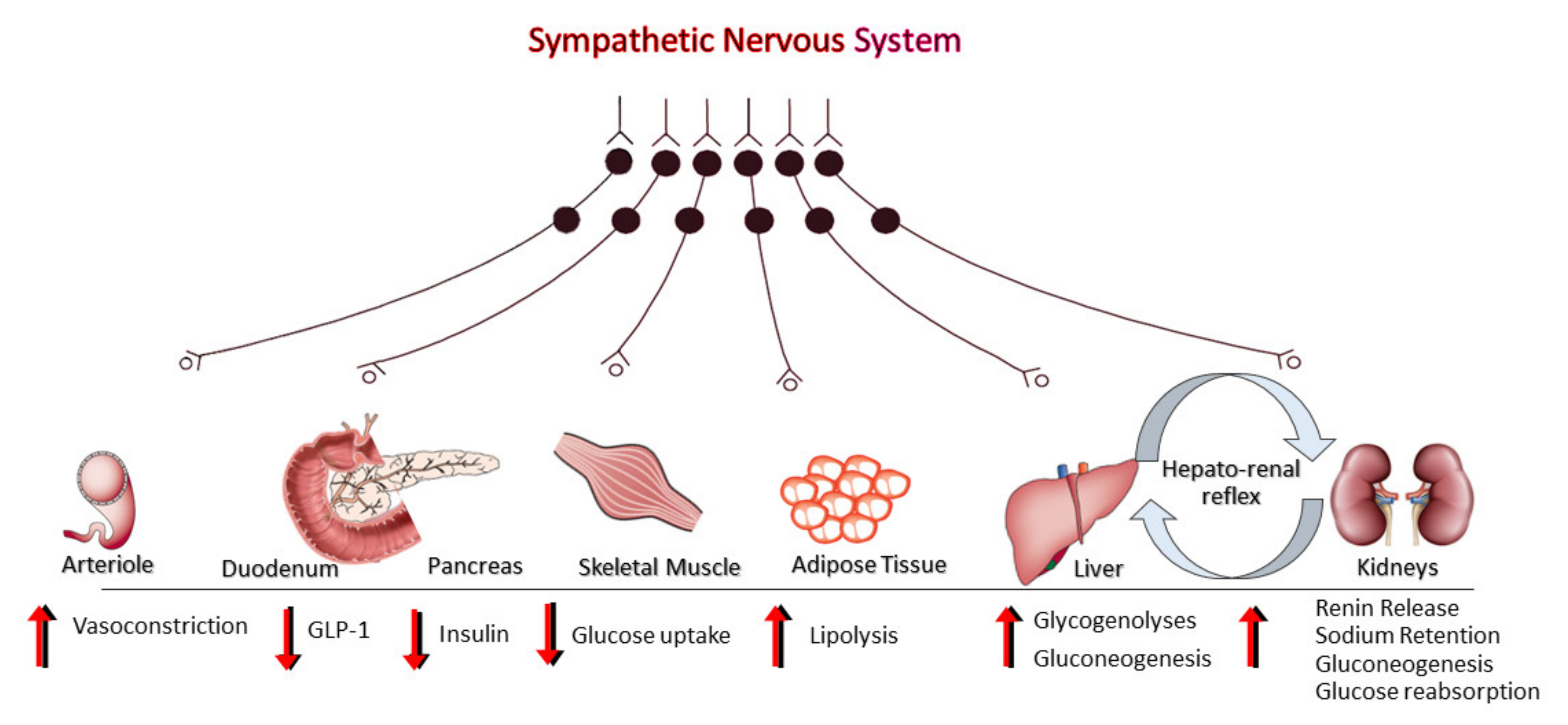
3. Sympathetic Nervous System (SNS) Activation, Metabolic Dysregulation and MAFLD
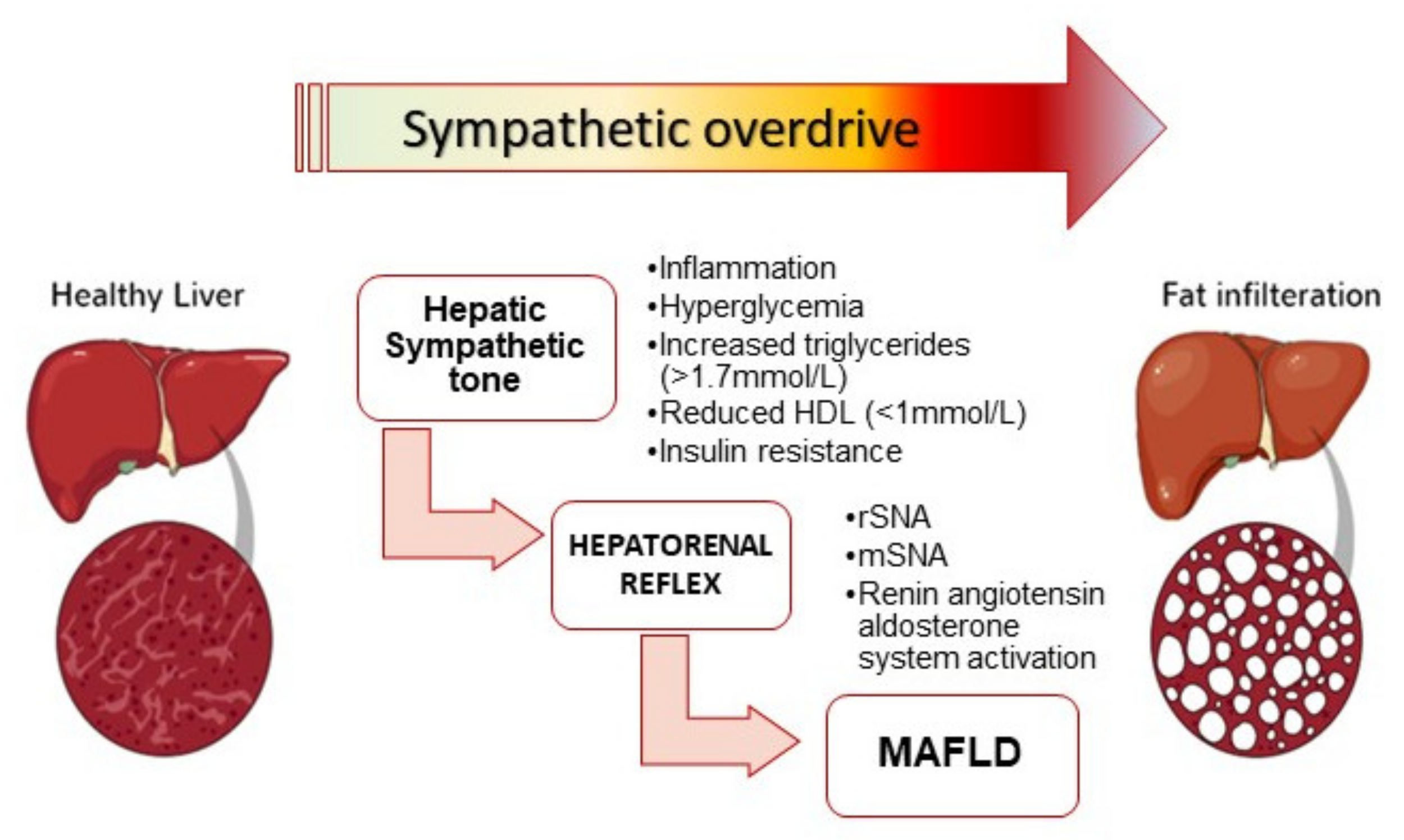
4. Sympathetic Activation and MAFLD Progression
5. Insights from Experimental Studies: Hepatic Fibrosis Is Sympathetically Driven
6. Potential Therapeutic Implications
6.1. The Sympathetic Nervous System as a Target for Therapy
6.2. Weight Loss and Exercise
7. Pharmacotherapy
8. Device-Based Approaches
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, L.A.; Roberts, S.K.; Strasser, S.I.; Mahady, S.E.; Powell, E.; Estes, C.; Razavi, H.; George, J. Nonalcoholic fatty liver disease burden: Australia, 2019–2030. J. Gastroenterol. Hepatol. 2020, 35, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Streba, L.A.M.; Vere, C.C.; Rogoveanu, I.; Streba, C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. World J. Gastroenterol. 2015, 21, 4103–4110. [Google Scholar] [CrossRef]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Dixon, J.B.; Schlaich, M.P. Sympathetic nervous activation in obesity and the metabolic syndrome—Causes, consequences and therapeutic implications. Pharmacol. Ther. 2010, 126, 159–172. [Google Scholar] [CrossRef]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef]
- Mancia, G.; Bombelli, M.; Corrao, G.; Facchetti, R.; Madotto, F.; Giannattasio, C.; Trevano, F.Q.; Grassi, G.; Zanchetti, A.; Sega, R. The metabolic syndrome in the PAMELA population: Daily life blood pressure, cardiac damage and prognosis. Hypertension 2007, 49, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.C.D.S.; Pinto, I.S.D.J.; Mourão, A.A.; Fajemiroye, J.O.; Colombari, E.; Reis, Â.D.S.; Freiria-Oliveira, A.H.; Ferreira-Neto, M.L.; Pedrino, G.R. Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front. Physiol. 2015, 6, 234. [Google Scholar] [CrossRef]
- Carnagarin, R.; Matthews, V.; Zaldivia, M.T.K.; Peter, K.; Schlaich, M.P. The bidirectional interaction between the sympathetic nervous system and immune mechanisms in the pathogenesis of hypertension. Br. J. Pharmacol. 2019, 176, 1839–1852. [Google Scholar] [CrossRef]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome Pandemic. Arter. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Sigala, B.; McKee, C.; Soeda, J.; Pazienza, V.; Morgan, M.; Lin, C.-I.; Selden, C.; Borght, S.V.; Mazzoccoli, G.; Roskams, T.; et al. Sympathetic Nervous System Catecholamines and Neuropeptide Y Neurotransmitters Are Upregulated in Human NAFLD and Modulate the Fibrogenic Function of Hepatic Stellate Cells. PLoS ONE 2013, 8, e72928. [Google Scholar]
- Oben, J.A.; Yang, S.; Lin, H.; Ono, M.; Diehl, A.M. Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochem. Biophys. Res. Commun. 2003, 302, 685–690. [Google Scholar] [CrossRef]
- Oben, J.A.; Roskams, T.; Yang, S.; Lin, H.; Sinelli, N.; Torbenson, M.; Smedh, U.; Moran, T.H.; Li, Z.; Huang, J.; et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 2004, 53, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Oben, J.A.; Roskams, T.; Yang, S.; Lin, H.; Sinelli, N.; Li, Z.; Torbenson, M.; Huang, J.; Guarino, P.; Kafrouni, M.; et al. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology 2003, 38, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, L.; Desmoulière, A.; Decourt, B.; Evadé, L.; Bedin, C.; Boussarie, L.; Barrier, L.; Vidaud, M.; Rosenbaum, J. Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology 2002, 35, 325–331. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Lambert, G.W.; Masuo, K.; Esler, M.D.; Nestel, P.J. Effects of Dietary Weight Loss on Sympathetic Activity and Cardiac Risk Factors Associated with the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5998–6005. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Grima, M.T.; Eikelis, N.; Nestel, P.J.; Dawood, T.; Schlaich, M.P.; Chopra, R.; Masuo, K.; Esler, M.D.; Sari, C.I.; et al. The Effects of Weight LossVersusWeight Loss Maintenance on Sympathetic Nervous System Activity and Metabolic Syndrome Components. J. Clin. Endocrinol. Metab. 2011, 96, E503–E508. [Google Scholar] [CrossRef]
