Aptamers for Anti-Viral Therapeutics and Diagnostics
Abstract
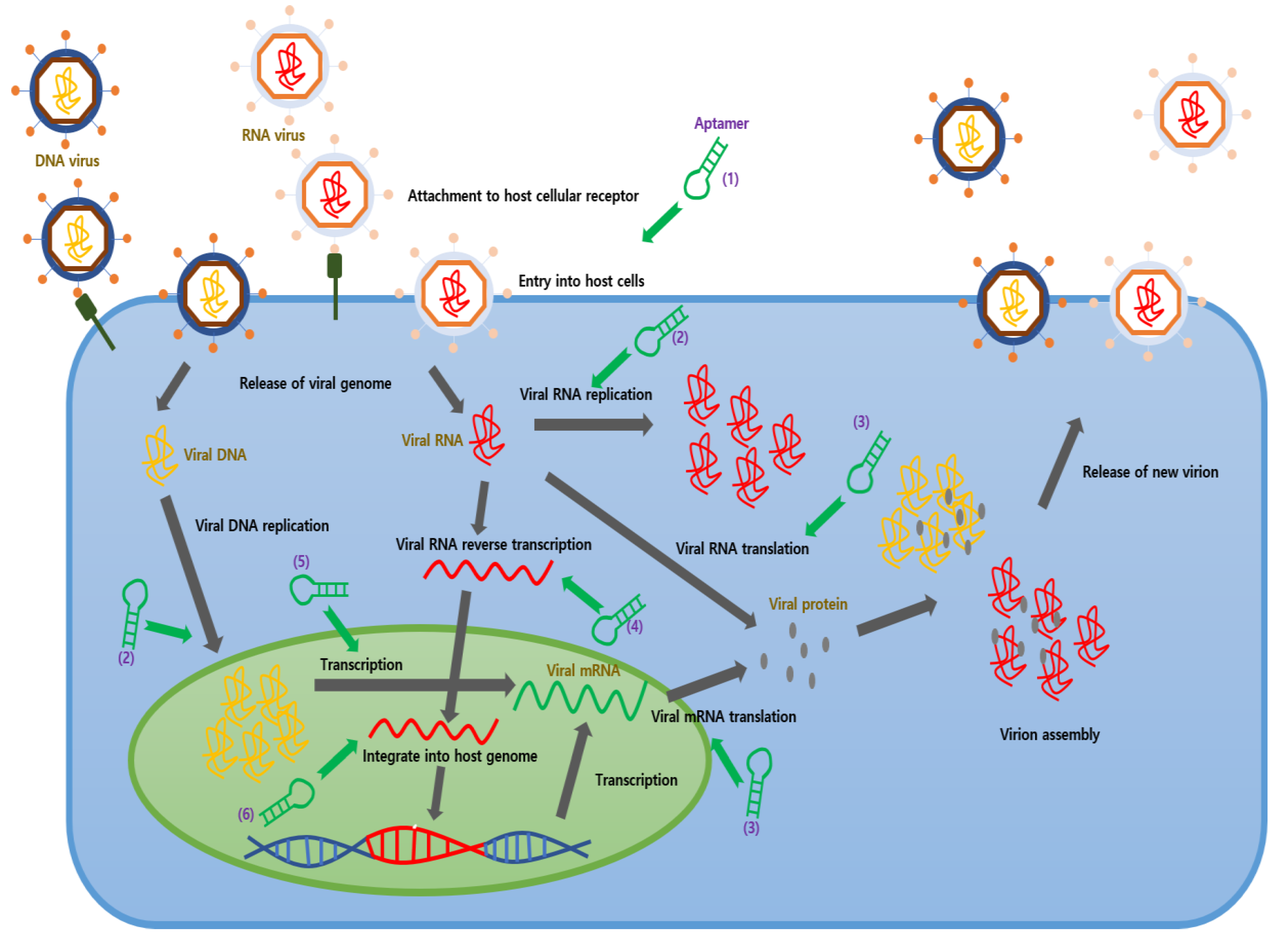
1. Introduction
2. Aptamers
3. Aptamers for Double-Stranded DNA Viruses
3.1. Aptamers for Human Papillomavirus (HPV)
3.1.1. Aptamers for HPV Diagnostics
3.1.2. Aptamers for HPV Therapeutics
3.2. Aptamers for Herpes Simplex Virus (HSV)
4. Aptamers for Positive Sense Single-Stranded RNA Viruses
4.1. Aptamers for the Hepatitis C Virus (HCV)
4.1.1. Aptamers for HCV Diagnostics
4.1.2. Aptamers for HCV Therapeutics
Therapeutic Aptamers for Targeting the HCV Viral Genome
Therapeutic Aptamers for Targeting HCV Viral Proteins
4.2. Aptamers for Zika Virus (ZIKV)
4.3. Aptamers for Dengue Virus (DENV)
4.3.1. Aptamers for DENV Diagnostics
4.3.2. Aptamers for DENV Therapeutics
4.4. Aptamers for Japanese Encephalitis Virus (JEV)
4.5. Aptamers for Tick-Borne Encephalitis Virus (TBEV)
4.6. Aptamers for Norovirus (NoV)
4.7. Aptamers for Coronavirus (CoV)
4.7.1. Aptamers for SARS-CoV
4.7.2. Aptamers for SARS-CoV2
5. Aptamers for Negative Sense Single-Stranded RNA Viruses
5.1. Aptamers for Influenza Virus
5.1.1. Aptamers for Influenza Virus Diagnostics
Aptamers for Influenza A Virus Diagnostics
Aptamers for Avian Influenza Virus (AIV) Diagnostics
5.1.2. Aptamers for Influenza Virus Therapeutics
5.2. Aptamers for Rift Valley Fever Virus (RVFV)
5.3. Aptamers for Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV)
5.4. Aptamers for Ebola Virus (EBOV)
6. Aptamers for Single-Stranded RNA Viruses with DNA Intermediate
6.1. Aptamers for Human Immunodeficiency Virus (HIV)
6.1.1. Aptamers for HIV Diagnostics
6.1.2. Aptamers for HIV Therapeutics
Therapeutic Aptamers for Targeting the HIV Viral Genome
Therapeutic Aptamers for Targeting HIV Viral Proteins
Therapeutic Aptamers for Targeting HIV Related Cellular Factors
7. Aptamers for Single-Stranded DNA Viruses with RNA Intermediate
7.1. Aptamers for Hepatitis B Virus (HBV)
7.1.1. Aptamers for HBV Diagnostics
7.1.2. Aptamers for HBV Therapeutics
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chang, C.P.; Lee, G.B. Integrated microfluidic device using a single universal aptamer to detect multiple types of influenza viruses. Biosens. Bioelectron. 2016, 86, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Ljubojevic, S.; Skerlev, M. HPV-associated diseases. Clin. Dermatol. 2014, 32, 227–234. [Google Scholar] [CrossRef]
- Toscano-Garibay, J.D.; Benitez-Hess, M.L.; Alvarez-Salas, L.M. Isolation and characterization of an RNA aptamer for the HPV-16 E7 oncoprotein. Arch. Med. Res. 2011, 42, 88–96. [Google Scholar] [CrossRef]
- Leija-Montoya, A.G.; Benitez-Hess, M.L.; Toscano-Garibay, J.D.; Alvarez-Salas, L.M. Characterization of an RNA aptamer against HPV-16 L1 virus-like particles. Nucleic Acid Ther. 2014, 24, 344–355. [Google Scholar] [CrossRef]
- Trausch, J.J.; Shank-Retzlaff, M.; Verch, T. Development and Characterization of an HPV Type-16 Specific Modified DNA Aptamer for the Improvement of Potency Assays. Anal. Chem. 2017, 89, 3554–3561. [Google Scholar] [CrossRef]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide-based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef]
- Nicol, C.; Bunka, D.H.; Blair, G.E.; Stonehouse, N.J. Effects of single nucleotide changes on the binding and activity of RNA aptamers to human papillomavirus 16 E7 oncoprotein. Biochem. Biophys. Res. Commun. 2011, 405, 417–421. [Google Scholar] [CrossRef]
- Nicol, C.; Cesur, O.; Forrest, S.; Belyaeva, T.A.; Bunka, D.H.; Blair, G.E.; Stonehouse, N.J. An RNA aptamer provides a novel approach for the induction of apoptosis by targeting the HPV16 E7 oncoprotein. PLoS ONE 2013, 8, e64781. [Google Scholar] [CrossRef]
- Cesur, O.; Nicol, C.; Groves, H.; Mankouri, J.; Blair, G.E.; Stonehouse, N.J. The Subcellular Localisation of the Human Papillomavirus (HPV) 16 E7 Protein in Cervical Cancer Cells and Its Perturbation by RNA Aptamers. Viruses 2015, 7, 3443–3461. [Google Scholar] [CrossRef]
- Gourronc, F.A.; Rockey, W.M.; Thiel, W.H.; Giangrande, P.H.; Klingelhutz, A.J. Identification of RNA aptamers that internalize into HPV-16 E6/E7 transformed tonsillar epithelial cells. Virology 2013, 446, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, T.A.; Nicol, C.; Cesur, O.; Trave, G.; Blair, G.E.; Stonehouse, N.J. An RNA Aptamer Targets the PDZ-Binding Motif of the HPV16 E6 Oncoprotein. Cancers 2014, 6, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Resendiz, D.G.; Palomino-Vizcaino, G.; Tapia-Vieyra, J.V.; Benitez-Hess, M.L.; Leija-Montoya, A.G.; Alvarez-Salas, L.M. Inhibition of Human Papillomavirus Type 16 Infection Using an RNA Aptamer. Nucleic Acid Ther. 2018, 28, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, F.; Deback, C.; Agut, H. Herpes simplex encephalitis: From virus to therapy. Infect. Disord. Drug Targets 2011, 11, 235–250. [Google Scholar] [CrossRef]
- Moore, M.D.; Bunka, D.H.J.; Forzan, M.; Spear, P.G.; Stockley, P.G.; McGowan, I.; James, W. Generation of neutralizing aptamers against herpes simplex virus type 2: Potential components of multivalent microbicides. J. Gen. Virol. 2011, 92, 1493–1499. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Hayashi, K.; Kumar, P.K. Aptamer that binds to the gD protein of herpes simplex virus 1 and efficiently inhibits viral entry. J. Virol. 2012, 86, 6732–6744. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Agelidis, A.; Jaishankar, D.; Mangano, K.; Thakkar, N.; Penmetcha, K.; Shukla, D. Targeting Herpes Simplex Virus-1 gD by a DNA Aptamer Can Be an Effective New Strategy to Curb Viral Infection. Mol. Ther. Nucleic Acids 2017, 9, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Baumert, T.F. HCV entry and neutralizing antibodies: Lessons from viral variants. Future Microbiol. 2009, 4, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.S.; Jo, M.; Jin, M.; Lee, D.K.; Kim, S. Chip-based detection of hepatitis C virus using RNA aptamers that specifically bind to HCV core antigen. Biochem. Biophys. Res. Commun. 2007, 358, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, Y.; Li, D.; Chen, H.; Zhang, X.L. CS-SELEX generates high-affinity ssDNA aptamers as molecular probes for hepatitis C virus envelope glycoprotein E2. PLoS ONE 2009, 4, e8142. [Google Scholar] [CrossRef]
- Park, J.H.; Jee, M.H.; Kwon, O.S.; Keum, S.J.; Jang, S.K. Infectivity of hepatitis C virus correlates with the amount of envelope protein E2: Development of a new aptamer-based assay system suitable for measuring the infectious titer of HCV. Virology 2013, 439, 13–22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Shen, X. Development of a nucleic acid lateral flow strip for detection of hepatitis C virus (HCV) core antigen. Nucleosides Nucleotides Nucleic Acids 2013, 32, 59–68. [Google Scholar] [CrossRef]
- Ghanbari, K.; Roushani, M.; Azadbakht, A. Ultra-sensitive aptasensor based on a GQD nanocomposite for detection of hepatitis C virus core antigen. Anal. Biochem. 2017, 534, 64–69. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Bayzyanova, J.M.; Anashkina, A.S.; Uchaikin, V.F.; Ziborov, V.S.; Konev, V.A.; Archakov, A.I.; Ivanov, Y.D. The detection of hepatitis c virus core antigen using afm chips with immobolized aptamers. J. Virol. Methods 2018, 251, 99–105. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of Hepatitis C Virus Core Protein in Serum Using Aptamer-Functionalized AFM Chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R.; Panel, A.-I.H.C.G. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H.; Lee, S.W. Prospects for nucleic acid-based therapeutics against hepatitis C virus. World J. Gastroenterol. 2013, 19, 8949–8962. [Google Scholar] [CrossRef]
