Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts
Abstract
1. Introduction
2. Results
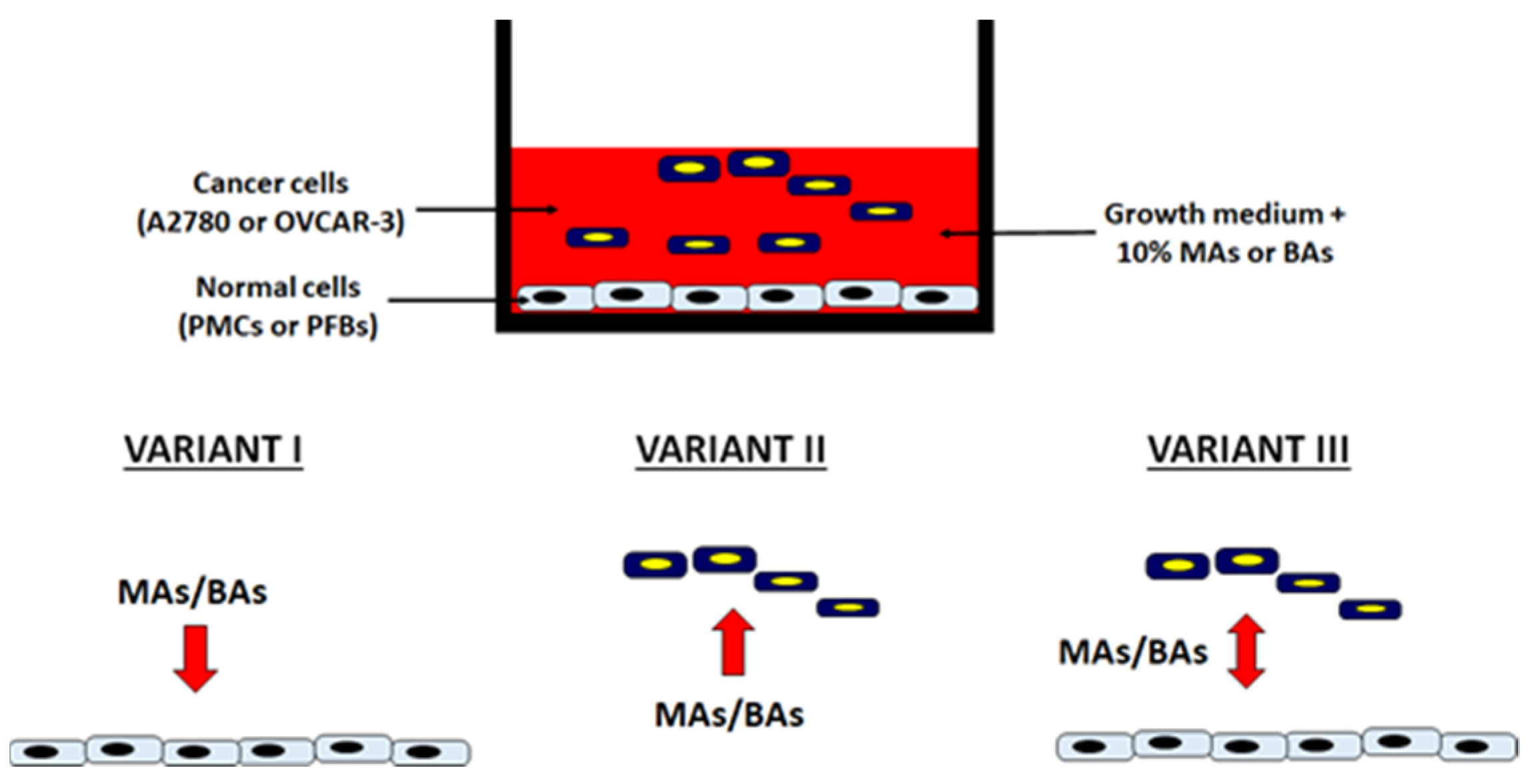
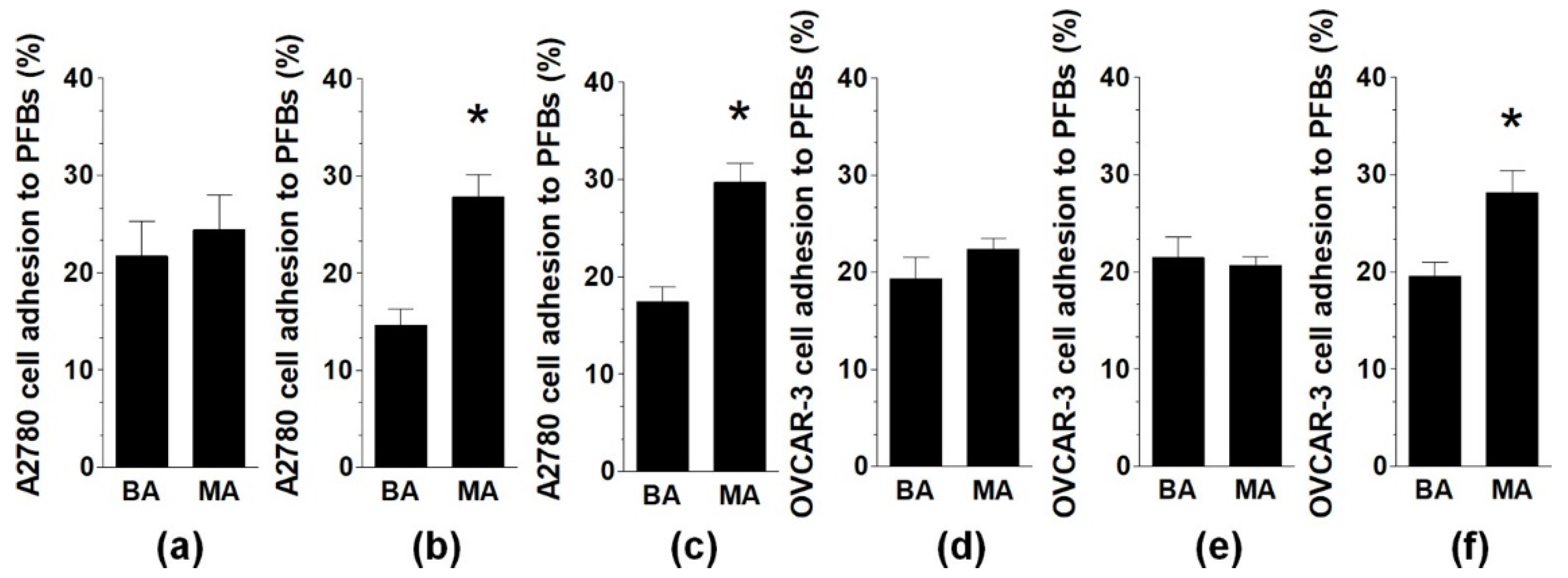
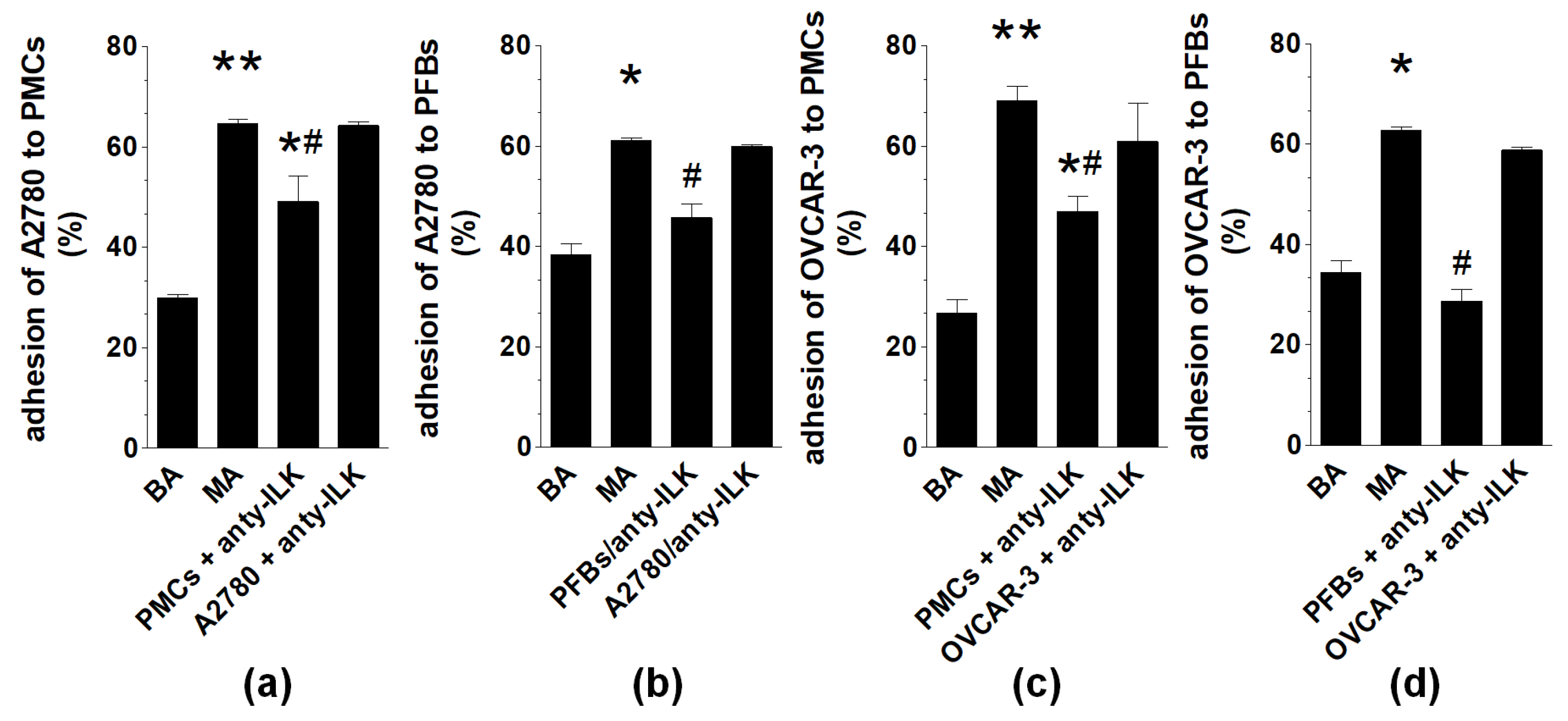
2.1. Malignant Ascites Stimulate Adhesion of Ovarian Cancer Cells to PMCs and PFBs
2.2. Soluble MAs-Derived Proteins Are Responsible for the Proadhesive Potential of the Fluid
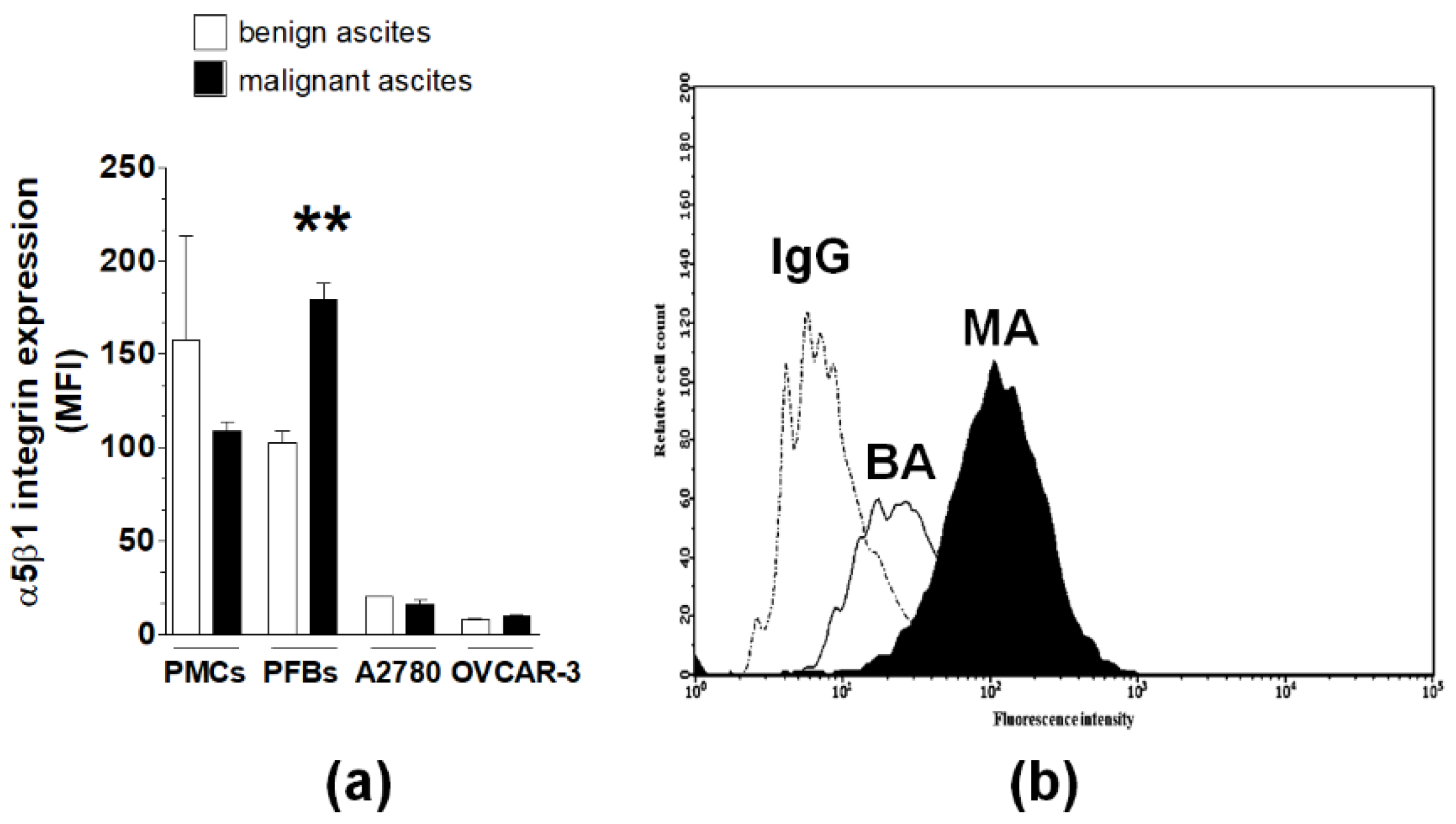
2.3. Analysis of α5β1 Integrin and Integrin-Linked Kinase Engagement
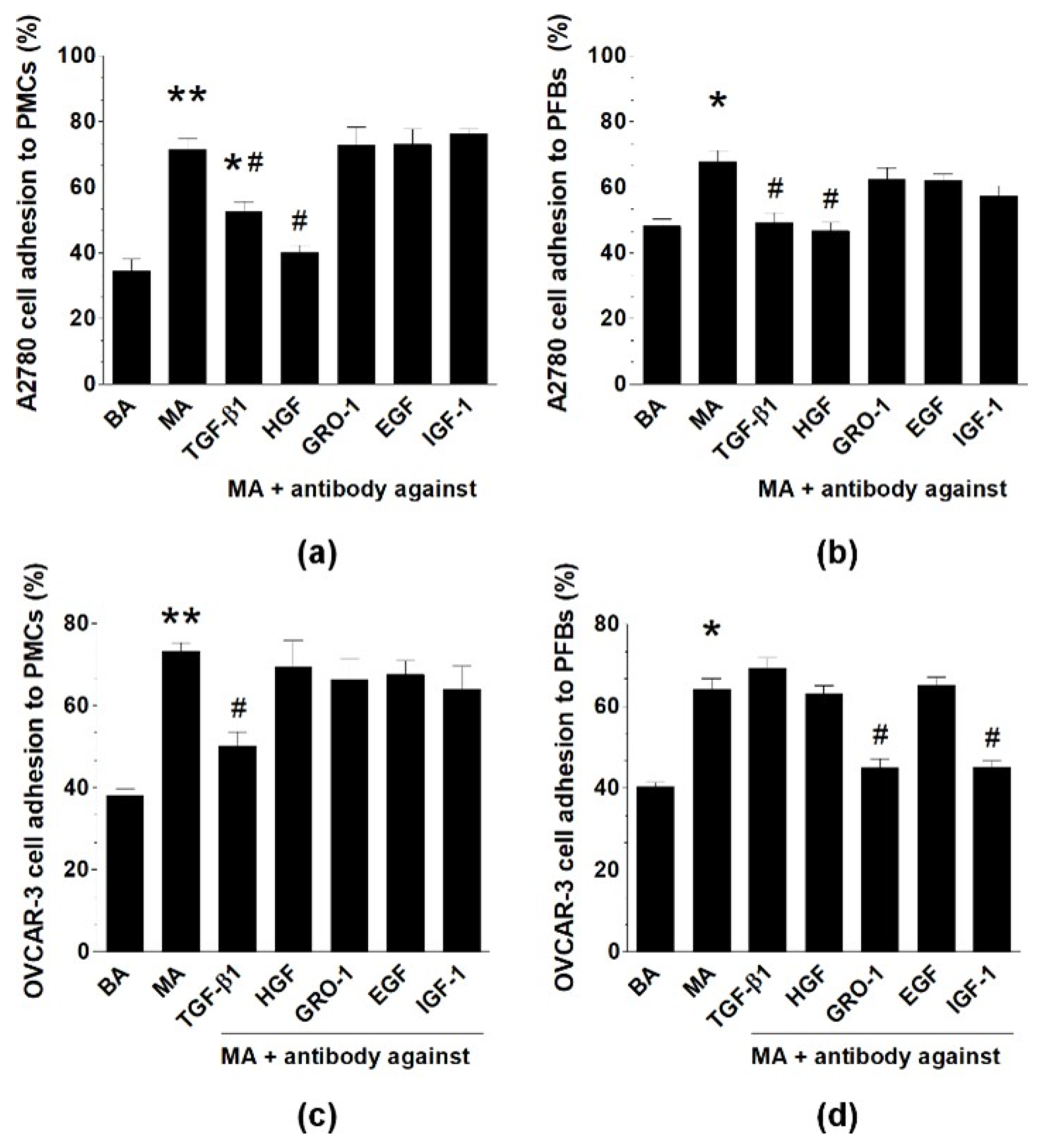
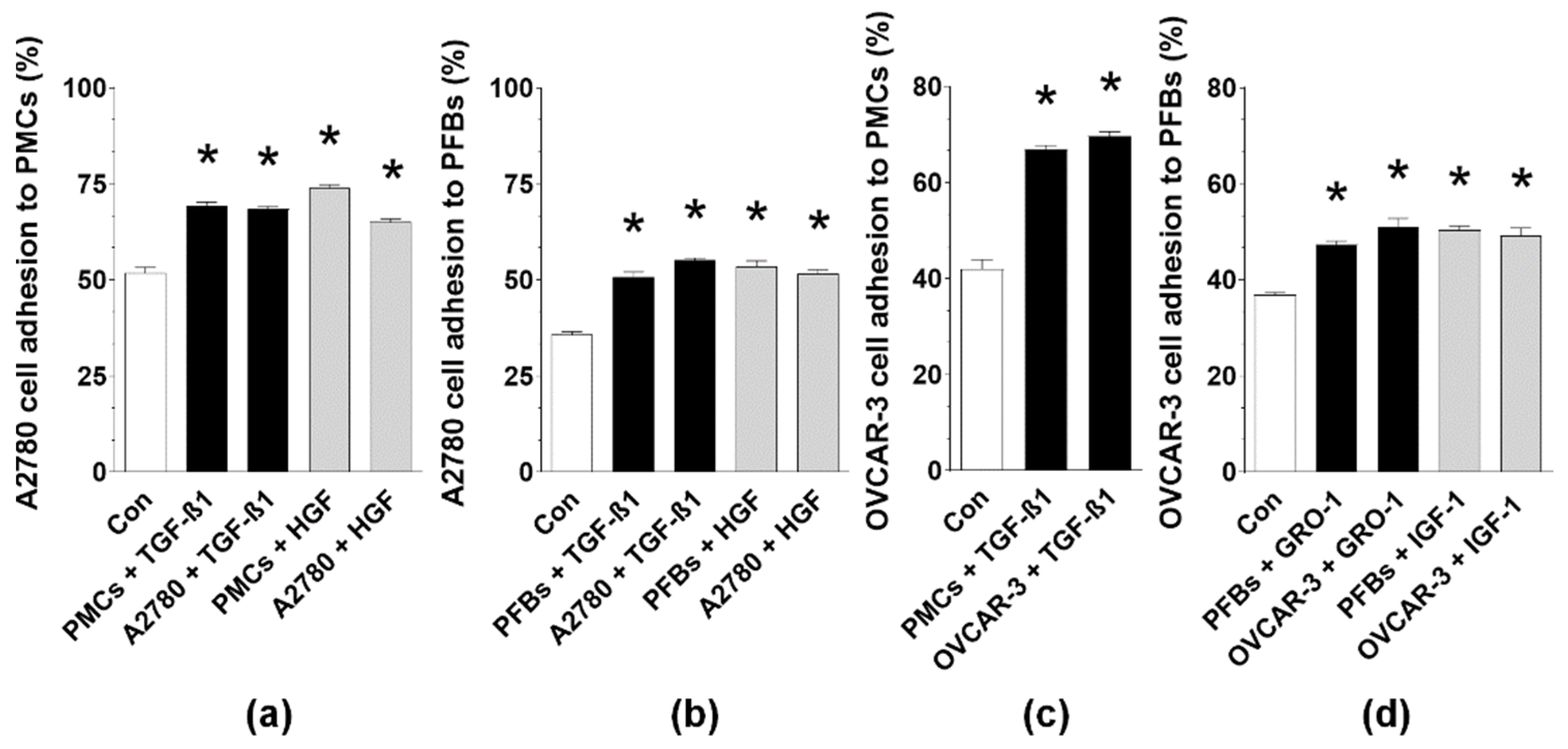
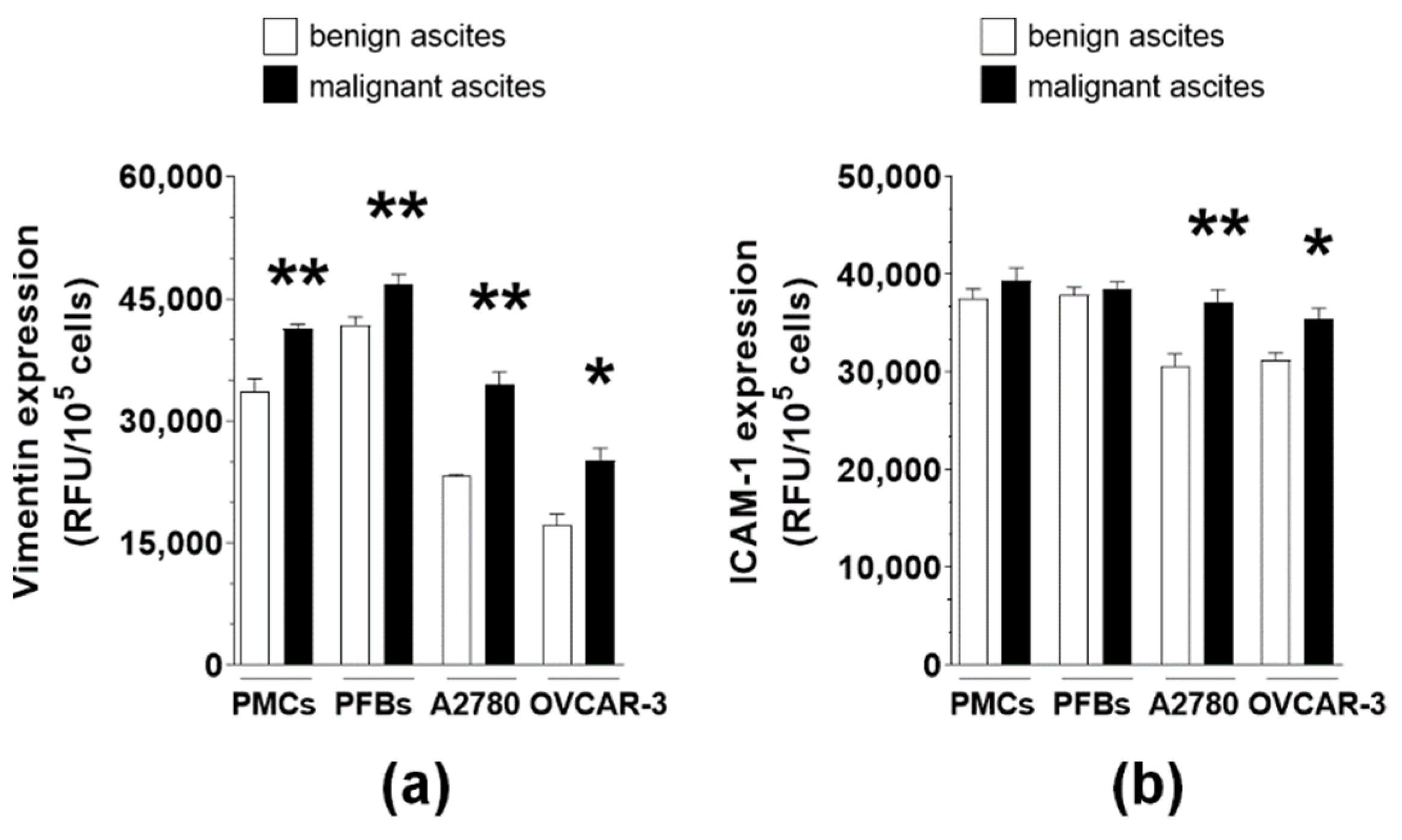
2.4. MAs Alter the Expression of Surface Adhesion Molecules
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. Malignant and Benign Ascites
4.4. Cell Adhesion Assay
4.5. Immunofluorescence
4.6. Flow Cytometry
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Pickel, H.; Lahousen, M.; Girardi, F.; Tamussino, H.; Stettner, H. Intraperitoneal and retroperitoneal spread of ovarian cancer. In Ovarian Cancer: Biologic and Therapeutic Challenges; Sharp, C., Mason, W., Leake, R., Eds.; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Ahmed, N.; Stenvers, K.L. Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Tykarski, A.; Ksiazek, K. The peritoneal “soil” for a cancerous “seed”: A comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell. Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Simpson-Abelson, M.R.; Loyall, J.L.; Lehman, H.K.; Barnas, J.L.; Minderman, H.; O’Loughlin, K.L.; Wallace, P.K.; George, T.C.; Peng, P.; Kelleher, R.J., Jr.; et al. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013, 13, 14. [Google Scholar]
- Mikula-Pietrasik, J.; Uruski, P.; Szubert, S.; Maksin, K.; Moszynski, R.; Szpurek, D.; Wozniak, A.; Sajdak, S.; Tykarski, A.; Ksiazek, K. The Proangiogenic Capabilities of Malignant Ascites Generated by Aggressive Ovarian Tumors. Biomed. Res. Int. 2017, 2017, 2592496. [Google Scholar] [CrossRef]
- Pakula, M.; Mikula-Pietrasik, J.; Witucka, A.; Kostka-Jeziorny, K.; Uruski, P.; Moszynski, R.; Naumowicz, E.; Sajdak, S.; Tykarski, A.; Ksiazek, K. The Epithelial-Mesenchymal Transition Initiated by Malignant Ascites Underlies the Transmesothelial Invasion of Ovarian Cancer Cells. Int. J. Mol. Sci. 2019, 20, 137. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Matuszkiewicz, K.; Szubert, S.; Moszynski, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell Oncol. 