An Overview of Long Non-Coding (lnc)RNAs in Neuroblastoma
Abstract
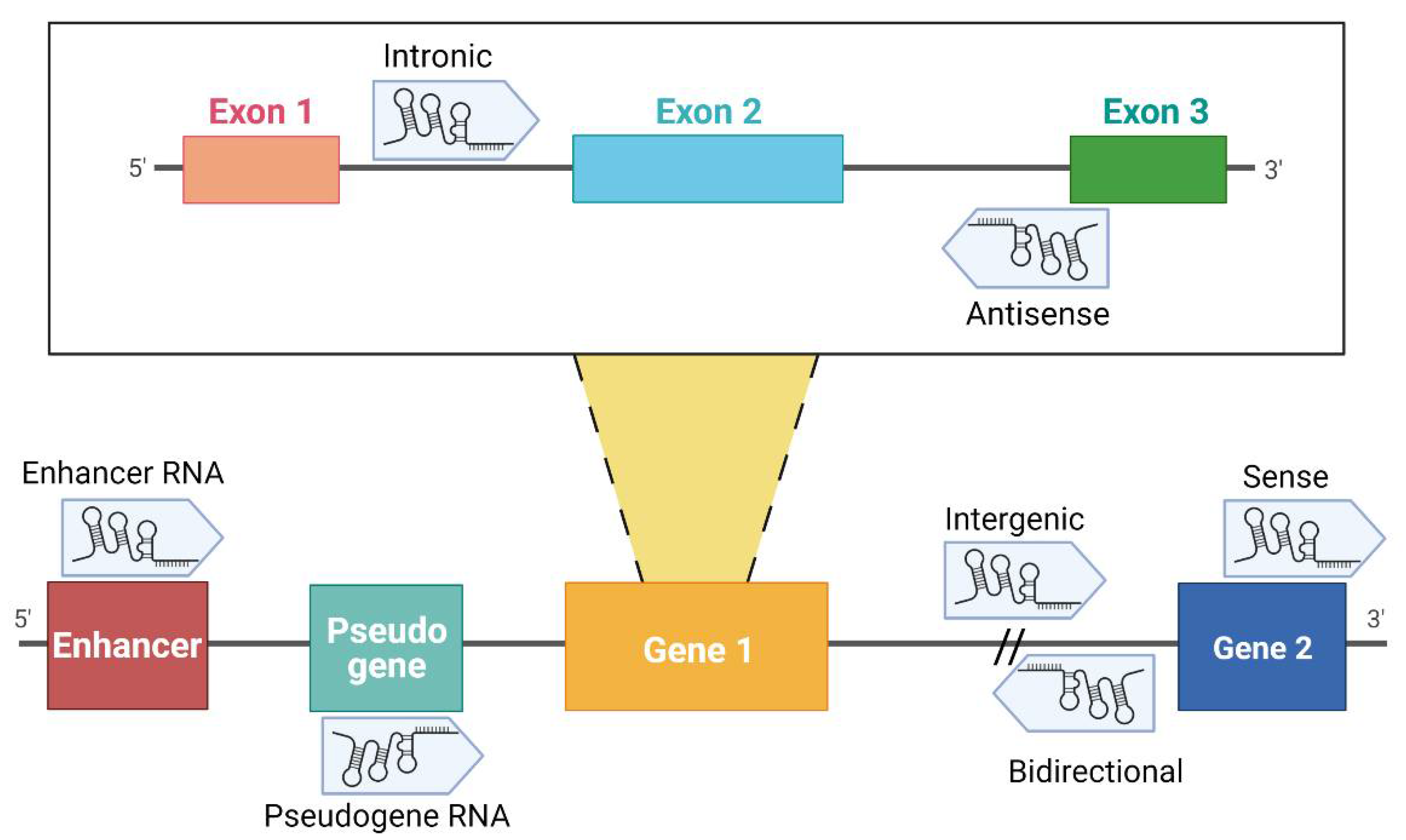
1. Introduction
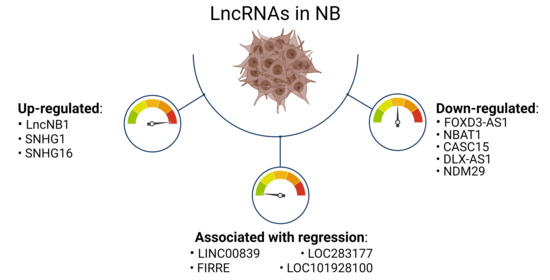
2. Main lncRNAs Down-Regulated in Neuroblastoma
2.1. FOXD3-AS1
2.2. NBAT1 and CASC15
2.3. DLX6-AS1
2.4. NDM29
3. Main lncRNAs Up-Regulated in Neuroblastoma and Correlated with MYCN Amplification
3.1. LncNB1
3.2. SNHG1
3.3. SNHG16
4. LncRNA Associated with NB Regression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsubota, S.; Kadomatsu, K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018, 372, 211–221. [Google Scholar] [CrossRef]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef]
- Trigg, R.M.; Turner, S.D. ALK in neuroblastoma: Biological and therapeutic implications. Cancers 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Cartolano, M.; Hero, B.; Welte, A.; Kahlert, Y.; Roderwieser, A.; Bartenhagen, C.; Walter, E.; Gecht, J.; Kerschke, L.; et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 2018, 1170, 1165–1170. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Prajapati, B.; Fatma, M.; Fatima, M.; Khan, M.T.; Sinha, S.; Seth, P.K. Identification of lncRNAs Associated With Neuroblastoma in Cross-Sectional Databases: Potential Biomarkers. Front. Mol. Neurosci. 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef] [PubMed]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.K.V. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef]
- Sait, S.; Modak, S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef]
- Dondero, A.; Morini, M.; Cangelosi, D.; Mazzocco, K.; Serra, M.; Spaggiari, G.M.; Rotta, G.; Tondo, A.; Locatelli, F.; Castellano, A.; et al. Multiparametric flow cytometry highlights B7-H3 as a novel diagnostic/therapeutic target in GD2neg/low neuroblastoma variants. J. Immunother. Cancer 2021, 9, e002293. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef]
- Modak, S.; Kramer, K.; Gultekin, S.H.; Guo, H.F.; Cheung, N.K.V. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001, 61, 4048–4054. [Google Scholar]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef]
- Irwin, M.S.; Park, J.R. Neuroblastoma: Paradigm for precision medicine. Pediatr. Clin. N. Am. 2015, 62, 225–256. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, 17–29. [Google Scholar] [CrossRef]
- Louro, R.; Smirnova, A.S.; Verjovski-Almeida, S. Long intronic noncoding RNA transcription: Expression noise or expression choice? Genomics 2009, 93, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Natoli, G.; Andrau, J.C. Noncoding transcription at enhancers: General principles and functional models. Annu. Rev. Genet. 2012, 46, 1–19. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.C.; Wicks, K.; Caley, D.P.; Punch, E.K.; Jacobs, L.; Raul, D.; Carter, F. Pseudogenes: Pseudo-functional or key regulators in health and disease? RNA 2011, 17, 792–798. [Google Scholar] [CrossRef]
- Tutar, Y. Pseudogenes. Comp. Funct. Genom. 2012, 2012. [Google Scholar] [CrossRef]