- Kikuchi, K.; Umehara, T.; Fukuda, K.; Hwang, J.; Kuno, A.; Hasegawa, T.; Nishikawa, S. Structure-inhibition analysis of RNA aptamers that bind to HCV IRES. Nucleic Acids Symp. Ser. 2003, 13, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Umehara, T.; Fukuda, K.; Kuno, A.; Hasegawa, T.; Nishikawa, S. A hepatitis C virus (HCV) internal ribosome entry site (IRES) domain III-IV-targeted aptamer inhibits translation by binding to an apical loop of domain IIId. Nucleic Acids Res. 2005, 33, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Fukuda, K.; Umehara, T.; Hwang, J.; Kuno, A.; Hasegawa, T.; Nishikawa, S. In vitro selection of RNA aptamers that bind to domain II of HCV IRES. Nucleic Acids Symp. Ser. 2002, 2, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Umehara, T.; Fukuda, K.; Hwang, J.; Kuno, A.; Hasegawa, T.; Nishikawa, S. RNA aptamers targeted to domain II of hepatitis C virus IRES that bind to its apical loop region. J. Biochem. 2003, 133, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Umehara, T.; Nishikawa, F.; Fukuda, K.; Hasegawa, T.; Nishikawa, S. Increased inhibitory ability of conjugated RNA aptamers against the HCV IRES. Biochem. Biophys. Res. Commun. 2009, 386, 118–123. [Google Scholar] [CrossRef]
- Fukuda, K.; Toyokawa, Y.; Kikuchi, K.; Konno, K.; Ishihara, R.; Fukazawa, C.; Nishikawa, S.; Hasegawa, T. Isolation of RNA aptamers specific for the 3’ X tail of HCV. Nucleic Acids Symp. Ser. 2008, 52, 205–206. [Google Scholar] [CrossRef]
- Marton, S.; Berzal-Herranz, B.; Garmendia, E.; Cueto, F.J.; Berzal-Herranz, A. Anti-HCV RNA Aptamers Targeting the Genomic cis-Acting Replication Element. Pharmaceuticals 2011, 5, 49–60. [Google Scholar] [CrossRef]
- Marton, S.; Romero-Lopez, C.; Berzal-Herranz, A. RNA aptamer-mediated interference of HCV replication by targeting the CRE-5BSL3.2 domain. J. Viral. Hepat. 2013, 20, 103–112. [Google Scholar] [CrossRef]
- Konno, K.; Nishikawa, S.; Hasegawa, T.; Fukuda, K. Isolation of RNA aptamers specific for the HCV minus-IRES domain I. Nucleic Acids Symp. Ser. 2007, 51, 393–394. [Google Scholar] [CrossRef]
- Konno, K.; Fujita, S.; Iizuka, M.; Nishikawa, S.; Hasegawa, T.; Fukuda, K. Isolation and characterization of RNA aptamers specific for the HCV minus-IRES domain I. Nucleic Acids Symp. Ser. 2008, 493–494. [Google Scholar] [CrossRef]
- Konno, K.; Iizuka, M.; Fujita, S.; Nishikawa, S.; Hasegawa, T.; Fukuda, K. An RNA aptamer containing two binding sites against the HCV minus-IRES domain I. Nucleosides Nucleotides Nucleic Acids 2011, 30, 185–202. [Google Scholar] [CrossRef]
- Yang, D.; Meng, X.; Yu, Q.; Xu, L.; Long, Y.; Liu, B.; Fang, X.; Zhu, H. Inhibition of hepatitis C virus infection by DNA aptamer against envelope protein. Antimicrob. Agents Chemother. 2013, 57, 4937–4944. [Google Scholar] [CrossRef]
- Shi, S.; Yu, X.; Gao, Y.; Xue, B.; Wu, X.; Wang, X.; Yang, D.; Zhu, H. Inhibition of hepatitis C virus production by aptamers against the core protein. J. Virol. 2014, 88, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Gao, Y.; Yu, X.; Xue, B.; Zhou, F.; Wang, X.; Yang, D.; Liu, N.; Xu, L.; Fang, X.; et al. Inhibition of Hepatitis C Virus Infection by DNA Aptamer against NS2 Protein. PLoS ONE 2014, 9, e90333. [Google Scholar] [CrossRef]
- Kumar, P.K.R.; Machida, K.; Urvil, P.T.; Kakiuchi, N.; Vishnuvardhan, D.; Shimotohno, K.; Taira, K.; Nishikawa, S. Isolation of RNA aptamers specific to the NS3 protein of hepatitis C virus from a pool of completely random RNA. Virology 1997, 237, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Cho, J.S.; Yeo, H.J.; Kim, J.H.; Chung, K.M.; Han, K.; Jang, S.K.; Lee, S.W. Isolation of specific and high-affinity RNA aptamers against NS3 helicase domain of hepatitis C virus. RNA 2004, 10, 1277–1290. [Google Scholar] [CrossRef]
- Urvil, P.T.; Kakiuchi, N.; Zhou, D.M.; Shimotohno, K.; Kumar, P.K.; Nishikawa, S. Selection of RNA aptamers that bind specifically to the NS3 protease of hepatitis C virus. Eur. J. Biochem. 1997, 248, 130–138. [Google Scholar] [CrossRef]
- Fukuda, K.; Vishnuvardhan, D.; Sekiya, S.; Hwang, J.; Kakiuchi, N.; Taira, K.; Shimotohno, K.; Kumar, P.K.R.; Nishikawa, S. Isolation and characterization of RNA aptamers specific for the hepatitis C virus nonstructural protein 3 protease. Eur. J. Biochem. 2000, 267, 3685–3694. [Google Scholar] [CrossRef]
- Nishikawa, F.; Kakiuchi, N.; Funaji, K.; Fukuda, K.; Sekiya, S.; Nishikawa, S. Inhibition of HCV NS3 protease by RNA aptamers in cells. Nucleic Acids Res. 2003, 31, 1935–1943. [Google Scholar] [CrossRef]
- Sekiya, S.; Nishikawa, F.; Fukuda, K.; Nishikawa, S. Structurefunction analysis of an RNA aptamer for hepatitis C virus NS3 protease. J. Biochem. 2003, 133, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Nishikawa, F.; Fukuda, K. In vitro selection of RNA aptamers against HCV-NS3 helicase and their structural similarity with 3’(+)UTR of HCV. Nucleic Acids Symp. Ser. 2003, 3, 241–242. [Google Scholar] [CrossRef]
- Nishikawa, F.; Funaji, K.; Fukuda, K.; Nishikawa, S. In vitro selection of RNA aptamers against the HCV NS3 helicase domain. Oligonucleotides 2004, 14, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Umehara, T.; Sekiya, S.; Kunio, K.; Hasegawa, T.; Nishikawa, S. An RNA ligand inhibits hepatitis C virus NS3 protease and helicase activities. Biochem. Biophys. Res. Commun. 2004, 325, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Umehara, T.; Fukuda, K.; Nishikawa, F.; Sekiya, S.; Kohara, M.; Hasegawa, T.; Nishikawa, S. Designing and analysis of a potent bi-functional aptamers that inhibit protease and helicase activities of HCV NS3. Nucleic Acids Symp. Ser. 2004, 48, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Umehara, T.; Fukuda, K.; Nishikawa, F.; Kohara, M.; Hasegawa, T.; Nishikawa, S. Rational design of dual-functional aptamers that inhibit the protease and helicase activities of HCV NS3. J. Biochem. 2005, 137, 339–347. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Y.; Xue, B.; Wang, X.; Yang, D.; Qin, Y.; Yu, R.; Liu, N.; Xu, L.; Fang, X.; et al. Inhibition of hepatitis C virus infection by NS5A-specific aptamer. Antivir. Res. 2014, 106, 116–124. [Google Scholar] [CrossRef]
- Biroccio, A.; Hamm, J.; Incitti, I.; De Francesco, R.; Tomei, L. Selection of RNA aptamers that are specific and high-affinity ligands of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 2002, 76, 3688–3696. [Google Scholar] [CrossRef]
- Bellecave, P.; Andreola, M.L.; Ventura, M.; Tarrago-Litvak, L.; Litvak, S.; Astier-Gin, T. Selection of DNA aptamers that bind the RNA-dependent RNA polymerase of hepatitis C virus and inhibit viral RNA synthesis in vitro. Oligonucleotides 2003, 13, 455–463. [Google Scholar] [CrossRef]
- Bellecave, P.; Cazenave, C.; Rumi, J.; Staedel, C.; Cosnefroy, O.; Andreola, M.L.; Ventura, M.; Tarrago-Litvak, L.; Astier-Gin, T. Inhibition of hepatitis C virus (HCV) RNA polymerase by DNA aptamers: Mechanism of inhibition of in vitro RNA synthesis and effect on HCV-infected cells. Antimicrob. Agents Chemother. 2008, 52, 2097–2110. [Google Scholar] [CrossRef]