2016, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Chiang, C.Y.; White, E.A.; Schryver, E.M.; Habis, M.; Romero, I.L.; Ladanyi, A.; Penicka, C.V.; George, J.; Matlin, K.; et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J. Clin. Investig. 2014, 124, 4614–4628. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Tang, H.; Xu, L.; Wang, X.; Yang, C.; Ruan, S.; Guo, J.; Hu, S.; Wang, Z. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 2012, 33, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Ruff, L.E.; Skubitz, A.P. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Ksiazek, K.; Mikula-Pietrasik, J.; Korybalska, K.; Dworacki, G.; Jorres, A.; Witowski, J. Senescent peritoneal mesothelial cells promote ovarian cancer cell adhesion: The role of oxidative stress-induced fibronectin. Am. J. Pathol. 2009, 174, 1230–1240. [Google Scholar] [CrossRef]
- Widmaier, M.; Rognoni, E.; Radovanac, K.; Azimifar, S.B.; Fassler, R. Integrin-linked kinase at a glance. J. Cell Sci. 2012, 125, 1839–1843. [Google Scholar] [CrossRef]
- Ren, J.; Xiao, Y.J.; Singh, L.S.; Zhao, X.; Zhao, Z.; Feng, L.; Rose, T.M.; Prestwich, G.D.; Xu, Y. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006, 66, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Szubert, S.; Moszynski, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Biochemical composition of malignant ascites determines high aggressiveness of undifferentiated ovarian tumors. Med. Oncol. 2016, 33, 94. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Taucher, V.; Haybaeck, J. Malignant ascites in ovarian cancer and the role of targeted therapeutics. Anticancer Res. 2014, 34, 1553–1561. [Google Scholar] [PubMed]
- Tudrej, P.; Olbryt, M.; Zembala-Nozynska, E.; Kujawa, K.A.; Cortez, A.J.; Fiszer-Kierzkowska, A.; Piglowski, W.; Nikiel, B.; Glowala-Kosinska, M.; Bartkowska-Chrobok, A.; et al. Establishment and Characterization of the Novel High-Grade Serous Ovarian Cancer Cell Line OVPA8. Int. J. Mol. Sci. 2018, 19, 2080. [Google Scholar] [CrossRef]
- Lee, J.G.; Ahn, J.H.; Jin Kim, T.; Ho Lee, J.; Choi, J.H. Mutant p53 promotes ovarian cancer cell adhesion to mesothelial cells via integrin beta4 and Akt signals. Sci. Rep. 2015, 5, 12642. [Google Scholar] [CrossRef]
