Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma
Abstract
1. Introduction
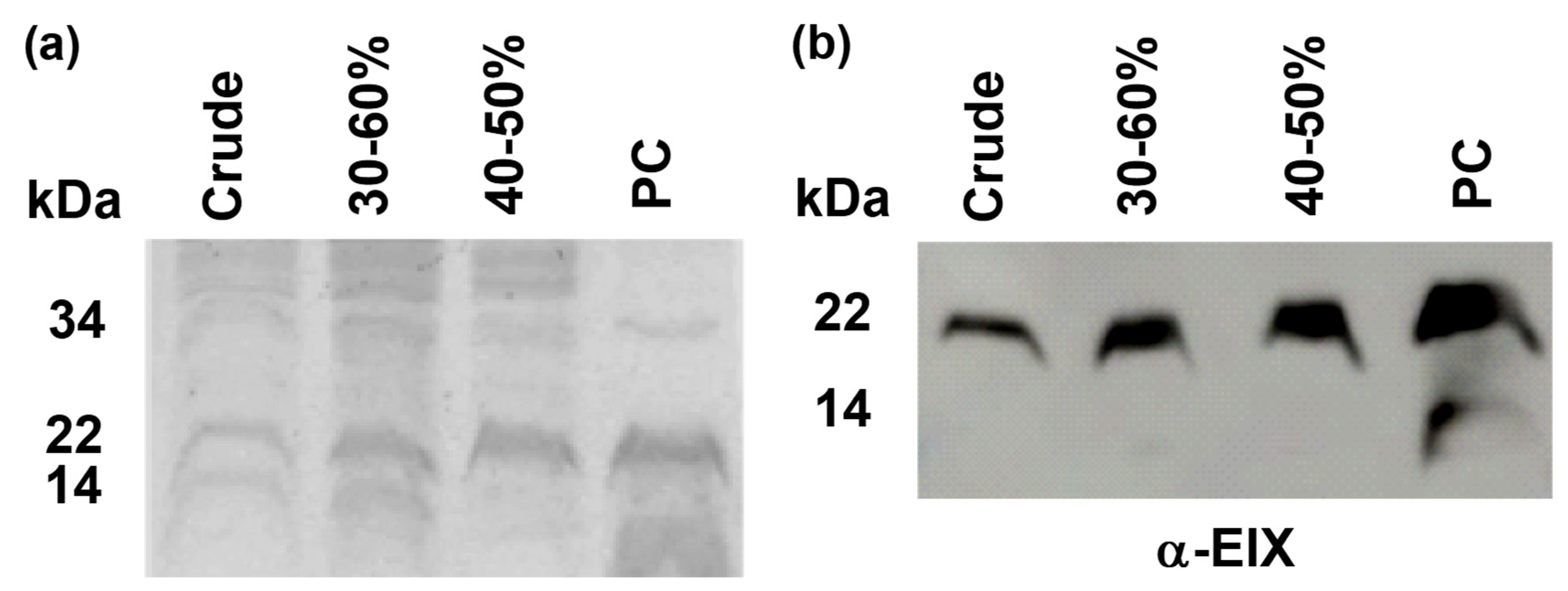
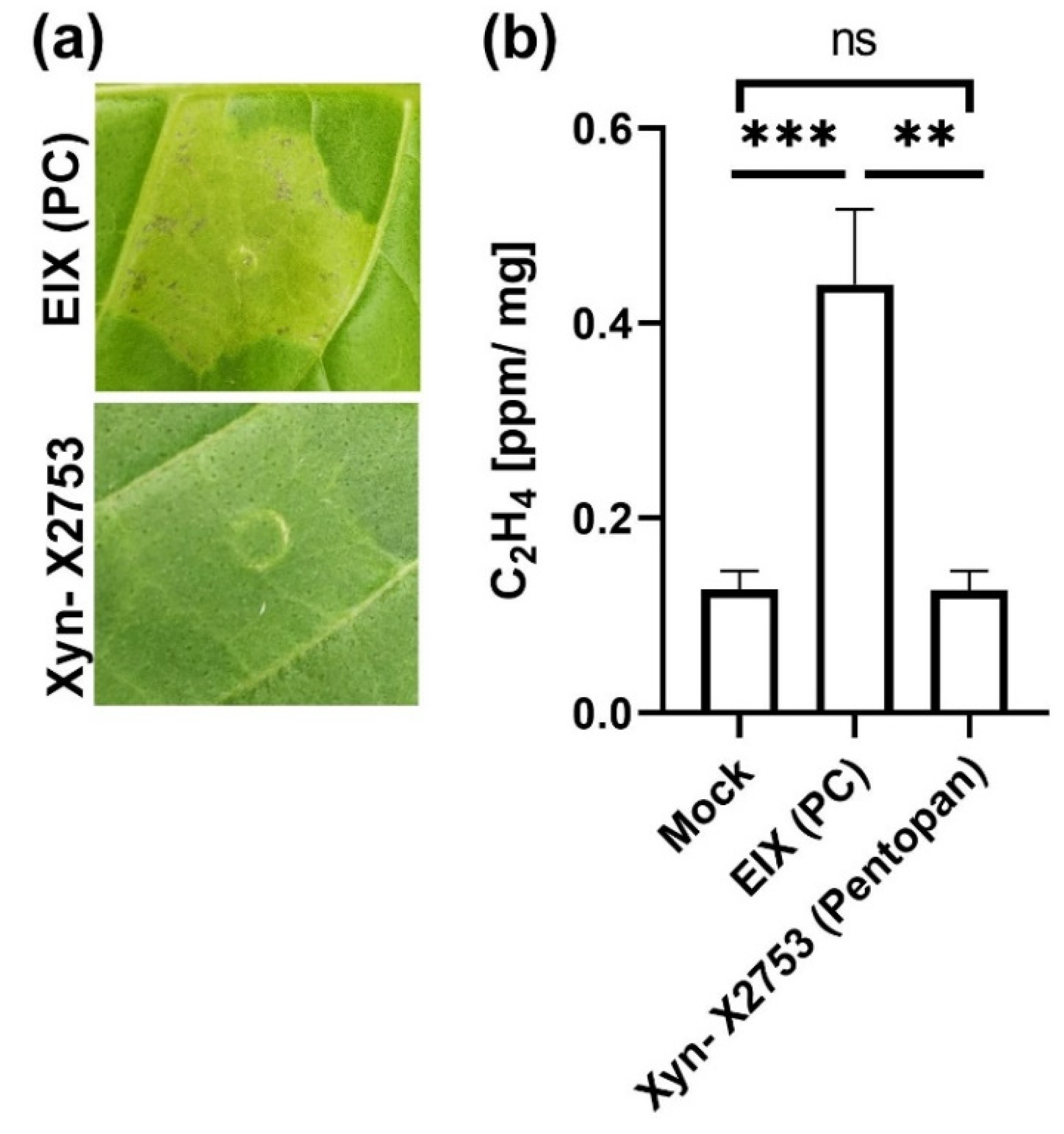
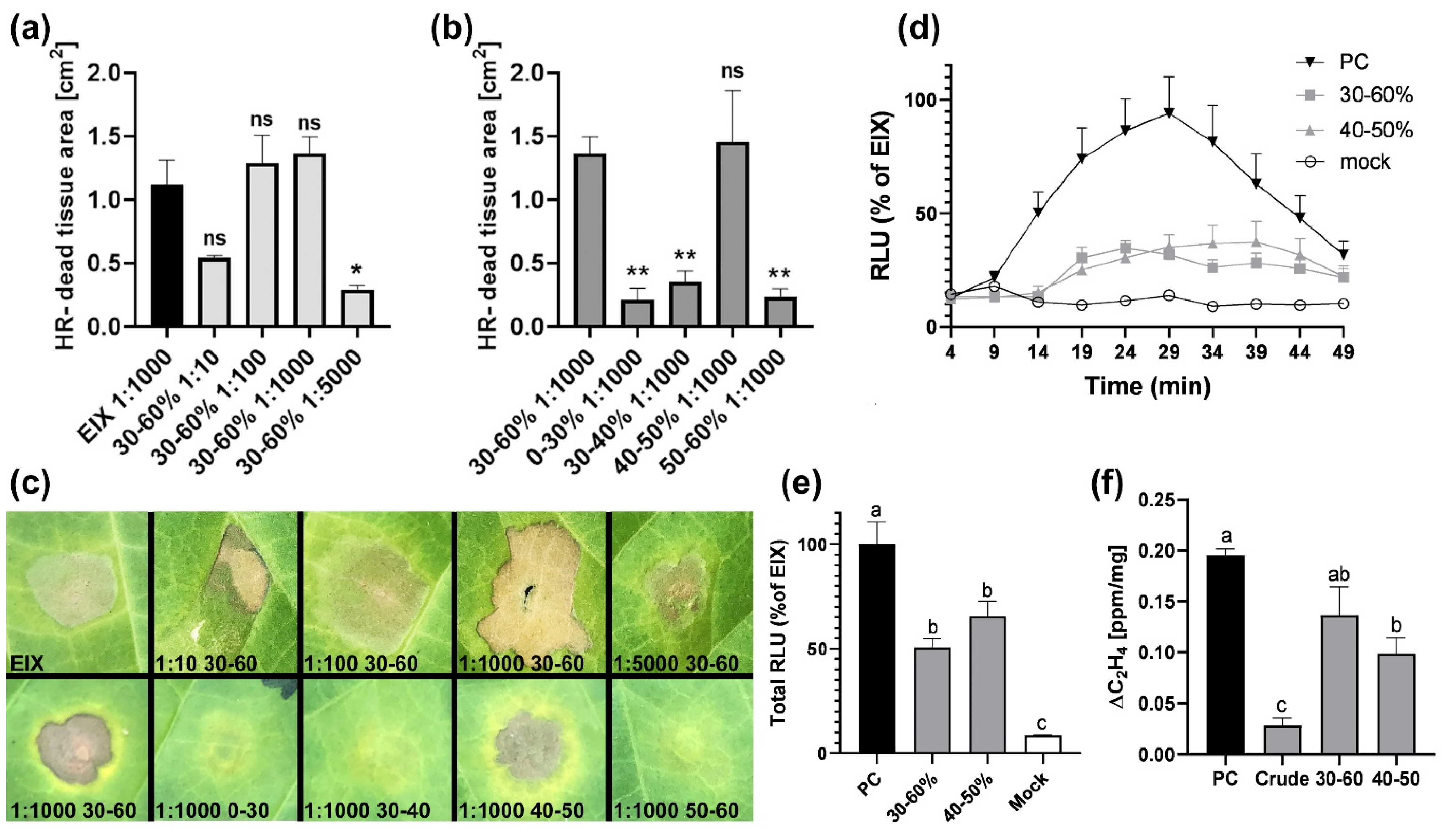
2. Results
3. Discussion
4. Materials and Methods
4.1. Microorganism and Culture Condition
4.2. Production of Xylanase from T. harzianum
4.3. Secreted Protein Extraction
4.4. Enzyme Purification
4.5. Coomassie Staining and Western Blot
4.6. Xylanase Assay
4.7. ROS Measurement
4.8. Ethylene Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Noman, A.; Aqeel, M.; Lou, Y. PRRs and NB-LRRs: From Signal Perception to Activation of Plant Innate Immunity. Int. J. Mol. Sci. 2019, 20, 1882. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit–pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Perazzolli, M.; Zago, E.D.; Delledonne, M. Nitric oxide signalling functions in plant-pathogen interactions. Cell. Microbiol. 2004, 6, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Canessa, P.; Hoppe, G.; Retamal, I.; Moyano, T.C.; Canales, J.; Gutiérrez, R.A.; Rubilar, J. Transcriptome analysis reveals regulatory networks underlying differential susceptibility to Botrytis cinerea in response to nitrogen availability in Solanum lycopersicum. Front. Plant Sci. 2015, 6, 911. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Yang, J.; Yang, W. Comparative Transcriptome Analysis of Resistant and Susceptible Tomato Lines in Response to Infection by Xanthomonas perforans Race T3. Front. Plant Sci. 2015, 6, 1173. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Bailey, B.A.; Dean, J.F.D.; Anderson, J.D. An ethylene biosynthesis-inducing endoxylanase elicits electrolyte leakage and necrosis in Nicotiana tabacum cv xanthi leaves. Plant Physiol. 1990, 94, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Dong, Y.; Qiu, D. The Botrytis cinerea Xylanase BcXyl1 Modulates Plant Immunity. Front. Microbiol. 2018, 9, 2535. [Google Scholar] [CrossRef]