- Jones, L.A.; Clancy, L.E.; Rawlinson, W.D.; White, P.A. High-affinity aptamers to subtype 3a hepatitis C virus polymerase display genotypic specificity. Antimicrob. Agents Chemother. 2006, 50, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Yuhashi, K.; Uchiyama, Y.; Kodama, T.; Ohnishi, S. In vitro selection of RNA aptamers that bind the RNA-dependent RNA polymerase of hepatitis C virus: A possible role of GC-rich RNA motifs in NS5B binding. Virology 2009, 388, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, Y.J.; Kim, J.H.; Lim, J.H.; Kim, J.H.; Han, W.; Lee, S.H.; Noh, G.J.; Lee, S.W. Inhibition of hepatitis C virus (HCV) replication by specific RNA aptamers against HCV NS5B RNA replicase. J. Virol. 2013, 87, 7064–7074. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.H.; Kim, J.H.; Noh, Y.H.; Noh, G.J.; Lee, S.W. Pharmacokinetics of a Cholesterol-conjugated Aptamer Against the Hepatitis C Virus (HCV) NS5B Protein. Mol. Ther. Nucleic Acids 2015, 4, e254. [Google Scholar] [CrossRef]
- Malone, R.W.; Homan, J.; Callahan, M.V.; Glasspool-Malone, J.; Damodaran, L.; Schneider Ade, B.; Zimler, R.; Talton, J.; Cobb, R.R.; Ruzic, I.; et al. Zika Virus: Medical Countermeasure Development Challenges. PLoS Negl. Trop. Dis. 2016, 10, e0004530. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Hilgenfeld, R. Zika virus NS1, a pathogenicity factor with many faces. EMBO J. 2016, 35, 2631–2633. [Google Scholar] [CrossRef]
- Lee, K.H.; Zeng, H. Aptamer-Based ELISA Assay for Highly Specific and Sensitive Detection of Zika NS1 Protein. Anal. Chem. 2017, 89, 12743–12748. [Google Scholar] [CrossRef]
- Kim, D.T.H.; Bao, D.T.; Park, H.; Ngoc, N.M.; Yeo, S.J. Development of a novel peptide aptamer-based immunoassay to detect Zika virus in serum and urine. Theranostics 2018, 8, 3629–3642. [Google Scholar] [CrossRef]
- Dolai, S.; Tabib-Azar, M. Whole virus detection using aptamers and paper-based sensor potentiometry. Med. Devices Sens. 2020, e10112. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Phillips, L.W.; Milligan, A.S.; Rodda, S.J. Toward specific detection of Dengue virus serotypes using a novel modular biosensor. Biosens. Bioelectron. 2010, 26, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.R.; Crulhas, B.P.; Magro, M.; Vianello, F.; Pedrosa, V.A. A new immunoassay of hybrid nanomater conjugated to aptamers for the detection of dengue virus. Talanta 2019, 197, 482–490. [Google Scholar] [CrossRef]
- Gandham, S.H.; Volk, D.E.; Lokesh, G.L.; Neerathilingam, M.; Gorenstein, D.G. Thioaptamers targeting dengue virus type-2 envelope protein domain III. Biochem. Biophys. Res. Commun. 2014, 453, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Hsiao, W.H.; Lee, H.C.; Wu, S.C.; Cheng, J.W. Selection and Characterization of DNA Aptamers Targeting All Four Serotypes of Dengue Viruses. PLoS ONE 2015, 10, e0131240. [Google Scholar] [CrossRef]
- Jung, J.I.; Han, S.R.; Lee, S.W. Development of RNA aptamer that inhibits methyltransferase activity of dengue virus. Biotechnol. Lett. 2018, 40, 315–324. [Google Scholar] [CrossRef]
- Cnossen, E.J.; Silva, A.G.; Marangoni, K.; Arruda, R.A.; Souza, E.G.; Santos, F.A.; Fujimura, P.T.; Yokosawa, J.; Goulart, L.R.; Neves, A.F. Characterization of oligonucleotide aptamers targeting the 5’-UTR from dengue virus. Future Med. Chem. 2017, 9, 541–552. [Google Scholar] [CrossRef]
- Wang, H.; Liang, G. Epidemiology of Japanese encephalitis: Past, present, and future prospects. Ther. Clin. Risk Manag. 2015, 11, 435–448. [Google Scholar] [CrossRef]
- Han, S.R.; Lee, S.W. Inhibition of Japanese encephalitis virus (JEV) replication by specific RNA aptamer against JEV methyltransferase. Biochem. Biophys. Res. Commun. 2017, 483, 687–693. [Google Scholar] [CrossRef]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Kondratov, I.G.; Khasnatinov, M.A.; Potapova, U.V.; Potapov, V.V.; Levitskii, S.A.; Leonova, G.N.; Pavlenko, E.V.; Solovarov, I.S.; Denikina, N.N.; Kulakova, N.V.; et al. Obtaining aptamers to a fragment of surface protein E of tick-borne encephalitis virus. Dokl. Biochem. Biophys. 2013, 448, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Vinje, J.; Green, J.; Lewis, D.C.; Gallimore, C.I.; Brown, D.W.; Koopmans, M.P. Genetic polymorphism across regions of the three open reading frames of “Norwalk-like viruses”. Arch. Virol. 2000, 145, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Phan, K.; Teng, I.; Pu, J.; Watanabe, T. A systematic review and meta-analysis of the prevalence of norovirus in cases of gastroenteritis in developing countries. Medicine 2017, 96, e8139. [Google Scholar] [CrossRef]
- Escudero-Abarca, B.I.; Suh, S.H.; Moore, M.D.; Dwivedi, H.P.; Jaykus, L.A. Selection, characterization and application of nucleic acid aptamers for the capture and detection of human norovirus strains. PLoS ONE 2014, 9, e106805. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.D.; Escudero-Abarca, B.I.; Suh, S.H.; Jaykus, L.A. Generation and characterization of nucleic acid aptamers targeting the capsid P domain of a human norovirus GII.4 strain. J. Biotechnol. 2015, 209, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Z.; Yin, Y.; Jia, F.; Wu, Q.; Tian, P.; Wang, D. Development and evaluation of a novel in situ target-capture approach for aptamer selection of human noroviruses. Talanta 2019, 193, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, A.; Labib, M.; Hassan, E.M.; Tetro, J.A.; Springthorpe, S.; Sattar, S.A.; Berezovski, M.V.; DeRosa, M.C. Ultrasensitive norovirus detection using DNA aptasensor technology. PLoS ONE 2013, 8, e79087. [Google Scholar] [CrossRef]
- Chand, R.; Neethirajan, S. Microfluidic platform integrated with graphene-gold nano-composite aptasensor for one-step detection of norovirus. Biosens. Bioelectron. 2017, 98, 47–53. [Google Scholar] [CrossRef]
- Kim, B.; Chung, K.W.; Lee, J.H. Non-stop aptasensor capable of rapidly monitoring norovirus in a sample. J. Pharm. Biomed. Anal. 2018, 152, 315–321. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Jang, K.J.; Lee, N.R.; Yeo, W.S.; Jeong, Y.J.; Kim, D.E. Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase. Biochem. Biophys. Res. Commun. 2008, 366, 738–744. [Google Scholar] [CrossRef]
- Shum, K.T.; Tanner, J.A. Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. Chembiochem 2008, 9, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.G.; Jeon, I.J.; Kim, J.D.; Song, M.S.; Han, S.R.; Lee, S.W.; Jung, H.; Oh, J.W. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst 2009, 134, 1896–1901. [Google Scholar] [CrossRef]
- Cho, S.J.; Woo, H.M.; Kim, K.S.; Oh, J.W.; Jeong, Y.J. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011, 112, 535–540. [Google Scholar] [CrossRef]
- Roh, C.; Jo, S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, X.; Liu, X.; Ou, H.; Zhang, H.; Wang, J.; Li, Q.; Cheng, H.; Zhang, W.; Luo, Z. Discovery of sandwich type COVID-19 nucleocapsid protein DNA aptamers. Chem. Commun. 2020, 56, 10235–10238. [Google Scholar] [CrossRef]
- Arbeitskreis Blut, U. Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar] [CrossRef]
- Cox, N.J.; Subbarao, K. Global Epidemiology of Influenza Past and Present. Annu. Rev. Med. 2000, 51, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Baltimore, D.; Doherty, P.C.; Markel, H.; Morens, D.M.; Webster, R.G.; Wilson, I.A. Reconstruction of the 1918 influenza virus: Unexpected rewards from the past. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Tharakaraman, K.; Sasisekharan, R. Influenza surveillance: 2014-2015 H1N1 “swine”-derived influenza viruses from India. Cell Host Microbe 2015, 17, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Kopylov, A. Aptamers to Hemagglutinin: A Novel Tool for Influenza Virus Recognition and Neutralization. Curr. Pharm. Des. 2016, 22, 4835–4853. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Q.; Ren, Q.; Wei, H.P.; Chen, Z.; Deng, J.Y.; Zhang, Z.P.; Zhang, X.E. Quantum dot-aptamer nanoprobes for recognizing and labeling influenza A virus particles. Nanoscale 2011, 3, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, J.; He, Y.; Chen, S.; Jiang, Y.; Zhao, Y.; Zhao, S. Protein-binding aptamer assisted signal amplification for the detection of influenza A (H1N1) DNA sequences based on quantum dot fluorescence polarization analysis. Analyst 2013, 138, 4722–4727. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Wang, C.H.; Chang, C.P.; Lee, G.B. Integrated microfluidic system for rapid detection of influenza H1N1 virus using a sandwich-based aptamer assay. Biosens. Bioelectron. 2016, 82, 105–111. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, J.; Ryu, I.; Woo, H.M.; Lee, T.G.; Jung, W.; Yim, S.; Jeong, Y.J. An Aptamer-Based Electrochemical Sensor That Can Distinguish Influenza Virus Subtype H1 from H5. J. Microbiol. Biotechnol. 2017, 27, 2037–2043. [Google Scholar] [CrossRef]