- Mancia, G.; Bousquet, P.; Elghozi, J.L.; Esler, M.; Grassi, G.; Julius, S.; Reid, J.; Van Zwieten, P.A. The sympathetic nervous system and the metabolic syndrome. J. Hypertens. 2007, 25, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.C.; Ciccariello, L.; Perrone, F.; Stoico, V.; Gussoni, G.; Scotti, A.; Muggeo, M. Clinical efficacy and tolerability of alpha-blocker doxazozin as add-on therapy in patients with hypertension and impaired glucose metabolism. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 137–147. [Google Scholar] [CrossRef]
- Sannajust, F.; Head, G.A. Involvement of imidazoline-preferring receptors in regulation of sympathetic tone. Am. J. Cardiol. 1994, 74, A7–A19. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving Concepts in the Pathogenesis of NASH: Beyond Steatosis and Inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Tsujikawa, H.; Effendi, K.; Ojima, H.; Harada, K.; Zen, Y.; Kondo, F.; Nakano, M.; Kage, M.; Sumida, Y.; et al. Pathological findings of nonalcoholic steatohepatitis and nonalcoholic fatty liver disease. Pathol. Int. 2016, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in Adipose Tissue and Hepatic Lipid Kinetics in Obese Men and Women with Nonalcoholic Fatty Liver Disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Kozakova, M.; Højlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. The RISC Investigators Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic Neural Activation in Visceral Obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef]
- Florian, J.P.; Pawelczyk, J.A. Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin. Sci. 2009, 118, 61–69. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Pei, L.; Ackermans, M.T.; Foppen, E.; Borgers, A.J.; Kwakkel, J.; Alkemade, A.; Fliers, E.; Kalsbeek, A. Hypothalamic Neuropeptide Y (NPY) Controls Hepatic VLDL-Triglyceride Secretion in Rats via the Sympathetic Nervous System. Diabetes 2012, 61, 1043–1050. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.W.; Kim, J.E.; Kim, J.Y. Age-associated changes in fat metabolism in the rat and its relation to sympathetic activity. Life Sci. 2006, 79, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Ioannou, G.N.; Boyko, E.J.; Utzschneider, K.M. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: Results of a US national survey in three ethnic groups. J. Gastroenterol. Hepatol. 2013, 28, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, N.E.; Eikelis, N.; Lambert, E.A.; Esler, M.D. Mediators of sympathetic activation in metabolic syndrome obesity. Curr. Hypertens. Rep. 2008, 10, 440–447. [Google Scholar] [CrossRef]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-Alcoholic Fatty Liver Disease (NAFLD) and Its Connection with Insulin Resistance, Dyslipidemia, Atherosclerosis and Coronary Heart Disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoek, A.M.; van Heijningen, C.; Schroder-van der Elst, J.P.; Ouwens, D.M.; Havekes, L.M.; Romijn, J.A.; Kalsbeek, A.; Pijl, H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 2008, 57, 2304–2310. [Google Scholar] [CrossRef]