- Leibman-Markus, M.; Pizarro, L.; Bar, M.; Coaker, G.; Avni, A. NRC proteins—A critical node for pattern and effector mediated signaling. Plant Signal. Behav. 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Pizarro, L.; Leibman-Markus, M.; Schuster, S.; Bar, M.; Avni, A. Tomato Dynamin Related Protein 2A Associates With LeEIX2 and Enhances PRR Mediated Defense by Modulating Receptor Trafficking. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Switchgrass for bioethanol and other value-added applications: A review. Bioresour. Technol. 2009, 100, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 1–36. [Google Scholar] [CrossRef]
- Bar, M.; Sharfman, M.; Avni, A. LeEix1 functions as a decoy receptor to attenuate LeEix2 signaling. Plant Signal. Behav. 2011, 6, 455–457. [Google Scholar] [CrossRef][Green Version]
- Fuchs, Y.; Saxena, A.; Ray Gamble, H.; Anderson, J.D. Ethylene biosynthesis-inducing protein from cellulysin is an endoxylanase. Plant Physiol. 1989, 89, 138–143. [Google Scholar] [CrossRef]
- Gupta, R.; Bar, M. Plant Immunity, Priming, and Systemic Resistance as Mechanisms for Trichoderma spp. Biocontrol. In Trichoderma, Host Pathogen Interactions and Applications; Sharma, A.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 81–110. [Google Scholar]
- Ron, M.; Kantety, R.; Martin, G.B.; Avidan, N.; Eshed, Y.; Zamir, D.; Avni, A. High-resolution linkage analysis and physical characterization of the EIX-responding locus in tomato. Theor. Appl. Genet. 2000, 100, 184–189. [Google Scholar] [CrossRef]
- Ron, M.; Avni, A. The Receptor for the Fungal Elicitor Ethylene-Inducing Xylanase Is a Member of a Resistance-Like Gene Family in Tomato. Plant Cell 2004, 16, 1604–1615. [Google Scholar] [CrossRef]