- Tsagakis, I.; Douka, K.; Birds, I.; Aspden, J.L. Long non-coding RNAs in development and disease: Conservation to mechanisms. J. Pathol. 2020, 250, 480–495. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Bhan, A.; Mandal, S.S. Long noncoding RNAs: Emerging stars in gene regulation, epigenetics and human disease. ChemMedChem 2014, 9, 1932–1956. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Beckedorff, F.C.; Sena Amaral, M.; Deocesano-Pereira, C.; Verjovski-Almeida, S. Long non-coding RNAs and their implications in cancer epigenetics. Biosci. Rep. 2013, 33, 667–675. [Google Scholar] [CrossRef]
- Buechner, J.; Einvik, C. N-myc and noncoding RNAs in neuroblastoma. Mol. Cancer Res. 2012, 10, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, D.; Huang, D.; Song, H.; Mei, H.; Fang, E.; Wang, X.; Yang, F.; Zheng, L.; Huang, K.; et al. Risk-Associated Long Noncoding RNA FOXD3-AS1 Inhibits Neuroblastoma Progression by Repressing PARP1-Mediated Activation of CTCF. Mol. Ther. 2018, 26, 755–773. [Google Scholar] [CrossRef]
- Guan, Y.; Bhandari, A.; Xia, E.; Yang, F.; Xiang, J.; Wang, O. lncRNA FOXD3-AS1 is associated with clinical progression and regulates cell migration and invasion in breast cancer. Cell Biochem. Funct. 2019, 37, 239–244. [Google Scholar] [CrossRef]
- Ma, W.; Shi, S.; Chen, L.; Lou, G.; Feng, X. SP1-induced lncRNA FOXD3-AS1 contributes to tumorigenesis of cervical cancer by modulating the miR-296-5p/HMGA1 pathway. J. Cell. Biochem. 2021, 122, 235–248. [Google Scholar] [CrossRef]
- Yang, X.; Du, H.; Bian, W.; Li, Q.; Sun, H. FOXD3-AS1/miR-128-3p/LIMK1 axis regulates cervical cancer progression. Oncol. Rep. 2021, 45, 1–12. [Google Scholar] [CrossRef]
- Wang, L. ELF1-activated FOXD3-AS1 promotes the migration, invasion and EMT of osteosarcoma cells via sponging miR-296-5p to upregulate ZCCHC3. J. Bone Oncol. 2021, 26, 100335. [Google Scholar] [CrossRef]
- Hu, J.; Pan, J.; Luo, Z.; Duan, Q.; Wang, D. Long non-coding RNA FOXD3-AS1 silencing exerts tumor suppressive effects in nasopharyngeal carcinoma by downregulating FOXD3 expression via microRNA-185-3p upregulation. Cancer Gene Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tonini, G.P. The Origin of Neuroblastoma. In Neuroblastoma—Current State and Recent Updates; InTech: London, UK, 2017. [Google Scholar]
- Lee, Y.H.; Kim, J.H.; Song, G.G. Genome-wide pathway analysis in neuroblastoma. Tumor Biol. 2014, 35, 3471–3485. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Lee, J.W.; Yoo, J.E.; Joung, J.G.; Yoo, K.H.; Koo, H.H.; Song, Y.M.; Sung, K.W. Genome-wide association study for the identification of novel genetic variants associated with the risk of neuroblastoma in Korean children. Cancer Res. Treat. 2020, 52, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Barr, E.; Applebaum, M. Genetic Predisposition to Neuroblastoma. Children 2018, 5, 119. [Google Scholar] [CrossRef]
- Decock, A.; Ongenaert, M.; Vandesompele, J.; Speleman, F. Neuroblastoma epigenetics: From candidate gene approaches to genome-wide screenings. Epigenetics 2011, 6, 962–970. [Google Scholar] [CrossRef]
- Maris, J.M.; Mosse, Y.P.; Bradfield, J.P.; Hou, C.; Monni, S.; Scott, R.H.; Asgharzadeh, S.; Attiyeh, E.F.; Diskin, S.J.; Laudenslager, M.; et al. Chromosome 6p22 Locus Associated with Clinically Aggressive Neuroblastoma. N. Engl. J. Med. 2008, 358, 2585–2593. [Google Scholar] [CrossRef]