- Mabuchi, S.; Ohmichi, M.; Kimura, A.; Nishio, Y.; Arimoto-Ishida, E.; Yada-Hashimoto, N.; Tasaka, K.; Murata, Y. Estrogen inhibits paclitaxel-induced apoptosis via the phosphorylation of apoptosis signal-regulating kinase 1 in human ovarian cancer cell lines. Endocrinology 2004, 145, 49–58. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Sosinska, P.; Kucinska, M.; Murias, M.; Maksin, K.; Malinska, A.; Ziolkowska, A.; Piotrowska, H.; Wozniak, A.; Ksiazek, K. Peritoneal mesothelium promotes the progression of ovarian cancer cells in vitro and in a mice xenograft model in vivo. Cancer Lett. 2014, 355, 310–315. [Google Scholar] [CrossRef]
- Beviglia, L.; Kramer, R.H. HGF induces FAK activation and integrin-mediated adhesion in MTLn3 breast carcinoma cells. Int. J. Cancer 1999, 83, 640–649. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, G.; Wu, H.; Sun, K.; Chen, J.; Feng, Y.; Chen, C.; Cai, S.; Xu, J.; et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin beta1/FAK/AKT signaling. Cancer Lett. 2017, 385, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Podar, K.; Catley, L.; Tseng, Y.H.; Akiyama, M.; Shringarpure, R.; Burger, R.; Hideshima, T.; Chauhan, D.; Mitsiades, N.; et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3’-kinase/AKT signaling. Cancer Res. 2003, 63, 5850–5858. [Google Scholar]