- Driss, D.; Bhiri, F.; Siela, M.; Bessess, S.; Chaabouni, S.; Ghorbel, R. Improvement of Breadmaking Quality by Xylanase GH11 from Penicillium occitanis Pol6. J. Texture Stud. 2013, 44, 75–84. [Google Scholar] [CrossRef]
- Rosmine, E.; Sainjan, N.C.; Silvester, R.; Alikkunju, A.; Varghese, S.A. Statistical optimisation of xylanase production by estuarine Streptomyces sp. and its application in clarification of fruit juice. J. Genet. Eng. Biotechnol. 2017, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, R.; Khosravinia, H.; Afzali, N. Effects of feed form and xylanase supplementation on metabolizable energy partitioning in broiler chicken fed wheat-based diets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Singh, R. Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem. Eng. J. 2012, 61, 49–58. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Garg, G.; Sharma, J.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Reduction in Acute Ecotoxicity of Paper Mill Effluent by Sequential Application of Xylanase and Laccase. PLoS ONE 2014, 9, e102581. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Prema, P. Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 2002, 22, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Haltrich, D.; Nidetzky, B.; Kulbe, K.D.; Steiner, W.; Župančič, S. Production of fungal xylanases. Bioresour. Technol. 1996, 58, 137–161. [Google Scholar] [CrossRef]
- Graminha, E.B.N.; Gonçalves, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; Da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. App. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Sources, Production, and Classification of Xylanases. In Xylanolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2014; pp. 43–52. [Google Scholar]