- Bai, C.; Lu, Z.; Jiang, H.; Yang, Z.; Liu, X.; Ding, H.; Li, H.; Dong, J.; Huang, A.; Fang, T.; et al. Aptamer selection and application in multivalent binding-based electrical impedance detection of inactivated H1N1 virus. Biosens. Bioelectron. 2018, 110, 162–167. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Chaudhary, N.; Kim, H.; Jang, J. Subtyping of influenza A H1N1 virus using a label-free electrochemical biosensor based on the DNA aptamer targeting the stem region of HA protein. Anal. Chim. Acta 2019, 1064, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Park, S.G.; Choi, N.; Moon, J.I.; Dang, H.; Das, A.; Lee, S.; Kim, D.G.; Chen, L.; Choo, J. SERS imaging-based aptasensor for ultrasensitive and reproducible detection of influenza virus A. Biosens. Bioelectron. 2020, 167, 112496. [Google Scholar] [CrossRef]
- Le, T.T.; Adamiak, B.; Benton, D.J.; Johnson, C.J.; Sharma, S.; Fenton, R.; McCauley, J.W.; Iqbal, M.; Cass, A.E. Aptamer-based biosensors for the rapid visual detection of flu viruses. Chem. Commun. 2014, 50, 15533–15536. [Google Scholar] [CrossRef]
- Chen, C.; Zou, Z.; Chen, L.; Ji, X.; He, Z. Functionalized magnetic microparticle-based colorimetric platform for influenza A virus detection. Nanotechnology 2016, 27, 435102. [Google Scholar] [CrossRef]
- Le, T.T.; Chang, P.; Benton, D.J.; McCauley, J.W.; Iqbal, M.; Cass, A.E.G. Dual Recognition Element Lateral Flow Assay toward Multiplex Strain Specific Influenza Virus Detection. Anal. Chem. 2017, 89, 6781–6786. [Google Scholar] [CrossRef]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef]
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors 2012, 12, 12506–12518. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenza virus. Biosens. Bioelectron. 2013, 42, 148–155. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.; Hargis, B.; Tung, S.; Bottje, W.; Lu, H.; Li, Y. An Impedance Aptasensor with Microfluidic Chips for Specific Detection of H5N1 Avian Influenza Virus. Sensors 2015, 15, 18565–18578. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Rong, Z.; Wang, J.; Xiao, R.; Wang, S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF). Biosens. Bioelectron. 2015, 66, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Karash, S.; Wang, R.; Kelso, L.; Lu, H.; Huang, T.J.; Li, Y. Rapid detection of avian influenza virus H5N1 in chicken tracheal samples using an impedance aptasensor with gold nanoparticles for signal amplification. J. Virol. Methods 2016, 236, 147–156. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Seo, H.B.; Kim, B.C.; Kim, S.K.; Song, C.S.; Gu, M.B. Highly sensitive sandwich-type SPR based detection of whole H5Nx viruses using a pair of aptamers. Biosens. Bioelectron. 2016, 86, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.H. Aptamer-Based Field-Effect Transistor for Detection of Avian Influenza Virus in Chicken Serum. Anal. Chem. 2020, 92, 5524–5531. [Google Scholar] [CrossRef]
- Shum, K.T.; Zhou, J.; Rossi, J.J. Aptamer-based therapeutics: New approaches to combat human viral diseases. Pharmaceuticals 2013, 6, 1507–1542. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kayhan, B.; Ben-Yedidia, T.; Arnon, R. A DNA aptamer prevents influenza infection by blocking the receptor binding region of the viral hemagglutinin. J. Biol. Chem. 2004, 279, 48410–48419. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Sakamaki, Y.; Kawasaki, K.; Kumar, P.K. An efficient RNA aptamer against human influenza B virus hemagglutinin. J. Biochem. 2006, 139, 837–846. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Misono, T.S.; Kawasaki, K.; Mizuno, T.; Imai, M.; Odagiri, T.; Kumar, P.K.R. An RNA aptamer that distinguishes between closely related human influenza viruses and inhibits haemagglutinin-mediated membrane fusion. J. Gen. Virol. 2006, 87, 479–487. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Kumar, P.K. Aptamers that bind to the hemagglutinin of the recent pandemic influenza virus H1N1 and efficiently inhibit agglutination. Acta Biomater. 2013, 9, 8932–8941. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Dong, J.; Yao, L.; Chen, A.; Jia, R.; Huan, L.; Guo, J.; Shu, Y.; Zhang, Z. Potent inhibition of human influenza H5N1 virus by oligonucleotides derived by SELEX. Biochem. Biophys. Res. Commun. 2008, 366, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Lee, C.; Lee, K.S.; Choe, S.Y.; Mo, I.P.; Seong, R.H.; Hong, S.; Jeon, S.H. DNA aptamers against the receptor binding region of hemagglutinin prevent avian influenza viral infection. Mol. Cells 2011, 32, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, S.; Yoon, H.; Kim, K.B.; Kalme, S.S.; Oh, S.; Song, C.S.; Kim, D.E. Selection of an antiviral RNA aptamer against hemagglutinin of the subtype H5 avian influenza virus. Nucleic Acid. Ther. 2011, 21, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Musafia, B.; Oren-Banaroya, R.; Noiman, S. Designing anti-influenza aptamers: Novel quantitative structure activity relationship approach gives insights into aptamer-virus interaction. PLoS ONE 2014, 9, e97696. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Lee, K.H.; Han, B.W.; Han, M.R.; Kim, D.H.; Kim, D.E. An RNA aptamer that specifically binds to the glycosylated hemagglutinin of avian influenza virus and suppresses viral infection in cells. PLoS ONE 2014, 9, e97574. [Google Scholar] [CrossRef]
- Suenaga, E.; Kumar, P.K. An aptamer that binds efficiently to the hemagglutinins of highly pathogenic avian influenza viruses (H5N1 and H7N7) and inhibits hemagglutinin-glycan interactions. Acta Biomater. 2014, 10, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Z.; Jiang, F.; Fu, P.; Shen, J.; Wu, W.; Li, J. Two DNA aptamers against avian influenza H9N2 virus prevent viral infection in cells. PLoS ONE 2015, 10, e0123060. [Google Scholar] [CrossRef]
- Li, W.; Feng, X.; Yan, X.; Liu, K.; Deng, L. A DNA Aptamer against Influenza A Virus: An Effective Inhibitor to the Hemagglutinin-Glycan Interactions. Nucleic Acid. Ther. 2016, 26, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.M.; Kim, K.S.; Lee, J.M.; Shim, H.S.; Cho, S.J.; Lee, W.K.; Ko, H.W.; Keum, Y.S.; Kim, S.Y.; Pathinayake, P.; et al. Single-stranded DNA aptamer that specifically binds to the influenza virus NS1 protein suppresses interferon antagonism. Antivir. Res. 2013, 100, 337–345. [Google Scholar] [CrossRef]
- Reich, S.; Guilligay, D.; Pflug, A.; Malet, H.; Berger, I.; Crepin, T.; Hart, D.; Lunardi, T.; Nanao, M.; Ruigrok, R.W.; et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 2014, 516, 361–366. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, N.; Singh, K.; Shuai, H.; Chu, H.; Zhou, J.; Chow, B.K.; Zheng, B.J. Cross-protection of influenza A virus infection by a DNA aptamer targeting the PA endonuclease domain. Antimicrob. Agents. Chemother. 2015, 59, 4082–4093. [Google Scholar] [CrossRef]