- Esler, M.; Dudley, F.; Jennings, G.; Debinski, H.; Lambert, G.; Jones, P.; Crotty, B.; Colman, J.; Willett, I. Increased Sympathetic Nervous Activity and the Effects of Its Inhibition with Clonidine in Alcoholic Cirrhosis. Ann. Intern. Med. 1992, 116, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Gregory, C.; Azzam, O.; Hillis, G.S.; Schultz, C.; Watts, G.F.; Bell, D.; Matthews, V.; Schlaich, M.P. The Role of Sympatho-Inhibition in Combination Treatment of Obesity-Related Hypertension. Curr. Hypertens. Rep. 2017, 19, 99. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Tsai, T.-H.; Huang, Y.-T.; Lee, T.-Y.; Chan, C.-C.; Lee, K.-C.; Lin, H.-C. Hepatic endothelin-1 and endocannabinoids-dependent effects of hyperleptinemia in nonalcoholic steatohepatitis-cirrhotic rats. Hepatology 2012, 55, 1540–1550. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Papatheodoridis, G.V.; Hadziyannis, E.; Georgiou, A.; Kafiri, G.; Tiniakos, D.G.; Manesis, E.K.; Archimandritis, A.J. Serum adipokine levels in chronic liver diseases: Association of resistin levels with fibrosis severity. Scand. J. Gastroenterol. 2008, 43, 1128–1136. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunology 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Kamada, Y.; Tamura, S.; Kiso, S.; Matsumoto, H.; Saji, Y.; Yoshida, Y.; Fukui, K.; Maeda, N.; Nishizawa, H.; Nagaretani, H.; et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 2003, 125, 1796–1807. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Papatheodoridis, G.V.; Archimandritis, A.J. The Evolving Role of Leptin and Adiponectin in Chronic Liver Diseases. Am. J. Gastroenterol. 2006, 101, 2629–2640. [Google Scholar] [CrossRef]
- Reaven, G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol. Metab. Clin. North Am. 2004, 33, 283–303. [Google Scholar] [CrossRef]
- Ming, Z.; Smyth, D.D.; Lautt, W.W. Intrahepatic adenosine triggers a hepatorenal reflex to regulate renal sodium and water excretion. Auton Neurosci. 2001, 93, 1–7. [Google Scholar] [CrossRef]
- Wider, M.D. Metabolic syndrome and the hepatorenal reflex. Surg. Neurol. Int. 2016, 7, 99. [Google Scholar] [CrossRef]
- Kostreva, D.R.; Castaner, A.; Kampine, J.P. Reflex effects of hepatic baroreceptors on renal and cardiac sympathetic nerve activity. Am. J. Physiol. Content 1980, 238, 390–394. [Google Scholar] [CrossRef]
- Ezzat, W.R.; Lautt, W.W. Hepatic arterial pressure-flow autoregulation is adenosine mediated. Am. J. Physiol. Circ. Physiol. 1987, 252, H836–H845. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Roden, M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol. Cell. Endocrinol. 2013, 379, 35–42. [Google Scholar] [CrossRef]
- Oberbach, A.; Neuhaus, J.; Inge, T.; Kirsch, K.; Schlichting, N.; Blüher, S.; Kullnick, Y.; Kugler, J.; Baumann, S.; Till, H. Bariatric surgery in severely obese adolescents improves major comorbidities including hyperuricemia. Metabolism 2014, 63, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Oben, J.A.; Diehl, A.M. Sympathetic nervous system regulation of liver repair. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2004, 280, 874–883. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Ring-Larsen, H.; Kanstrup, I.L.; Christensen, N.J. Splanchnic and renal elimination and release of catecholamines in cirrhosis. Evidence of enhanced sympathetic nervous activity in patients with decompensated cirrhosis. Gut 1984, 25, 1034–1043. [Google Scholar] [CrossRef]
- Wallace, K.; Burt, A.D.; Wright, M.C. Liver fibrosis. Biochem. J. 2008, 411, 1–18. [Google Scholar] [CrossRef]