- Sasaki, H.; Hayakawa, J.; Terai, Y.; Kanemura, M.; Tanabe-Kimura, A.; Kamegai, H.; Seino-Noda, H.; Ezoe, S.; Matsumura, I.; Kanakura, Y.; et al. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene 2008, 27, 2737–2745. [Google Scholar] [CrossRef]
- Cox, O.T.; O’Shea, S.; Tresse, E.; Bustamante-Garrido, M.; Kiran-Deevi, R.; O’Connor, R. IGF-1 Receptor and Adhesion Signaling: An Important Axis in Determining Cancer Cell Phenotype and Therapy Resistance. Front. Endocrinol. 2015, 6, 106. [Google Scholar] [CrossRef]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef]
- Yoshida, K.; Saito, T.; Kamida, A.; Matsumoto, K.; Saeki, K.; Mochizuki, M.; Sasaki, N.; Nakagawa, T. Transforming growth factor-beta transiently induces vimentin expression and invasive capacity in a canine mammary gland tumor cell line. Res. Vet. Sci. 2013, 94, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Yasuda, H.; Saigusa, S.; Matushita, K.; Fujikawa, H.; Tanaka, K.; Mohri, Y.; Inoue, Y.; Goel, A.; Kusunoki, M. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int. J. Cancer 2012, 130, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Spinelli, A.M.; Wang, R.; Anfinogenova, Y.; Singer, H.A.; Tang, D.D. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 2006, 281, 34716–34724. [Google Scholar] [CrossRef] [PubMed]