- Aragón, T.; de la Luna, S.; Novoa, I.; Carrasco, L.; Ortín, J.; Nieto, A. Eukaryotic Translation Initiation Factor 4GI Is a Cellular Target for NS1 Protein, a Translational Activator of Influenza Virus. Mol. Cell. Biol. 2000, 20, 6259–6268. [Google Scholar] [CrossRef] [PubMed]
- Burgui, I.; Aragon, T.; Ortin, J.; Nieto, A. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol. 2003, 84, 3263–3274. [Google Scholar] [CrossRef] [PubMed]
- Yanguez, E.; Rodriguez, P.; Goodfellow, I.; Nieto, A. Influenza virus polymerase confers independence of the cellular cap-binding factor eIF4E for viral mRNA translation. Virology 2012, 422, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Perez-Morgado, M.I.; Gonzalez, V.M.; Martin, M.E.; Nieto, A. Inhibition of Influenza Virus Replication by DNA Aptamers Targeting a Cellular Component of Translation Initiation. Mol. Ther. Nucleic Acids. 2016, 5, e308. [Google Scholar] [CrossRef]
- Balkhy, H.H.; Memish, Z.A. Rift Valley fever: An uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 2003, 21, 153–157. [Google Scholar] [CrossRef]
- Ruigrok, R.W.; Crepin, T.; Kolakofsky, D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr. Opin. Microbiol. 2011, 14, 504–510. [Google Scholar] [CrossRef]
- Ellenbecker, M.; St Goddard, J.; Sundet, A.; Lanchy, J.M.; Raiford, D.; Lodmell, J.S. Computational prediction and biochemical characterization of novel RNA aptamers to Rift Valley fever virus nucleocapsid protein. Comput. Biol. Chem. 2015, 58, 120–125. [Google Scholar] [CrossRef]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- Park, S.W.; Han, M.G.; Yun, S.M.; Park, C.; Lee, W.J.; Ryou, J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1880–1882. [Google Scholar] [CrossRef]
- Jiao, Y.; Zeng, X.; Guo, X.; Qi, X.; Zhang, X.; Shi, Z.; Zhou, M.; Bao, C.; Zhang, W.; Xu, Y.; et al. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2012, 50, 372–377. [Google Scholar] [CrossRef]
- Yeom, G.; Kang, J.; Jang, H.; Nam, H.Y.; Kim, M.G.; Park, C.J. Development of DNA Aptamers against the Nucleocapsid Protein of Severe Fever with Thrombocytopenia Syndrome Virus for Diagnostic Application: Catalytic Signal Amplification using Replication Protein A-Conjugated Liposomes. Anal. Chem. 2019, 91, 13772–13779. [Google Scholar] [CrossRef]
- Feldmann, H.; Geisbert, T.W. Ebola haemorrhagic fever. Lancet 2011, 377, 849–862. [Google Scholar] [CrossRef]
- Binning, J.M.; Wang, T.; Luthra, P.; Shabman, R.S.; Borek, D.M.; Liu, G.; Xu, W.; Leung, D.W.; Basler, C.F.; Amarasinghe, G.K. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 2013, 52, 8406–8419. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kasahara, Y.; Miyamoto, Y.; Okuda, T.; Kasai, T.; Onodera, K.; Kuwahara, M.; Oka, M.; Yoneda, Y.; Obika, S. Development of oligonucleotide-based antagonists of Ebola virus protein 24 inhibiting its interaction with karyopherin alpha 1. Org. Biomol. Chem. 2018, 16, 4456–4463. [Google Scholar] [CrossRef]
- Shubham, S.; Hoinka, J.; Banerjee, S.; Swanson, E.; Dillard, J.A.; Lennemann, N.J.; Przytycka, T.M.; Maury, W.; Nilsen-Hamilton, M. A 2’FY-RNA Motif Defines an Aptamer for Ebolavirus Secreted Protein. Sci. Rep. 2018, 8, 12373. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.L.; Xiang, M.Q.; Tang, M.; Pang, D.W.; Zhang, Z.L. Ebola Virus Aptamers: From Highly Efficient Selection to Application on Magnetism-Controlled Chips. Anal. Chem. 2019, 91, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A. How does HIV cause AIDS? Science 1993, 260, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Kim, P.S. HIV entry and its inhibition. Cell 1998, 93, 681–684. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Lovsin, N.; Peterlin, B.M. Newly identified host factors modulate HIV replication. Immunol. Lett. 2005, 97, 225–234. [Google Scholar] [CrossRef]
- Pollard, V.W.; Malim, M.H. The HIV-1 Rev protein. Annu. Rev. Microbiol. 1998, 52, 491–532. [Google Scholar] [CrossRef]
- Pavski, V.; Le, X.C. Detection of human immunodeficiency virus type 1 reverse transcriptase using aptamers as probes in affinity capillary electrophoresis. Anal. Chem. 2001, 73, 6070–6076. [Google Scholar] [CrossRef]
- Minunni, M.; Tombelli, S.; Gullotto, A.; Luzi, E.; Mascini, M. Development of biosensors with aptamers as bio-recognition element: The case of HIV-1 Tat protein. Biosens. Bioelectron. 2004, 20, 1149–1156. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Luzi, E.; Mascini, M. Aptamer-based biosensors for the detection of HIV-1 Tat protein. Bioelectrochemistry 2005, 67, 135–141. [Google Scholar] [CrossRef]
- Rahim Ruslinda, A.; Tanabe, K.; Ibori, S.; Wang, X.; Kawarada, H. Effects of diamond-FET-based RNA aptamer sensing for detection of real sample of HIV-1 Tat protein. Biosens. Bioelectron. 2013, 40, 277–282. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.O.; Ustundag, Z. Spectrophotometric ellipsometry based Tat-protein RNA-aptasensor for HIV-1 diagnosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117748. [Google Scholar] [CrossRef]
- Srisawat, C.; Engelke, D.R. Selection of RNA aptamers that bind HIV-1 LTR DNA duplexes: Strand invaders. Nucleic Acids. Res. 2010, 38, 8306–8315. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Luque, F.J.; Stich, M.; Manrubia, S.; Briones, C.; Berzal-Herranz, A. Efficient HIV-1 inhibition by a 16 nt-long RNA aptamer designed by combining in vitro selection and in silico optimisation strategies. Sci. Rep. 2014, 4, 6242. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Marchand, B.; Das, K.; Himmel, D.M.; Parniak, M.A.; Hughes, S.H.; Arnold, E. Structure and function of HIV-1 reverse transcriptase: Molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef]
- Chaloin, L.; Lehmann, M.J.; Sczakiel, G.; Restle, T. Endogenous expression of a high-affinity pseudoknot RNA aptamer suppresses replication of HIV-1. Nucleic Acids Res. 2002, 30, 4001–4008. [Google Scholar] [CrossRef]
- Held, D.M.; Kissel, J.D.; Saran, D.; Michalowski, D.; Burke, D.H. Differential susceptibility of HIV-1 reverse transcriptase to inhibition by RNA aptamers in enzymatic reactions monitoring specific steps during genome replication. J. Biol. Chem. 2006, 281, 25712–25722. [Google Scholar] [CrossRef] [PubMed]
- Held, D.M.; Kissel, J.D.; Thacker, S.J.; Michalowski, D.; Saran, D.; Ji, J.; Hardy, R.W.; Rossi, J.J.; Burke, D.H. Cross-clade inhibition of recombinant human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus SIVcpz reverse transcriptases by RNA pseudoknot aptamers. J. Virol. 2007, 81, 5375–5384. [Google Scholar] [CrossRef][Green Version]
- Joshi, P.; Prasad, V.R. Potent inhibition of human immunodeficiency virus type 1 replication by template analog reverse transcriptase inhibitors derived by SELEX (systematic evolution of ligands by exponential enrichment). J. Virol. 2002, 76, 6545–6557. [Google Scholar] [CrossRef]
- Joshi, P.J.; North, T.W.; Prasad, V.R. Aptamers directed to HIV-1 reverse transcriptase display greater efficacy over small hairpin RNAs targeted to viral RNA in blocking HIV-1 replication. Mol. Ther. 2005, 11, 677–686. [Google Scholar] [CrossRef]
- Kissel, J.D.; Held, D.M.; Hardy, R.W.; Burke, D.H. Active site binding and sequence requirements for inhibition of HIV-1 reverse transcriptase by the RT1 family of single-stranded DNA aptamers. Nucleic Acids Res. 2007, 35, 5039–5050. [Google Scholar] [CrossRef]