- Oben, J.A.; Roskams, T.; Yang, S.; Lin, H.; Sinelli, N.; Li, Z.; Torbenson, M.; Thomas, S.A.; Diehl, A.M. Norepinephrine induces hepatic fibrogenesis in leptin deficient ob/ob mice. Biochem. Biophys. Res. Commun. 2003, 308, 284–292. [Google Scholar] [CrossRef]
- Honda, H.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Lang, T.; Enomoto, N.; Kitamura, T.; Takei, Y.; Sato, N. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology 2002, 36, 12–21. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Schriemer, R.; Robertson, G.R. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J. Hepatol. 2002, 37, 206–213. [Google Scholar] [CrossRef]
- Saxena, N.K.; Ikeda, K.; Rockey, D.C.; Friedman, S.L.; Anania, F.A. Leptin in hepatic fibrosis: Evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 2002, 35, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Eikelis, N.; Schlaich, M.; Aggarwal, A.; Kaye, D.; Esler, M. Interactions Between Leptin and the Human Sympathetic Nervous System. Hypertension 2003, 41, 1072–1079. [Google Scholar] [CrossRef]
- Sancho-Bru, P.; Bataller, R.; Colmenero, J.; Gasull, X.; Moreno, M.; Arroyo, V.; Brenner, D.A.; Ginès, P. Norepinephrine induces calcium spikes and proinflammatory actions in human hepatic stellate cells. Am. J. Physiol. Liver Physiol. 2006, 291, G877–G884. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-T. The role of the sympathetic nervous system in promoting liver cirrhosis induced by carbon tetrachloride, using the essential hypertensive animal (SHR). J. Auton. Nerv. Syst. 1992, 37, 163–173. [Google Scholar] [CrossRef]
- Cruise, J.L.; Knechtle, S.J.; Bollinger, R.R.; Kuhn, C.; Michalopoulos, G. Alpha 1-adrenergic effects and liver regeneration. Hepatology 1987, 7, 1189–1194. [Google Scholar] [CrossRef]
- Kato, H.; Shimazu, T. Effect of Autonomic Denervation on DNA Synthesis during Liver Regeneration after Partial Hepatectomy. JBIC J. Biol. Inorg. Chem. 1983, 134, 473–478. [Google Scholar] [CrossRef]
- Kiba, T.; Tanaka, K.; Numata, K.; Hoshino, M.; Inoue, S. Facilitation of liver regeneration after partial hepatectomy by ventromedial hypothalamic lesions in rats. Pflügers Arch. Eur. J. Physiol. 1994, 428, 26–29. [Google Scholar] [CrossRef]
- Ohtake, M.; Sakaguchi, T.; Yoshida, K.; Muto, T. Hepatic branch vagotomy can suppress liver regeneration in partially he atectomized rats. HPB Surg. 1993, 6, 277–286. [Google Scholar] [CrossRef]
- Attar, B.M.; Van Thiel, D.H. Current Concepts and Management Approaches in Nonalcoholic Fatty Liver Disease. Sci. World J. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Pan, L.-L.; Ma, Z.-M.; Chen, Z.; Huang, Z.-F.; Sun, Q.; Lu, Y.; Han, C.-K.; Lin, M.-Z.; Li, X.-J.; et al. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: A 1-year follow-up study. Diabetes Obes. Metab. 2017, 19, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Abe, K.; Usami, K.; Imaizumi, H.; Hayashi, M.; Okai, K.; Kanno, Y.; Tanji, N.; Watanabe, H.; Ohira, H. Simple Resistance Exercise helps Patients with Non-alcoholic Fatty Liver Disease. Int. J. Sports Med. 2015, 36, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- De Luis, D.A.; Aller, R.; Izaola, O.; Gonzalez Sagrado, M.; Conde, R. Effect of two different hypocaloric diets in transaminases and insulin resistance in nonalcoholic fatty liver disease and obese patients. Nutr. Hosp. 2010, 25, 730–735. [Google Scholar] [PubMed]
- Haufe, S.; Engeli, S.; Kast, P.; Böhnke, J.; Utz, W.; Haas, V.; Hermsdorf, M.; Mähler, A.; Wiesner, S.; Birkenfeld, A.L.; et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011, 53, 1504–1514. [Google Scholar] [CrossRef]