- McInroy, L.; Maatta, A. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem. Biophys. Res. Commun. 2007, 360, 109–114. [Google Scholar] [CrossRef]
- Havel, L.S.; Kline, E.R.; Salgueiro, A.M.; Marcus, A.I. Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene 2015, 34, 1979–1990. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tanigaki, T.; Heimer, D.; Wang, W.; Ross, W.G.; Murphy, G.A.; Sakai, A.; Sussman, H.H.; Vu, T.H.; Raffin, T.A. TGF-beta 1 causes increased endothelial ICAM-1 expression and lung injury. J. Appl. Physiol. 1994, 77, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, K.; Mikula-Pietrasik, J.; Catar, R.; Dworacki, G.; Winckiewicz, M.; Frydrychowicz, M.; Dragun, D.; Staniszewski, R.; Jorres, A.; Witowski, J. Oxidative stress-dependent increase in ICAM-1 expression promotes adhesion of colorectal and pancreatic cancers to the senescent peritoneal mesothelium. Int. J. Cancer 2010, 127, 293–303. [Google Scholar] [CrossRef]
- Hollis, R.L.; Gourley, C. Genetic and molecular changes in ovarian cancer. Cancer Biol. Med. 2016, 13, 236–247. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uruski, P.; Mikuła-Pietrasik, J.; Pakuła, M.; Budkiewicz, S.; Drzewiecki, M.; Gaiday, A.N.; Wierzowiecka, M.; Naumowicz, E.; Moszyński, R.; Tykarski, A.; et al. Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 4222. https://doi.org/10.3390/ijms22084222
Uruski P, Mikuła-Pietrasik J, Pakuła M, Budkiewicz S, Drzewiecki M, Gaiday AN, Wierzowiecka M, Naumowicz E, Moszyński R, Tykarski A, et al. Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts. International Journal of Molecular Sciences. 2021; 22(8):4222. https://doi.org/10.3390/ijms22084222
Chicago/Turabian StyleUruski, Paweł, Justyna Mikuła-Pietrasik, Martyna Pakuła, Sylwia Budkiewicz, Marcin Drzewiecki, Andrey N. Gaiday, Małgorzata Wierzowiecka, Eryk Naumowicz, Rafał Moszyński, Andrzej Tykarski, and et al. 2021. "Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts" International Journal of Molecular Sciences 22, no. 8: 4222. https://doi.org/10.3390/ijms22084222
APA StyleUruski, P., Mikuła-Pietrasik, J., Pakuła, M., Budkiewicz, S., Drzewiecki, M., Gaiday, A. N., Wierzowiecka, M., Naumowicz, E., Moszyński, R., Tykarski, A., & Książek, K. (2021). Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts. International Journal of Molecular Sciences, 22(8), 4222. https://doi.org/10.3390/ijms22084222