- Kissel, J.D.; Held, D.M.; Hardy, R.W.; Burke, D.H. Single-stranded DNA aptamer RT1t49 inhibits RT polymerase and RNase H functions of HIV type 1, HIV type 2, and SIVCPZ RTs. AIDS Res. Hum. Retrovir. 2007, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, J.J.; Nair, G.R. Novel aptamer inhibitors of human immunodeficiency virus reverse transcriptase. Oligonucleotides 2008, 18, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Michalowski, D.; Chitima-Matsiga, R.; Held, D.M.; Burke, D.H. Novel bimodular DNA aptamers with guanosine quadruplexes inhibit phylogenetically diverse HIV-1 reverse transcriptases. Nucleic Acids Res. 2008, 36, 7124–7135. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.J.; Sharma, T.K.; Whatley, A.S.; Landon, L.A.; Tempesta, M.A.; Johnson, M.C.; Burke, D.H. Robust suppression of HIV replication by intracellularly expressed reverse transcriptase aptamers is independent of ribozyme processing. Mol. Ther. 2012, 20, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Shiang, Y.C.; Ou, C.M.; Chen, S.J.; Ou, T.Y.; Lin, H.J.; Huang, C.C.; Chang, H.T. Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale 2013, 5, 2756–2764. [Google Scholar] [CrossRef]
- Whatley, A.S.; Ditzler, M.A.; Lange, M.J.; Biondi, E.; Sawyer, A.W.; Chang, J.L.; Franken, J.D.; Burke, D.H. Potent Inhibition of HIV-1 Reverse Transcriptase and Replication by Nonpseudoknot, “UCAA-motif” RNA Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e71. [Google Scholar] [CrossRef]
- Lange, M.J.; Nguyen, P.D.M.; Callaway, M.K.; Johnson, M.C.; Burke, D.H. RNA-protein interactions govern antiviral specificity and encapsidation of broad spectrum anti-HIV reverse transcriptase aptamers. Nucleic Acids Res. 2017, 45, 6087–6097. [Google Scholar] [CrossRef]
- Nguyen, P.D.M.; Zheng, J.; Gremminger, T.J.; Qiu, L.; Zhang, D.; Tuske, S.; Lange, M.J.; Griffin, P.R.; Arnold, E.; Chen, S.J.; et al. Binding interface and impact on protease cleavage for an RNA aptamer to HIV-1 reverse transcriptase. Nucleic Acids Res. 2020, 48, 2709–2722. [Google Scholar] [CrossRef]
- Andreola, M.L.; Pileur, F.; Calmels, C.; Ventura, M.; Tarrago-Litvak, L.; Toulmé, J.J.; Litvak, S. DNA aptamers selected against the HIV-1 RNase H display in vitro antiviral activity. Biochemistry 2001, 40, 10087–10094. [Google Scholar] [CrossRef]
- de Soultrait, V.R.; Lozach, P.-Y.; Altmeyer, R.; Tarrago-Litvak, L.; Litvak, S.; Andréola, M.L. DNA Aptamers Derived from HIV-1 RNase H Inhibitors are Strong Anti-integrase Agents. J. Mol. Biol. 2002, 324, 195–203. [Google Scholar] [CrossRef]
- Chiu, T.K.; Davies, D.R. Structure and function of HIV-1 integrase. Curr. Top. Med. Chem. 2004, 4, 965–977. [Google Scholar] [CrossRef]
- Metifiot, M.; Leon, O.; Tarrago-Litvak, L.; Litvak, S.; Andreola, M.L. Targeting HIV-1 integrase with aptamers selected against the purified RNase H domain of HIV-1 RT. Biochimie 2005, 87, 911–919. [Google Scholar] [CrossRef]
- Ojwang, J.O.; Buckheit, R.W.; Pommier, Y.; Mazumder, A.; De Vreese, K.; Este, J.A.; Reymen, D.; Pallansch, L.A.; Lackman-Smith, C.; Wallace, T.L. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1995, 39, 2426–2435. [Google Scholar] [CrossRef]
- Marchand, C.; Maddali, K.; Metifiot, M.; Pommier, Y. HIV-1 IN Inhibitors 2010 Update and Perspectives. Curr. Top. Med. Chem. 2009, 9, 1016–1037. [Google Scholar] [CrossRef]
- Faure-Perraud, A.; Metifiot, M.; Reigadas, S.; Recordon-Pinson, P.; Parissi, V.; Ventura, M.; Andreola, M.L. The guanine-quadruplex aptamer 93del inhibits HIV-1 replication ex vivo by interfering with viral entry, reverse transcription and integration. Antivir. Ther. 2011, 16, 383–394. [Google Scholar] [CrossRef]
- Virgilio, A.; Amato, T.; Petraccone, L.; Esposito, F.; Grandi, N.; Tramontano, E.; Romero, R.; Haider, S.; Gomez-Monterrey, I.; Novellino, E.; et al. Improvement of the activity of the anti-HIV-1 integrase aptamer T30175 by introducing a modified thymidine into the loops. Sci. Rep. 2018, 8, 7447. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C. Understanding HIV-1 protease autoprocessing for novel therapeutic development. Future Med. Chem. 2013, 5, 1215–1229. [Google Scholar] [CrossRef]
- Duclair, S.; Gautam, A.; Ellington, A.; Prasad, V.R. High-affinity RNA Aptamers against the HIV-1 Protease Inhibit Both In Vitro Protease Activity and Late Events of Viral Replication. Mol. Ther. Nucleic Acids 2015, 4, e228. [Google Scholar] [CrossRef]
- Levin, J.G.; Mitra, M.; Mascarenhas, A.; Musier-Forsyth, K. Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription. RNA Biol. 2010, 7, 754–774. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.Y.; Lee, J.H.; You, J.C.; Jeong, S. Selection and stabilization of the RNA aptamers against the human immunodeficiency virus type-1 nucleocapsid protein. Biochem. Biophys. Res. Commun. 2002, 291, 925–931. [Google Scholar] [CrossRef]
- Kim, M.Y.; Jeong, S. Inhibition of the functions of the nucleocapsid protein of human immunodeficiency virus-1 by an RNA aptamer. Biochem. Biophys. Res. Commun. 2004, 320, 1181–1186. [Google Scholar] [CrossRef]
- Wyatt, R.; Kwong, P.D.; Desjardins, E.; Sweet, R.W.; Robinson, J.; Hendrickson, W.A.; Sodroski, J.G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 1998, 393, 705–711. [Google Scholar] [CrossRef]
- Khati, M.; Schuman, M.; Ibrahim, J.; Sattentau, Q.; Gordon, S.; James, W. Neutralization of infectivity of diverse R5 clinical isolates of human immunodeficiency virus type 1 by gp120-binding 2’F-RNA aptamers. J. Virol. 2003, 77, 12692–12698. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Griffiths, C.; Lea, S.M.; James, W. Structural characterization of an anti-gp120 RNA aptamer that neutralizes R5 strains of HIV-1. RNA 2005, 11, 873–884. [Google Scholar] [CrossRef]
- Dey, A.K.; Khati, M.; Tang, M.; Wyatt, R.; Lea, S.M.; James, W. An aptamer that neutralizes R5 strains of human immunodeficiency virus type 1 blocks gp120-CCR5 interaction. J. Virol. 2005, 79, 13806–13810. [Google Scholar] [CrossRef]
- Cohen, C.; Forzan, M.; Sproat, B.; Pantophlet, R.; McGowan, I.; Burton, D.; James, W. An aptamer that neutralizes R5 strains of HIV-1 binds to core residues of gp120 in the CCR5 binding site. Virology 2008, 381, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mufhandu, H.T.; Gray, E.S.; Madiga, M.C.; Tumba, N.; Alexandre, K.B.; Khoza, T.; Wibmer, C.K.; Moore, P.L.; Morris, L.; Khati, M. UCLA1, a synthetic derivative of a gp120 RNA aptamer, inhibits entry of human immunodeficiency virus type 1 subtype C. J. Virol. 2012, 86, 4989–4999. [Google Scholar] [CrossRef] [PubMed]
- Lochrie, M.A.; Waugh, S.; Pratt, D.G., Jr.; Clever, J.; Parslow, T.G.; Polisky, B. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 gag polyprotein. Nucleic Acids Res. 1997, 25, 2902–2910. [Google Scholar] [CrossRef]
- Ramalingam, D.; Duclair, S.; Datta, S.A.; Ellington, A.; Rein, A.; Prasad, V.R. RNA aptamers directed to human immunodeficiency virus type 1 Gag polyprotein bind to the matrix and nucleocapsid domains and inhibit virus production. J. Virol. 2011, 85, 305–314. [Google Scholar] [CrossRef]
- Dearborn, A.D.; Eren, E.; Watts, N.R.; Palmer, I.W.; Kaufman, J.D.; Steven, A.C.; Wingfield, P.T. Structure of an RNA Aptamer that Can Inhibit HIV-1 by Blocking Rev-Cognate RNA (RRE) Binding and Rev-Rev Association. Structure 2018, 26, 1187–1195 e1184. [Google Scholar] [CrossRef]
- Symensma, T.L.; Giver, L.; Zapp, M.; Takle, G.B.; Ellington, A.D. RNA aptamers selected to bind human immunodeficiency virus type 1 Rev in vitro are Rev responsive in vivo. J. Virol. 1996, 70, 179–187. [Google Scholar] [CrossRef]
- Konopka, K.; Lee, N.S.; Rossi, J.; Düzgüneş, N. Rev-binding aptamer and CMV promoter act as decoys to inhibit HIV replication. Gene 2000, 255, 235–244. [Google Scholar] [CrossRef]