- Mondal, T.; Juvvuna, P.K.; Kirkeby, A.; Mitra, S.; Kosalai, S.T.; Traxler, L.; Hertwig, F.; Wernig-Zorc, S.; Miranda, C.; Deland, L.; et al. Sense-Antisense lncRNA Pair Encoded by Locus 6p22.3 Determines Neuroblastoma Susceptibility via the USP36-CHD7-SOX9 Regulatory Axis. Cancer Cell 2018, 33, 417–434.e7. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.K.; Mitra, S.; Subhash, S.; Hertwig, F.; Kanduri, M.; Mishra, K.; Fransson, S.; Ganeshram, A.; Mondal, T.; Bandaru, S.; et al. The Risk-Associated Long Noncoding RNA NBAT-1 Controls Neuroblastoma Progression by Regulating Cell Proliferation and Neuronal Differentiation. Cancer Cell 2014, 26, 722–737. [Google Scholar] [CrossRef]
- Wang, D.L.; Yuan, P.; Tian, J.Y. Expression of long noncoding RNA NBAT1 is associated with the outcome of patients with non-small cell lung cancer. Rev. Assoc. Med. Bras. 2021, 66, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, P.; Hu, S.; Li, F.; Kong, K.; Zu, Y. LncRNA-NBAT-1 modulates esophageal cancer proliferation via PKM2. Am. J. Transl. Res. 2019, 11, 5978–5987. [Google Scholar]
- Wei, L.; Ling, M.; Yang, S.; Xie, Y.; Liu, C.; Yi, W. Long noncoding RNA NBAT1 suppresses hepatocellular carcinoma progression via competitively associating with IGF2BP1 and decreasing c-Myc expression. Hum. Cell 2021, 34, 539–549. [Google Scholar] [CrossRef]
- Xue, S.; Wang, S.; Li, J.; Guan, H.; Jiang, S.; Guo, Y.; Li, Q. LncRNA NBAT1 suppresses cell proliferation and migration via miR-346/GSK-3β axis in renal carcinoma. IUBMB Life 2019, 71, 1720–1728. [Google Scholar] [CrossRef]
- Xue, S.; Li, Q.W.; Che, J.P.; Guo, Y.; Yang, F.Q.; Zheng, J.H. Decreased expression of long non-coding RNA NBAT-1 is associated with poor prognosis in patients with clear cell renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3765–3774. [Google Scholar]
- Lei, T.; Lv, Z.Y.; Fu, J.F.; Wang, Z.; Fan, Z.; Wang, Y. LncRNA NBAT-1 is down-regulated in lung cancer and influences cell proliferation, apoptosis and cell cycle. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1958–1962. [Google Scholar]
- Hu, P.; Chu, J.; Wu, Y.; Sun, L.; Lv, X.; Zhu, Y.; Li, J.; Guo, Q.; Gong, C.; Liu, B.; et al. NBAT1 suppresses breast cancer metastasis by regulating DKK1 via PRC2. Oncotarget 2015, 6, 32410–32425. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.K.; Kanduri, C. Fighting Neuroblastomas with NBAT1. Oncoscience 2015, 2, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Muralidharan, S.V.; Di Marco, M.; Juvvuna, P.K.; Kosalai, S.T.; Reischl, S.; Jachimowicz, D.; Subhash, S.; Raimondi, I.; Kurian, L.; et al. Subcellular Distribution of p53 by the p53-Responsive lncRNA NBAT1 Determines Chemotherapeutic Response in Neuroblastoma. Cancer Res. 2021, 81, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, R.; Guo, H.; Cheng, J.; Zhang, R.; Liu, B.; Pang, J.; Cao, W. CASC15 Polymorphisms are Correlated With Breast Cancer Susceptibility in Chinese Han Women. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.M.; Tang, J.H.; Zhu, H.; Jing, Y. High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 21, 5661–5667. [Google Scholar]