- Rotblat, B.; Enshell-Seijffers, D.; Gershoni, J.M.; Schuster, S.; Avni, A. Identification of an essential component of the elicitation active site of the EIX protein elicitor. Plant J. 2002, 32, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, L.; Leibman-Markus, M.; Schuster, S.; Bar, M.; Meltz, T.; Avni, A. Tomato Prenylated RAB Acceptor Protein 1 Modulates Trafficking and Degradation of the Pattern Recognition Receptor LeEIX2, Affecting the Innate Immune Response. Front. Plant Sci. 2018, 9, 257. [Google Scholar] [CrossRef]
- Leibman-Markus, M.; Pizarro, L.; Schuster, S.; Lin, Z.J.D.D.; Gershony, O.; Bar, M.; Coaker, G.; Avni, A. The intracellular nucleotide-binding leucine-rich repeat receptor (SlNRC4a) enhances immune signalling elicited by extracellular perception. Plant Cell. Environ. 2018, 41, 2313–2327. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, L.; Leibman-Markus, M.; Gupta, R.; Kovetz, N.; Shtein, I.; Bar, E.; Davidovich-Rikanati, R.; Zarivach, R.; Lewinsohn, E.; Avni, A.; et al. A gain of function mutation in SlNRC4a enhances basal immunity resulting in broad-spectrum disease resistance. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Greenberg, J.T. Programed cell death in plant-pathogen interactions. Anu. Rev. Plant Physiol. 1997, 48, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Archana, A.; Satyanarayana, T. Xylanase production by thermophilic Bacillus licheniformis A99 in solid-state fermentation. Enzyme Microb.Technol. 1997, 21, 12–17. [Google Scholar] [CrossRef]
- Elad, Y.; Zimand, G.; Zaqs, Y.; Zuriel, S.; Chet, I. Use of Trichoderma harzianum in combination or alternation with fungicides to control cucumber grey mould (Botrytis cinerea) under commercial greenhouse conditions. Plant Pathol. 1993, 42, 324–332. [Google Scholar] [CrossRef]