- Zhou, J.; Satheesan, S.; Li, H.; Weinberg, M.S.; Morris, K.V.; Burnett, J.C.; Rossi, J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015, 22, 379–390. [Google Scholar] [CrossRef]
- Perrone, R.; Butovskaya, E.; Lago, S.; Garzino-Demo, A.; Pannecouque, C.; Palu, G.; Richter, S.N. The G-quadruplex-forming aptamer AS1411 potently inhibits HIV-1 attachment to the host cell. Int. J. Antimicrob. Agents 2016, 47, 311–316. [Google Scholar] [CrossRef]
- Couturier, J.; Orozco, A.F.; Liu, H.; Budhiraja, S.; Siwak, E.B.; Nehete, P.N.; Sastry, K.J.; Rice, A.P.; Lewis, D.E. Regulation of cyclin T1 during HIV replication and latency establishment in human memory CD4 T cells. Virol. J. 2019, 16, 22. [Google Scholar] [CrossRef]
- Um, H.J.; Kim, M.; Lee, S.H.; Kim, Y.H. Preventing the formation of positive transcription elongation factor b by human cyclin T1-binding RNA aptamer for anti-HIV transcription. AIDS 2012, 26, 1599–1605. [Google Scholar] [CrossRef]
- Suh, S.K.; Song, S.; Oh, H.B.; Hwang, S.H.; Hah, S.S. Aptamer-based competitive binding assay for one-step quantitation of hepatitis B surface antigen. Analyst 2014, 139, 4310–4314. [Google Scholar] [CrossRef]
- Xi, Z.; Huang, R.; Li, Z.; He, N.; Wang, T.; Su, E.; Deng, Y. Selection of HBsAg-Specific DNA Aptamers Based on Carboxylated Magnetic Nanoparticles and Their Application in the Rapid and Simple Detection of Hepatitis B Virus Infection. ACS Appl. Mater. Interfaces 2015, 7, 11215–11223. [Google Scholar] [CrossRef]
- Huang, R.; Xi, Z.; Deng, Y.; He, N. Fluorescence based Aptasensors for the determination of hepatitis B virus e antigen. Sci. Rep. 2016, 6, 31103. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Beck, J.; Nassal, M.; Hu, K.H. A SELEX-screened aptamer of human hepatitis B virus RNA encapsidation signal suppresses viral replication. PLoS ONE 2011, 6, e27862. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Pei, X.; Zhang, Q.; Lu, B.; Zhang, X.; Liu, J. An aptamer targets HBV core protein and suppresses HBV replication in HepG2.2.15 cells. Int. J. Mol. Med. 2014, 34, 1423–1429. [Google Scholar] [CrossRef]
- Orabi, A.; Bieringer, M.; Geerlof, A.; Bruss, V. An Aptamer against the Matrix Binding Domain on the Hepatitis B Virus Capsid Impairs Virion Formation. J. Virol. 2015, 89, 9281–9287. [Google Scholar] [CrossRef]
- Zheng, H.; Lang, Y.; Yu, J.; Han, Z.; Chen, B.; Wang, Y. Affinity binding of aptamers to agarose with DNA tetrahedron for removal of hepatitis B virus surface antigen. Colloids Surf. B Biointerfaces 2019, 178, 80–86. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef]

| Aptamer | Antibody | |
|---|---|---|
| Material | Nucleic Acid (DNA, RNA) | Protein |
| Target | Wide range, including proteins | A limited target composed of amino acids |
| Size | ~20 kDa | ~150 kDa |
| Binding manner | 3D structure recognition | Peptide sequence recognition |
| Immunogenicity | Minimal reported | Many reported |
| Development period | 6–8 weeks | Months |
| Manufacturing | Chemical synthesis | Biological manufacturing |
| Storage | Room temperature | Cold temperature |
| Group | Virus | Target | Name of Aptamer | Type | Kd | Sequence | Applications | References |
|---|---|---|---|---|---|---|---|---|
| Group I | HPV | HPV-16 VLPs | Sc5-c3 | RNA | 0.05 pM | 5′-GGG AAC AAA AGC UGC ACA GGU UAC CCC CGC UUG GGU CUC CCU AUA GUG AGU CGU AUU A-3′ | Inhibition of HPV-16 infection | [9,11,17] |
| HPV-07 | DNA | 400 ± 30 pM | n.d. | Specific detection of HPV-16 | [10] | |||
| HPV-16 E6/E7-HTECs | C5 | RNA * | n.d. | 5′-GGG AGG ACG AUG CGG AAG CAU CAA GGG UGA UCG UUU GAC CCU CCC CAG ACG ACU CGC CCG A-3′ | Internalize aptamers into target cells | [15] | ||
| E6 | F2 and F4 | RNA * | n.d. | 5′-UGA UAA UAC GAC UCA CUA UAG GGA AUG GAU CCA CAU ACU ACG AAU AUU CAA CAU UCG AGG UGG AUG CUA CGA AUC AAC UUC ACU GCA GAC UUG ACG AAG CUU-3′ (F2) | Blocking E6–p53 interaction in cells | [16] | ||
| E7 | G5a3N | RNA | 1.9 uM | 5′-UAA UAC GAC UCA CUA UAG GGA GAC CCA AGC CGA UUU AUU UUG UGC AGC UUU UGU UCC CUU UAG UGA GGG UUA AUU-3′ | Detection of HPV-16 | [8] | ||
| A2 | RNA * | 107 nM | 5′-CCC UUC AUC AUU AAC CCG UCC ACG CGC-3′ | Induce apoptosis in HPV infected cells | [12,13,14] | |||
| HSV | gD protein | F2, D5 and G7a | RNA | n.d. | 5′-GGG AGA GAG AGAG UCC AGU AAG CGC UCG UCA CACCCA CUA CGA GUG CCA UGC AUA UCU GCA ACA UCA CUU UAG GCC GAA UAA CGC AGA GGU AGA UGG-3′ (F2) | Inhibition of HSV-2 infection in cells | [19] | |
| aptamer-1 and -5 | RNA * | 109 nM | 5′-AGU AAU ACG ACU CAC UAU AGG GCA CGA GAG AGG UCG UCC-3′ (aptamer-1) | Inhibition of HSV-1 entry in cells | [20] | |||
| DApt | DNA | 50 nM | 5′-AGT AAT ACG ACT CA C TAT AGG GCA CGA GAG AGG TCG TCC-3′ | Inhibition of HSV-1 entry in mice | [21] | |||
| Group IV | HCV | 5′-UTR IRES | 2-02 and 3-07 | RNA | 110 ± 10 nM | 5′-GGG AGA AUU CCG ACC AGA AGG AGC GUU GCA GGG AAU GUA UGG CUA AUU CCC CUU UCC UCU CUC CUU CCU CUU CU-3′ (2-02) | Inhibition of HCV replication in vitro | [32,33,34,35,36] |
| 3′-UTR | cSL1, cSL2 and cSL3 | RNA | n.d. | n.d. | Inhibition of HCV replication in vitro | [37] | ||
| P-58 and P-78 | RNA | 0.47 ± 0.12 uM | 5′-GGG AUA UUA UAG UAC AUA AAU GCC CCG CCU CAC AAA GGG AUG GGC UGU GGA GGU AGC GAA UUA AAG AGU AGU CGA A-3′ (P-58) | Inhibition of HCV replication in vitro | [38,39] | |||
| Negative strand IRES | AP30 | RNA | 32 nM | 5′-GUA CAC CAA AUC GCC CAC HCC CGU CUG GGA CUG GAU CAA C-3′ | Inhibition of HCV replication in vitro | [40,41,42] | ||
| Core | 9-14 and 9-15 | RNA * | 100 nM | 5′-GGG CCG UUC GAA CAC GAG CAU GUU GUC UAC GUU GUA GAA GCU GUU AUG GUA GGU ACU UCC ACG AGG UAU CAA CGG AGU UGG UGG ACA GUA CUC AGG UCA UCC UAG G-3′ (9-14) | Chip-based detection of HCV | [23] | ||
| C4, 7, 42, 97, 103 and 104 | DNA | n.d. | 5′-GCA CGC CAG ACC AGC CGT CCT CTC TTC ATC CGA GCC TTC ACC GAG C-3′ (C4) | Inhibition of HCV production in cells | [44] | |||
| GQ- based detection of HCV | [27] | |||||||
| 9-15 | DNA | n.d. | 5′-GAT CGA GGA TGG GAA CAC CCA GTA GGA GGA TGG GCA TGG CCG GAC CCA AAA TTA GCA GTA AAA AAA AAA AAA AA AAA-3′ | LFA-based detection of HCV | [26] | |||
| A12, A14 and A15 | DNA | n.d. | 5′-ACG CTC GGA TGC CAC TAC AGG CAC GCC AGA CCA GCC GTC CTC TCT TCA TCC GAG CCT TCA CCG AGC CTC ATG GAC GTG CTG GTG A-3′ (A12) | AFM-chip-based detection of HCV | [28,29] | |||
| E2 | ZE2 | DNA | 1.05 ± 1 nM | 5′-GAA TGA GGA ATA ATC TAG CTC CTT CG CTG A-3′ | Detection of HCV | [24] | ||
| E2-A, B, C and D | DNA | 0.8–4 nM | n.d. | Aptamer-based detection of HCV | [25] | |||
| E1/E2 | E1E2-6 | DNA | n.d. | 5′-CAC GTC TAT TAA GAT TGG GAC GTG-3′ | Inhibition of HCV infection in cells | [43] | ||
| NS2 | NS2-2 | DNA | n.d. | 5′-CAG GTA CCA CCT TCA TGG GCG CGG AAG ACG ATG GTG TAC TA-3′ | Blocking NS2–NS5A interaction in cells | [45] | ||
| G6–16 and G6–19 | RNA | 120 ± 18 nM | 5′-GGG AGA AUU CGA CCA GAA GCC UUG CUG UUG UUU CCC UGU UGU UUU GUC UCU CAA CUU UAU UGU GGU AAA GAU CAC UGG GUU GAU AAG GGC UAA CUC UAA UUU GAC UAC AUG GUC GGA CCA AUC AGU UCU UUG GGA GAU GCA UAU GUG CGU CUA CAU GGA UCC UCA-3′ (G6-16) | Inhibition of NS3 enzymatic activity in vitro | [46] | |||
| NS3 | SE RNA | RNA | 990 pM | 5′-GAA GCG UGC UGG GCC ACU AGU GUA UAC GGC UCG AA -3′ | Inhibition of NS3 enzymatic activity in cells | [47] | ||
| 10G-1 and G9-I, II and III | RNA | 8–12 nM | 5′-GGG AAC UCG AUG AAG CGA AUU CUG UUG GCG AAC UGU ACG CAA GUA CAC UGG AUG ACA GCC UAC CUA UCG GAU CCA CG-3′ (10G-1) | Inhibition of NS3 protease activity in cells | [48,49,50,51] | |||