- Krasnoff, J.B.; Painter, P.L.; Wallace, J.P.; Bass, N.M.; Merriman, R.B. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008, 47, 1158–1166. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Kelleni, M.; Geddawy, A. Nonalcoholic fatty liver disease: Current and potential therapies. Life Sci. 2013, 92, 114–118. [Google Scholar] [CrossRef]
- Stewart, K.E.; Haller, D.L.; Sargeant, C.; Levenson, J.L.; Puri, P.; Sanyal, A.J. Readiness for behaviour change in non-alcoholic fatty liver disease: Implications for multidisciplinary care models. Liver Int. 2015, 35, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, N.E.; Lambert, E.A.; Grima, M.T.; Eikelis, N.; Nestel, P.J.; Dawood, T.; Schlaich, M.P.; Masuo, K.; Chopra, R.; Sari, C.I.; et al. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabetes Obes. Metab. 2011, 14, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Bhathal, P.S.; Hughes, N.R.; O’Brien, P.E. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology 2004, 39, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Mummadi, R.R.; Kasturi, K.S.; Chennareddygari, S.; Sood, G.K. Effect of Bariatric Surgery on Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2008, 6, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Pareja, J.C.; Chaim, E.A. Nonalcoholic fatty liver disease and bariatric surgery: A comprehensive review. Sao Paulo Med. J. 2017, 135, 277–295. [Google Scholar] [CrossRef]
- Houglum, K.; Venkataramani, A.; Lyche, K.; Chojkier, M. A pilot study of the effects of d-α-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology 1997, 113, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer. The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A Randomized Controlled Trial of Metformin versus Vitamin E or Prescriptive Diet in Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Konopski, Z.; Eggesbø, H.B.; Von Volkmann, H.L.; Raschpichler, G.; Bjøro, K.; Haaland, T.; Løberg, E.M.; Birkeland, K. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand. J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; LaVine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. From Fat to Inflammation. Gastroenterology 2006, 130, 207–210. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2008, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ioannides-Demos, L.; Piccenna, L.; McNeil, J. Pharmacotherapies for obesity: Past, current, and future therapies. J. Obes. 2011, 2011, 18. [Google Scholar] [CrossRef]
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151. [Google Scholar] [CrossRef]
- Xu, J.; Cho, H.; O’Malley, S.; Park, J.H.Y.; Clarke, S.D. Dietary Polyunsaturated Fats Regulate Rat Liver Sterol Regulatory Element Binding Proteins-1 and -2 in Three Distinct Stages and by Different Mechanisms. J. Nutr. 2002, 132, 3333–3339. [Google Scholar] [CrossRef]
- Tanaka, N.; Sano, K.; Horiuchi, A.; Tanaka, E.; Kiyosawa, K.; Aoyama, T. Highly Purified Eicosapentaenoic Acid Treatment Improves Nonalcoholic Steatohepatitis. J. Clin. Gastroenterol. 2008, 42, 413–418. [Google Scholar] [CrossRef]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Coffee induces expression of glucuronosyl transferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology 2010, 139, 1699–1710. [Google Scholar] [CrossRef]
- Gressner, O.A. Less Smad2 is good for you! A scientific update on coffee’s liver benefits. Hepatology 2009, 50, 970–997. [Google Scholar] [CrossRef]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef]