- Elad, Y. Trichoderma harzianum T39 Preparation for Biocontrol of Plant Diseases-Control of Botrytis cinerea, Sclerotinia sclerotiorum and Cladosporium fulvum. Biocontrol Sci. Technol. 2000, 10, 499–507. [Google Scholar] [CrossRef]
- Anand, G.; Yadav, S.; Yadav, D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Leibman-Markus, M.; Schuster, S.; Avni, A. LeEIX2 Interactors’ Analysis and EIX-Mediated Responses Measurement. In Plant Pattern Recognition Receptors: Methods and Protocols; Shan, L., He, P., Eds.; Springer: New York, NY, USA, 2017; pp. 167–172. ISBN 978-1-4939-6859-6. [Google Scholar]

| Fraction | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg) | Purification Fold |
|---|---|---|---|---|
| Crude | 371.2 ± 25.3 | 240 | 1.546 | - |
| 30–60% | 40.7 ± 2.7 | 14.4 | 2.826 | 1.82 |
| 40–50% | 39.4 ± 2.9 | 9.0 | 4.377 | 2.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, G.; Leibman-Markus, M.; Elkabetz, D.; Bar, M. Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma. Int. J. Mol. Sci. 2021, 22, 4214. https://doi.org/10.3390/ijms22084214
Anand G, Leibman-Markus M, Elkabetz D, Bar M. Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma. International Journal of Molecular Sciences. 2021; 22(8):4214. https://doi.org/10.3390/ijms22084214
Chicago/Turabian StyleAnand, Gautam, Meirav Leibman-Markus, Dorin Elkabetz, and Maya Bar. 2021. "Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma" International Journal of Molecular Sciences 22, no. 8: 4214. https://doi.org/10.3390/ijms22084214
APA StyleAnand, G., Leibman-Markus, M., Elkabetz, D., & Bar, M. (2021). Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma. International Journal of Molecular Sciences, 22(8), 4214. https://doi.org/10.3390/ijms22084214