- Fernando, T.R.; Contreras, J.R.; Zampini, M.; Rodriguez-Malave, N.I.; Alberti, M.O.; Anguiano, J.; Tran, T.M.; Palanichamy, J.K.; Gajeton, J.; Ung, N.M.; et al. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol. Cancer 2017, 16, 126. [Google Scholar] [CrossRef]
- Sheng, L.; Wei, R. Long Non-Coding RNA-CASC15 Promotes Cell Proliferation, Migration, and Invasion by Activating Wnt/β-Catenin Signaling Pathway in Melanoma. Pathobiology 2020, 87, 20–29. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, S.; Zheng, Y.; Yao, M.; Ruan, F. Lncrna casc15 functions as an unfavorable predictor of ovarian cancer prognosis and inhibits tumor progression through regulation of mir-221/arid1a axis. Onco. Targets Ther. 2019, 12, 8725–8736. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, W.; Cai, Y.; Guo, C.; Zhou, G.; Yuan, C. CASC15: A Tumor-Associated Long Non-Coding RNA. Curr. Pharm. Des. 2021, 27, 127–134. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Xing, M.-Q.; Guo, J.; Zhao, J.-C.; Chen, X.; Jiang, Z.; Zhang, H.; Dong, Q. Long noncoding RNA DLX6-AS1 promotes neuroblastoma progression by regulating miR-107/BDNF pathway. Cancer Cell Int. 2019, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, S.; Yang, C. Long non-coding RNA DLX6-AS1 regulates neuroblastoma progression by targeting YAP1 via miR-497-5p. Life Sci. 2020, 252, 117657. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Zhao, W.; Yang, R.; Chen, S.; Bai, Y.; Dun, S.; Chen, X.; Du, Y.; Wang, Y.; et al. Expression of long non-coding RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell Int. 2015, 15, 48. [Google Scholar] [CrossRef]
- Fu, X.; Tian, Y.; Kuang, W.; Wen, S.; Guo, W. Long non-coding RNA DLX6-AS1 silencing inhibits malignant phenotypes of gastric cancer cells. Exp. Ther. Med. 2019, 17, 4715–4722. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, W.R.; Chen, B.; Wang, Y.Y.; Yang, N.; Wang, L.J.; Zhang, Y.L. The up-regulated lncRNA DLX6-AS1 in colorectal cancer promotes cell proliferation, invasion and migration via modulating PI3K/AKT/mTOR pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8321–8331. [Google Scholar] [PubMed]
- Zhao, P.; Guan, H.; Dai, Z.; Ma, Y.; Zhao, Y.; Liu, D. Long noncoding RNA DLX6-AS1 promotes breast cancer progression via miR-505-3p/RUNX2 axis. Eur. J. Pharmacol. 2019, 865, 172778. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, X.; Jiang, H.; Mao, F.; Zhong, B.; Jiang, X.; Fu, G. Long non-coding RNA DLX6-AS1 facilitates bladder cancer progression through modulating miR-195-5p/VEGFA signaling pathway. Aging 2020, 12, 16021–16034. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, C. LncRNA DLX6-AS1 aggravates the development of ovarian cancer via modulating FHL2 by sponging miR-195-5p. Cancer Cell Int. 2020, 20, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Meng, X.; Mei, L.; Zhao, C.; Chen, W. LncRNA DLX6-AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR-641/HOXA9 signaling pathway. J. Cell. Biochem. 2019, 120, 11478–11489. [Google Scholar] [CrossRef]