- Itskovitz, H.D. Alpha 1-blockade for the treatment of hypertension: A megastudy of terazosin in 2214 clinical practice settings. Clin. Ther. 1994, 16, 490–504. [Google Scholar]
- ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000, 283, 1967–1975. [Google Scholar] [CrossRef]
- Sharma, A.M.; Pischon, T.; Hardt, S.; Kunz, I.; Luft, F.C. Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension 2001, 37, 250–254. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bell, D.S.; Fonseca, V.; Katholi, R.E.; McGill, J.B.; Phillips, R.A.; Raskin, P.; Wright, J.T.; Bangalore, S.; Holdbrook, F.K.; et al. For the GEMINI Investigators: Body weight changes with beta-blocker use: Results from GEMINI. Am. J. Med. 2007, 120, 610–615. [Google Scholar] [CrossRef]
- Fonseca, V.; Bakris, G.L.; Bell, D.S.; McGill, J.B. for the GEMINI Investigators: Differential effect of beta-blocker therapy on insulin resistance as a function of insulin sensitizer use: Results from GEMINI. Diabet. Med. 2007, 24, 759–763. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Bakris, G.L.; McGill, J.B. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes Obes. Metab. 2009, 11, 234–238. [Google Scholar] [CrossRef]
- Bakris, G.L.; Fonseca, V.; Katholi, R.E.; McGill, J.B.; Messerli, F.; Phillips, R.A.; Raskin, P.; Wright, J.T.; Waterhouse, B.; Lukas, M.A.; et al. The GEMINI Investigators: Differential effects of beta-blockers on albuminuria in patients with type 2 diabetes. Hypertension 2005, 46, 1309–1315. [Google Scholar] [CrossRef]
- Hussain, M.; Saeed, M.; Babar, M.Z.M.; Atif, M.A.; Akhtar, L. Nebivolol Attenuates Neutrophil Lymphocyte Ratio: A Marker of Subclinical Inflammation in Hypertensive Patients. Int. J. Hypertens. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Chazova, I.; Almazov, V.A.; Shlyakhto, E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: A comparison with metformin. Diabetes Obes. Metab. 2006, 8, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Haenni, A.; Lithell, H. Moxonidine improves insulin sensitivity in insulin-resistant hypertensives. J. Hypertens Suppl. 1999, 17, S29–S35. [Google Scholar] [PubMed]
- Topal, E.; Cikim, A.S.; Cikim, K.; Temel, I.; Ozdemir, R.; Cıkım, A.S. The Effect of Moxonidine on Endothelial Dysfunction in Metabolic Syndrome. Am. J. Cardiovasc. Drugs 2006, 6, 343–348. [Google Scholar] [CrossRef]
- Strojek, K.; Grzeszczak, W.; Górska, J.; Leschinger, M.I.; Ritz, E. Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: Novel approach to prevent progression of diabetic nephropathy? J. Am. Soc. Nephrol. 2001, 12, 602–605. [Google Scholar]
- Vonend, O.; Marsalek, P.; Russ, H.; Wulkow, R.; Oberhauser, V.; Rump, L.C. Moxonidine treatment of hypertensive patients with advanced renal failure. J. Hypertens. 2003, 21, 1709–1717. [Google Scholar] [CrossRef]
- Lambert, E.A.; Sari, C.; Eikelis, N.; Phillips, S.E.; Grima, M.; Straznicky, N.E.; Dixon, J.B.; Schlaich, M.P.; Head, G.A.; Lambert, G.W. Effects of moxonidine and low calorie diet: Cardio-metabolic benefits from combination of both therapies. Obesity 2017, 217, 1894–1902. [Google Scholar] [CrossRef]
- Chazova, I.; Schlaich, M.P. Improved Hypertension Control with the Imidazoline Agonist Moxonidine in a Multinational Metabolic Syndrome Population: Principal Results of the MERSY Study. Int. J. Hypertens. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanjuliani, A.F.; De Abreu, V.G.; Francischetti, E.A. Selective imidazoline agonist moxonidine in obese hypertensive patients. Int. J. Clin. Pr. 2006, 60, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME investigators. 27 version 2, dated 25 June 2016 Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Carnagarin, R.; Kiuchi, M.G.; Goh, G.; Adams, L.; Cohen, N.; Kavnoudias, H.; Gan, S.K.; Van Schie, G.; Esler, M.D.; Matthews, V.B.; et al. Role of the sympathetic nervous system in cardiometabolic control. J. Hypertens. 2021. [Google Scholar] [CrossRef]
- Kiuchi, M.G.; Ganesan, K.; Keating, J.; Carnagarin, R.; Matthews, V.B.; Herat, L.Y.; Goh, G.; Adams, L.; Schlaich, M.P. Combined renal and common hepatic artery denervation as a novel approach to reduce cardiometabolic risk: Technical approach, feasibility and safety in a pre-clinical model. Clin. Res. Cardiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Krum, H.; Schlaich, M.; Whitbourn, R.; Sobotka, P.A.; Sadowski, J.; Bartus, K.; Kapelak, B.; Walton, A.; Sievert, H.; Thambar, S.; et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet 2009, 373, 1275–1281. [Google Scholar] [CrossRef]
- Hering, D.; Lambert, E.A.; Marusic, P.; Walton, A.S.; Krum, H.; Lambert, G.W.; Esler, M.D.; Schlaich, M.P. Substantial Reduction in Single Sympathetic Nerve Firing After Renal Denervation in Patients with Resistant Hypertension. Hypertension 2013, 61, 457–464. [Google Scholar] [CrossRef]
- Mahfoud, F.; Schlaich, M.; Kindermann, I.; Ukena, C.; Cremers, B.; Brandt, M.C.; Hoppe, U.C.; Vonend, O.; Rump, L.C.; Sobotka, P.A.; et al. Response to Letter Regarding Article, “Effect of Renal Sympathetic Denervation on Glucose Metabolism in Patients with Resistant Hypertension: A Pilot Study”. Circulation 2011, 124, 1940–1946. [Google Scholar] [CrossRef]
- Schlaich, M.; Hering, D.; Sobotka, P.; Krum, H.; Lambert, G.; Lambert, E.; Esler, M. Effects of Renal Denervation on Sympathetic Activation, Blood Pressure, and Glucose Metabolism in Patients with Resistant Hypertension. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Straznicky, N.; Grima, M.; Ika-Sari, C.; Dawood, T.; Mahfoud, F.; Lambert, E.; Chopra, R.; Socratous, F.; Hennebry, S.; et al. Renal denervation: A potential new treatment modality for polycystic ovary syndrome? J. Hypertens. 2011, 29, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, A.; Prejbisz, A.; Florczak, E.; Kądziela, J.; Śliwiński, P.; Bieleń, P.; Michałowska, I.; Kabat, M.; Warchoł, E.; Januszewicz, M.; et al. Effects of Renal Sympathetic Denervation on Blood Pressure, Sleep Apnea Course, and Glycemic Control in Patients with Resistant Hypertension and Sleep Apnea. Hypertension 2011, 58, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Kraft, G.; Vrba, A.; Scott, M.; Allen, E.; Edgerton, D.S.; Williams, P.E.; Vafai, S.B.; Azamian, B.R.; Cherrington, A.D. Sympathetic Denervation of the Common Hepatic Artery Lessens Glucose Intolerance in the Fat- and Fructose-Fed Dog. Diabetes 2019, 68, 1143–1155. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Eliveld, J.; Foppen, E.; Busker, S.; Ackermans, M.T.; Fliers, E.; Kalsbeek, A. Hepatic denervation and dyslipidemia in obese Zucker (fa/fa) rats. Int. J. Obes. 2015, 39, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef]
- Wallbach, M.; Lehnig, L.-Y.; Helms, H.-J.; Schroer, C.; Müller, G.A.; Wachter, R.; Koziolek, M.J. Long-term effects of baroreflex activation therapy on glucose metabolism. Acta Diabetol. 2014, 52, 829–835. [Google Scholar] [CrossRef]
- Lohmeier, T.E.; Dwyer, T.M.; Irwin, E.D.; Rossing, M.A.; Kieval, R.S. Prolonged Activation of the Baroreflex Abolishes Obesity-Induced Hypertension. Hypertension 2007, 49, 1307–1314. [Google Scholar] [CrossRef]
| Intervention Strategy | Mechanism of Action | Outcomes |
|---|---|---|
| Pharmacotherapeutic Strategies | ||