- Pagano, A.; Castelnuovo, M.; Tortelli, F.; Ferrari, R.; Dieci, G.; Cancedda, R. New small nuclear RNA gene-like transcriptional units as sources of regulatory transcripts. PLoS Genet. 2007, 3, 0174–0184. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, M.; Massone, S.; Tasso, R.; Fiorino, G.; Gatti, M.; Robello, M.; Gatta, E.; Berger, A.; Strub, K.; Florio, T.; et al. An Alu-like RNA promotes cell differentiation and reduces malignancy of human neuroblastoma cells. FASEB J. 2010, 24, 4033–4046. [Google Scholar] [CrossRef] [PubMed]
- Alloisio, S.; Garbati, P.; Viti, F.; Dante, S.; Barbieri, R.; Arnaldi, G.; Petrelli, A.; Gigoni, A.; Giannoni, P.; Quarto, R.; et al. Generation of a Functional Human Neural Network by NDM29 Overexpression in Neuroblastoma Cancer Cells. Mol. Neurobiol. 2017, 54, 6097–6106. [Google Scholar] [CrossRef]
- Vella, S.; Penna, I.; Longo, L.; Pioggia, G.; Garbati, P.; Florio, T.; Rossi, F.; Pagano, A. Perhexiline maleate enhances antitumor efficacy of cisplatin in neuroblastoma by inducing over-expression of NDM29 ncRNA. Sci. Rep. 2015, 5, 18144. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Gigoni, A.; Würth, R.; Cancedda, R.; Florio, T.; Pagano, A. Metformin inhibition of neuroblastoma cell proliferation is differently modulated by cell differentiation induced by retinoic acid or overexpression of NDM29 non-coding RNA. Cancer Cell Int. 2014, 14, 59. [Google Scholar] [CrossRef]
- Gavazzo, P.; Vella, S.; Marchetti, C.; Nizzari, M.; Cancedda, R.; Pagano, A. Acquisition of neuron-like electrophysiological properties in neuroblastoma cells by controlled expression of NDM29 ncRNA. J. Neurochem. 2011, 119, 989–1001. [Google Scholar] [CrossRef]
- Garbati, P.; Barbieri, R.; Cangelosi, D.; Zanon, C.; Costa, D.; Eva, A.; Thellung, S.; Calderoni, M.; Baldini, F.; Tonini, G.P.; et al. Mcm2 and carbonic anhydrase 9 are novel potential targets for neuroblastoma pharmacological treatment. Biomedicines 2020, 8, 471. [Google Scholar] [CrossRef]
- Liu, P.Y.; Tee, A.E.; Milazzo, G.; Hannan, K.M.; Maag, J.; Mondal, S.; Atmadibrata, B.; Bartonicek, N.; Peng, H.; Ho, N.; et al. The long noncoding RNA lncNB1 promotes tumorigenesis by interacting with ribosomal protein RPL35. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Thin, K.Z.; Tu, J.C.; Raveendran, S. Long non-coding SNHG1 in cancer. Clin. Chim. Acta 2019, 494, 38–47. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Kang, Y.J.; Wang, C.; Xu, Y.; Zhang, Y.; Jiang, Z. Long non-coding RNA SNHG1 predicts a poor prognosis and promotes colon cancer tumorigenesis. Oncol. Rep. 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Li, T.; Yu, X.; Zhu, Y.; Ding, F.; Li, D.; Yang, T. Long noncoding RNA SNHG1 predicts a poor prognosis and promotes hepatocellular carcinoma tumorigenesis. Biomed. Pharm. 2016, 80, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, F.; Zhu, C.; Geng, L.; Tian, T.; Liu, H. Upregulated lncRNA SNHG1 contributes to progression of nonsmall cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 17785–17794. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, Y.; Ma, Y.; Wu, C.; Zheng, Y.; Xie, N. LncRNA SNHG1 promotes α-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology 2018, 68, 212–221. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, F.L.; Ma, T.S.; Zeng, Z.D.; Zhang, J.J. LncRNA SNHG1 contributes to tumorigenesis and mechanism by targeting MIR-338-3p to regulate PLK4 in human neuroblastoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8971–8983. [Google Scholar] [PubMed]
- Sahu, D.; Hsu, C.L.; Lin, C.C.; Yang, T.W.; Hsu, W.M.; Ho, S.Y.; Juan, H.F.; Huang, H.C. Co-expression analysis identifies long noncoding RNA SNHG1 as a novel predictor for event-free survival in neuroblastoma. Oncotarget 2016, 7, 58022–58037. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Tang, R.; Nan, W.; Zhou, K.S.; Zhang, H.H. Role of SNHG16 in human cancer. Clin. Chim. Acta 2020, 503, 175–180. [Google Scholar] [CrossRef]
- Feng, F.; Chen, A.; Huang, J.; Xia, Q.; Chen, Y.; Jin, X. Long noncoding RNA SNHG16 contributes to the development of bladder cancer via regulating miR-98/STAT3/Wnt/β-catenin pathway axis. J. Cell. Biochem. 2018, 119, 9408–9418. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Huo, Q.; Wang, X.; Chen, B.; Yang, Q. SNHG16 contributes to breast cancer cell migration by competitively binding miR-98 with E2F5. Biochem. Biophys. Res. Commun. 2017, 485, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Liu, H.; Ding, Z.; Xi, H.P.; Wang, G.W. lncRNA SNHG16 promotes glioma tumorigenicity through miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics 2020, 112, 1021–1029. [Google Scholar] [CrossRef]
- Xie, X.; Xu, X.; Sun, C.; Yu, Z. Long intergenic noncoding RNA SNHG16 interacts with miR-195 to promote proliferation, invasion and tumorigenesis in hepatocellular carcinoma. Exp. Cell Res. 2019, 383, 111501. [Google Scholar] [CrossRef]
- Su, P.; Mu, S.; Wang, Z. Long Noncoding RNA SNHG16 Promotes Osteosarcoma Cells Migration and Invasion via Sponging miRNA-340. DNA Cell Biol. 2019, 38, 170–175. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Liu, K.; Liu, Y. LncRNA SNHG16 promotes tumor growth of pancreatic cancer by targeting miR-218-5p. Biomed. Pharm. 2019, 114, 108862. [Google Scholar] [CrossRef]
- Tao, L.; Wang, X.; Zhou, Q. Long noncoding RNA SNHG16 promotes the tumorigenicity of cervical cancer cells by recruiting transcriptional factor SPI1 to upregulate PARP9. Cell Biol. Int. 2020, 44, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, F.; Yang, Y.; Jin, Y.; Shi, J.; Han, S.; Chu, P.; Lu, J.; Tai, J.; Wang, S.; et al. LncRNA SNHG16 is associated with proliferation and poor prognosis of pediatric neuroblastoma. Int. J. Oncol. 2019, 55, 93–102. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Wang, D.; Sun, H.; Liu, X. SNHG16 promotes tumorigenesis and cisplatin resistance by regulating miR-338-3p/PLK4 pathway in neuroblastoma cells. Cancer Cell Int. 2020, 20, 236. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, S.; Meng, Q.; Qin, T. SNHG16 Silencing Inhibits Neuroblastoma Progression by Downregulating HOXA7 via Sponging miR-128-3p. Neurochem. Res. 2020, 45, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gong, X.; Dong, Y.; Tang, C. Long non coding RNA SNHG16 facilitates proliferation, migration, invasion and autophagy of neuroblastoma cells via sponging miR-542-3p and upregulating ATG5 expression. Onco. Targets Ther. 2020, 13, 263–275. [Google Scholar] [CrossRef]