| Vitamin E (α-tocopherol) | Free radical scavenger—inhibits oxidative stress | Reductions in serum aminotransferases levels and improvement in hepatic inflammation resolution of steatohepatitis [89,90]. However, vitamin E supplement (400 IU/day) was associated with all cause mortality [91] and prostate cancer [92] |
| Metformin | Insulin sensitizer | Varied outcomes with improvement in hepatocellular inflammation, steatosis and fibrosis, however inconclusive [2,93,94,95] |
| Thiazolidinediones (TZDs), e.g., pioglitazone | Enhanced insulin sensitivity by acting on peroxisome proliferator-activated receptor gamma and increasing circulating adiponectin prevent the activation of adipocyte c-jun kinase, a kinase that when activated impairs adipocyte responsiveness to insulin and adipocyte storage of TG [96,97,98] | Significantly improved aminotransferase levels, hepatic inflammation and steatosis but did not alter the stage of fibrosis [57,65,96,97] The long-term use of TZDs is associated with side effects such as weight gain (average 4 Kg),congestive heart failure (CHF), other cardiovascular morbidity, bone loss (fracture risk) and urinary bladder cancers [99] |
| Statins | Inhibitors of cholesterol synthesis | Lack of evidence and increased risk of drug induced liver injury [75] |
| Weight loss medication | Weight loss mediated beneficial effect on MAFLD | No medication for weight loss has yet been identified, to have long-term safety, efficacy and tolerability [100] |
| Dietary interventions | ||
| n − 3/n − 6 polyunsaturated fatty acids (PUFA) dietary ratio | low n − 3/n − 6 PUFA ratio in MAFLD | Supplementation of omega-3 [101] and other PUFAs in diet [102] have shown a beneficial effect on both hepatic lipogenesis and steatosis [101,102,103] |
| Trans-fat enriched foods High fructose foods, e.g., corn syrup | Insulin resistance Hepatic steatosis hepatic fructose metabolism favors ATP depletion, lipotoxicity, insulin resistance and enhances enhanced TNF expression [104] | |
| Coffee (caffeine) | Caffeine alters TGFβ signaling pathways to reduce the transcription of connective tissue growth factor (CTGF), a major stimulator of fibrosis [105,106,107]. | Reduction of hepatic inflammation and fibrosis in morbidly obese MAFLD patients [105,106,107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnagarin, R.; Tan, K.; Adams, L.; Matthews, V.B.; Kiuchi, M.G.; Marisol Lugo Gavidia, L.; Lambert, G.W.; Lambert, E.A.; Herat, L.Y.; Schlaich, M.P. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation. Int. J. Mol. Sci. 2021, 22, 4241. https://doi.org/10.3390/ijms22084241
Carnagarin R, Tan K, Adams L, Matthews VB, Kiuchi MG, Marisol Lugo Gavidia L, Lambert GW, Lambert EA, Herat LY, Schlaich MP. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation. International Journal of Molecular Sciences. 2021; 22(8):4241. https://doi.org/10.3390/ijms22084241
Chicago/Turabian StyleCarnagarin, Revathy, Kearney Tan, Leon Adams, Vance B. Matthews, Marcio G. Kiuchi, Leslie Marisol Lugo Gavidia, Gavin W. Lambert, Elisabeth A. Lambert, Lakshini Y. Herat, and Markus P. Schlaich. 2021. "Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation" International Journal of Molecular Sciences 22, no. 8: 4241. https://doi.org/10.3390/ijms22084241
APA StyleCarnagarin, R., Tan, K., Adams, L., Matthews, V. B., Kiuchi, M. G., Marisol Lugo Gavidia, L., Lambert, G. W., Lambert, E. A., Herat, L. Y., & Schlaich, M. P. (2021). Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation. International Journal of Molecular Sciences, 22(8), 4241. https://doi.org/10.3390/ijms22084241