- Meng, X.; Fang, E.; Zhao, X.; Feng, J. Identification of prognostic long noncoding RNAs associated with spontaneous regression of neuroblastoma. Cancer Med. 2020, 9, 3800–3815. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shen, H.; Zhu, X.; Liu, Y.; Yang, H.; Chen, H.; Xiong, S.; Chi, H.; Xu, W. A nuclear lncRNA Linc00839 as a Myc target to promote breast cancer chemoresistance via PI3K/AKT signaling pathway. Cancer Sci. 2020, 111, 3279–3291. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Ma, L.; Chen, X.; Chen, G. Long noncoding rna linc00839 promotes the malignant progression of osteosarcoma by competitively binding to microrna-454-3p and consequently increasing c-met expression. Cancer Manag. Res. 2020, 12, 8975–8987. [Google Scholar] [CrossRef] [PubMed]
- Corallo, D.; Donadon, M.; Pantile, M.; Sidarovich, V.; Cocchi, S.; Ori, M.; De Sarlo, M.; Candiani, S.; Frasson, C.; Distel, M.; et al. LIN28B increases neural crest cell migration and leads to transformation of trunk sympathoadrenal precursors. Cell Death Differ. 2020, 27, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cui, Z.; Liu, X.; Wu, S.; Wu, Y.; Fang, F.; Zhao, H. Biochemical and Biophysical Research Communications LncRNA FIRRE is activated by MYC and promotes the development of diffuse large B-cell lymphoma via Wnt/b -catenin signaling pathway. Biochem. Biophys. Res. Commun. 2019, 510, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, S.; Wei, Z.; Yang, B.O. A putative competing endogenous RNA network in cisplatin—resistant lung adenocarcinoma cells identifying potentially rewarding research targets. Oncol. Lett. 2020, 19, 4040–4052. [Google Scholar] [CrossRef]
- Nobili, L.; Ronchetti, D.; Taiana, E.; Neri, A. Long non-coding RNAs in B-cell malignancies: A comprehensive overview. Oncotarget 2017, 8, 60605–60623. [Google Scholar] [CrossRef] [PubMed]
- Conde, L.; Riby, J.; Zhang, J.; Bracci, P.M.; Skibola, C.F. Copy number variation analysis on a non-hodgkin lymphoma case-control study identifies an 11q25 duplication associated with diffuse large B-cell lymphoma. PLoS ONE 2014, 9, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, C.; Zhu, Q.; Yuan, D.; Sun, M.; Gu, X.; Wu, G.; Lv, T.; Song, Y. Upregulation of long intergenic noncoding RNA 00673 promotes tumor proliferation via LSD1 interaction and repression of NCALD in non-small-cell lung cancer. Oncotarget 2016, 7, 25558–25575. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A neural crest derived embryonal malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
| LncRNA | Role in NB | Role in Other Tumors |
|---|---|---|
| FOXD3-AS1 | Reduced expression | Reduced expression led to induction of p53, c-Myc, and retinoblastoma |
| Positive prognostic marker for positive outcome | Reduced expression in colon cancer | |
| Increased invasion and proliferation rates | ||
| NBAT1 | Independent prognostic marker for clinical outcome | Involved in clear cell renal cell carcinoma, ovarian cancer and breast cancer |
| Lower expression in HR-NB Tumor suppressor activity through the regulation of SOX9, OSMR and VCAN | Emerging role in pathobiology of glioblastoma | |
| CASC15 | Low expression directly correlated with poor diagnosis Neuronal differentiation | High expression in other tumors, including CC, breast cancer, HCC, gastric cancer and melanoma Down-regulated in ovarian cancer and glioma |
| DLX-AS1 | Oncogenic role | Involvement in liver cancer and lung cancer |
| Positive correlation with poor differentiation and poor outcome | Aberrant expression in other tumor tissues and associated with tumor progression | |
| Improper neuronal differentiation | ||
| NDM29 | Neuronal differentiation | ND |
| LncNB1 | Cell proliferation regulation | Moderate expression in melanoma |
| Poor prognosis prediction | Absent in other tumors | |
| SNHG1 | miR338-3p inhibitor | Oncogene and regulator of Wnt/β-catenin axis in CRC |
| Proliferation, migration and invasion regulator | p53 inhibitor in HCC | |
| Positively correlated with MYCN amplification | miR-101-3p/SOX9/Wnt/β-catenin axis regulation in NSCLC | |
| SNHG16 | Associated with poor prognosis Regulates cell cycle progression, cell proliferation, migration and autophagy | Correlated with poor prognosis in different types of cancer, including bladder cancer, breast cancer, glioma, HCC, and osteosarcoma |
| LINC00839 | Correlated with bad prognosis and tumor progression | Involved in breast cancer and osteosarcoma |
| FIRRE | Correlated with bad prognosis and tumor progression | Correlated with poor survival in diffuse large B-cell lymphoma, kidney renal clear cell carcinoma, kidney renal papillary cell carcinoma, pancreatic adenocarcinoma, glioma, HCC, and mesothelioma |
| LOC283177 | Associated with spontaneous regression and neuronal differentiation | Involved in diffuse large B-cell lymphoma |
| LOC101928100 | Associated with spontaneous regression and neuronal differentiation | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, F.; Calderoni, M.; Vergani, L.; Modesto, P.; Florio, T.; Pagano, A. An Overview of Long Non-Coding (lnc)RNAs in Neuroblastoma. Int. J. Mol. Sci. 2021, 22, 4234. https://doi.org/10.3390/ijms22084234
Baldini F, Calderoni M, Vergani L, Modesto P, Florio T, Pagano A. An Overview of Long Non-Coding (lnc)RNAs in Neuroblastoma. International Journal of Molecular Sciences. 2021; 22(8):4234. https://doi.org/10.3390/ijms22084234
Chicago/Turabian StyleBaldini, Francesca, Matilde Calderoni, Laura Vergani, Paola Modesto, Tullio Florio, and Aldo Pagano. 2021. "An Overview of Long Non-Coding (lnc)RNAs in Neuroblastoma" International Journal of Molecular Sciences 22, no. 8: 4234. https://doi.org/10.3390/ijms22084234
APA StyleBaldini, F., Calderoni, M., Vergani, L., Modesto, P., Florio, T., & Pagano, A. (2021). An Overview of Long Non-Coding (lnc)RNAs in Neuroblastoma. International Journal of Molecular Sciences, 22(8), 4234. https://doi.org/10.3390/ijms22084234