| NEO-35-s41 and G925-s50 | RNA | 0.19 ± 0.16 nM | 5′-CGU CCC CAA AAA AAA AGG AGA GAG GAA AGG UAG UC-3′ (NEO-35-s41) | Inhibition of NS3 enzymatic activity in cells | [54,55,56] | |||
| NS5A | NS5A-4 and -5 | DNA | n.d. | 5′-GCT ATC TTA TGG AAA TTT CGT GTA GGG TTT GGT GTG GCG GGG CTA-3′ (NS5A-4)) | Inhibition of HCV replication in cells | [57] | ||
| NS5B | 27v and 127v | RNA | 132.3 ± 20 nM | 5′-ACG TAC ACT AGT GGT CCG GGC GGG GCT CAT TGT CC-3′ (27v) | Inhibition of HCV replication in vitro and cells | [59,60] | ||
| r10/43 and r10/47 | DNA | 1.3–23.5 nM | 5′-GGG AGA CAA GAA TAA ACG CTC AAG GGC GTG GTG GGT GGG GTA ATA ATA ATG TGC GTT TGT TCG ACA GGA CCG TCA CAA CAG GC-3′ (r10/43) | Inhibition of HCV genotype 3a replication | [61] | |||
| R-OH and R-F | RNA * | 2.62 nM | 5′-CCU UGA ACG AUU GGU AGU AGA AUA UCG UCA GUG AAC GGC AGU-3′ (R-F) | Inhibition of HCV replication in cells | [63,64] | |||
| ZIKV | Capsid protein | n.d. | DNA | n.d. | n.d. | Detection of ZIKV using paper-based sensor | [70] | |
| NS1 | Aptamers 2 and 10 | DNA | 45 pM | 5′-CTA GGT TGC AGG GGA CTG CTC GGG ATT GCG GAT CAA CCT AG-3′ (Aptamer 2) | Detection of ZIKV using aptamer-based ELISA | [68] | ||
| DNA | 45 pM | 5′-CTA GGT TGC AGG GGA CTG CTC GGG ATT GCG GAT CAA CCT AG-3′ (Aptamer 2) | Detection of ZIKV in serum and urine | [69] | ||||
| DENV | DENV | n.d. | DNA | n.d. | 5′-CCC GCA CCG GGC AGG ACG TCC GGG GTC CTC GGG GGG CGG G-3′ | Detection of DENV using nanoparticles | [74] | |
| Viral RNA | DEN-4 Linker | DNA | n.d. | 5′-CGA GTT CAA CAT TCC TGT TTG CCC AAT CAT AGT TGA ACT CGT CTT G-3′ | Detection of DENV using aptasensor | [73] | ||
| 5′-UTR | A03, B07 and C10 | RNA | n.d. | 5′-GGA GGU AGA GAG GGA GGG UUG AGG GGA AGG UUU ACC UCU UUA UUG-3′ (A03) | Potential for DENV diagnosis and therapy | [78] | ||
| E protein | DENTA-1 | DNA | 99 ± 5 nM | 5′-CGG CAT TCT CCT GCT ACG AGG CGC TGC GGT ACA CCC CGA CTC CAC GAG CCA CTG TCT ACG GAC ATC TG-3′ | Inhibition of DENV infection | [75] | ||
| S15 | DNA | 292 nM | 5′-GCA CCG GGC AGG GAC GTC CGG GTC CTC GGG C-3′ | Inhibition of 4 serotypes of DENV | [76] | |||
| MTase | n.d. | RNA * | 15.6 ± 1.03 nM | 5′-GGU UGG GCA CAU AUA GAC UGU GUA AUU CGU AUA GUG UGC AUA ACC-3′ | Inhibition of DENV MTase activity in vitro | [77] | ||
| JEV | MTase | G2 | RNA ** | 16 nM | 5′-GAU GCG CAU GGA GAC GAC AGC AUC-3′ | Inhibition of JEV MTase activity and in cells | [80] | |
| TBEV | E protein | n.d. | DNA | n.d. | n.d. | Inhibition of TBEV infection in cells | [82] | |
| NoV | VLPs | Aptamer 25 | DNA | 232 nM | 5′-CAT CTG TGT GAA GAC TAT ATG GCG CTC ACA TAT TTC TTT C-3′ | Detection of NoV using ELASA | [85] | |
| n.d. | DNA | n.d. | 5′-GGG GGT TTT CAT CTG TGT GAA GAC TAT ATG GCG-3′ | Detection of NoV using non-stop aptasensor | [90] | |||
| AG3 | DNA | 290 nM | 5′-GCT AGC GAA TTC CGT ACG AAG GGC GAA TTC CAC ATT GGG CTG CAG CCC GGG GGA TCC-3′ | Detection of Nov using DNA aptasensor | [88] | |||
| Capsid protein | M1 and M6-2 | DNA | n.d. | 5′-TGT TTA TGG GGA TAA ACG TAT CTA ATT CGT GTA CTA ATC A-3′ (M1) | Detection of NoV using ELASA | [86] | ||
| APTL-1 | DNA | 148.13 ± 6.53 nM | 5′-CGA TCA AAC GTT CAA GCG GGG CCC GGA GGCGTG ACT TGG ACA GGC AGG CGT TAC GAT GCA TCC CGC AAA TGA CGC ATG A-3′ | Detection of NoV in clinical samples | [87] | |||
| n.d. | DNA | n.d. | 5′-AGT ATA CCG TAT TAC CTG CAG CCA TGT TTT GTA GGT GTA ATA GGT CAT GTT AGG GTT TCT GCG ATA TCT CGG AGA TCT TGC-3′ | Detection of NoV using microfluidic platform | [89] | |||
| CoV | SARS-CoV nsP10 | ES15 | RNA | n.d. | 5′-GAU AAU ACG ACU CAC UAU AGG GUU CAC UGC AGA CUU GAC GAA GCU UGC AGA AAA GGG GGA AGA AGA GGG UGA UUC AGG CGA GAG AAU GGA UCC ACA UCU ACG AAU UC-3′ | Inhibition of helicase activity in vitro | [94] | |
| NG1 | DNA | 20.8 nM | 5′-CCG TAA TAC GAC TCA CTA TAG GGG AGC TCG GTA CCG AAT TCG TGT GAG GGT GAG ATG TGT GTG TAT TTG TCA AGC TTT GCA GAG AGG ATC CTT-3′ | Inhibition of helicase activity in vitro | [95] | |||
| SARS-CoV NP | Aptamer 1 and 2 | RNA * | 1.65 ± 0.41 nM | 5′-GGG AGA GCG GAA GCG UGC UGG GCG UGU CGU UCG CUG UCU UGC UAC GUU ACG UUA CAC GGU UGG CAU AAC CCA GAG GUC GAU GG-3′ (Aptamer 1) | RNA aptamer-based detection of SARS-CoV | [96] | ||
| Aptamer1 | DNA | 4.93 ± 0.30 nM | 5′-GCA ATG CTA CGG TAC TTC CGG ATC CGG AAA CTG GCT AAT TGG TGA GGC TGG GGC GGT CGT GCA GCA AAA GTG CAC GCT ACT TTG CTA A-3′ | Detection of SARS-CoV | [97] | |||
| n.d. | RNA | n.d. | 5′-GGG AGA GCG GAA GCG UGC UGG CCC UGU CGU UCG CUG UCU UGC UAC GUU ACG UUA CAC GGU UGG CAU AAC CCA CAG GUC GAU GG-3′ | Detection of SARS-CoV using quantum-dots | [98] | |||
| SARS-CoV2 spike | CoV2-RBD-4C | DNA | 19.9 nM | 5′-ATC CAG AGT GAC GCA GCA TTT CAT CGG GTC CAA AAG GGG CTG CTC GGG ATT GCG GAT ATC GAC ACG T-3′ | Provide new strategy to SARS-CoV2 | [99] | ||
| SARS-CoV2 NP | A48 | DNA | 0.49 nM | 5′-GCT GGA TGT CGC TTA CGA CAA TAT TCC TTA GGG GCA CCG CTA CAT TGA CAC ATC CAG C-3′ | Provide new strategy to SARS-CoV2 | [100] | ||
| Group V | Influenza Virus | H1N1 virus | Apt-DNA | DNA | n.d. | 5′-ACA CAA ATC CTA TTG ACC GCT GTG TGA CGC AAC ACT CAA T-3′ | Detection of virus using quantum dots (QDs) | [108] |
| n.d. | DNA | n.d. | 5′-GGC AGG AAG ACA AAC AGC CAG CGT GAC AGC GAC GCG TAG GGA CCG GCA TCC GCG GGT GGT CTG TGG TGC TGT-3 | Detection of virus using microfluidic system | [3,109] | |||
| A-20 | DNA | 6 nM | 5′-GGA CCA GTT GTC TTT CGG TCT CTA CCC CAG CCC GT-3′ | Detection of virus using EIS | [111] | |||
| AP-I | DNA | n.d. | 5′-GCA ATG GTA CGG TAC TTC CGG GTG GGT GGG AGG GGG TGG AGG TTG GGG GTT GGA CGC AGA GTG CCA AAA GTGC ACG CTA CTT TGC TAA-3′ | Distinguish virus subtype H1 form H5 | [110] | |||
| H1N1 HA | V46 | DNA | 19.2 nM | 5′-TAC TGC ACA CGA CAC CGA CTG TCA CCA TCA CCT CGG CGC A-3′ | Detection of virus by electrochemical sensor | [112] | ||
| n.d. | DNA | n.d. | 5′-GGG TTT GGG TTG GGT TGG GTT TTT GGG TTT GGG TTG GGT TGG GAA AAA-3′ | Detection of virus using SERS aptasensor | [113] | |||
| Aptamer 1 and 2 | DNA | 78 ± 1 nM | 5′-GGG AGC TCA GAA TAA ACG CTC AAG GCA CGG CAT GTG TGG TAT GTG GTG CCT GTA CTC GTT CGA CAT GAG GCC CGG ATC-3′ (Aptamer 1) | Blocking HA–Glycan interaction in cells | [137] | |||
| D-12 and D-26 | RNA * | 190 pM | 5′-GGA GCU CAG CCU UCA CUG C CA AAA AGU UAG GCC AGC AAA UUG CGA GCU GAU CCG GUG ACU GGC UAC AGG AGG CCU UGU CCA CGG CCG UAU U GG CAC CAC CGU CGG AUC C-3′ (D-12) | Inhibit agglutination of virus | [129] | |||
| H3N2 HA | A22 | DNA | 46.23 ± 5.46 nM | 5′-GCT GCA ATA CTC ATG GAC AGC CTC CTG GGG TCA GGC TCA GAC ATT GAT AAA GCG ACA TCG GTC TGG AGT ACG ACC CTG AA-3′ | Inhibition of viral infection in mice | [136] | ||
| Detection of virus using QDs | [107] | |||||||
| Detection of virus using colorimetric platform | [115] | |||||||
| P30-10-16 | RNA | n.d. | 5′-GGG AGA AUU CCG ACC AGA AGG GUU AGC AGU CGG CAU GCG GGU CAC GAC AGA CCU UUC CUC UCU CCU UCC UCU UCU-3′ | Inhibition of HA-mediated entry of virus | [128] | |||
| Detection of virus using aptasensor | [114] | |||||||
| Detection of virus using DRELFA | [116] | |||||||
| RHA0385 | DNA | n.d. | 5′–TTG GGG TTA TTT TGG GAG GGC GGG GGT T–3′ | Detection of virus using SERS aptasensor | [117] | |||
| H5N1 HA | Aptamer 1, 2 and 3 | DNA | 4.65 nM | 5′-GTG TGC ATG GAT AGC ACG TAA CGG TGT AGT AGA TAC GTG CGG GTA TGT TG-3′ (Aptamer 1) | Detection of virus using SPR aptasensor | [118] | ||
| Detection of virus using QCM aptasensor | [119] | |||||||
| Detection of virus using microfluidic chip | [120] | |||||||
| Detection of virus using gold nanoparticles | [122] | |||||||
| RHA0006 | DNA | n.d. | 3’-TTG GGG TTA TTT GGG AGG GCG GGG GTT-5’ | Detection of virus using fluorescent sensor | [121] | |||
| IF10 and IF22 | DNA | n.d. | 5′-CGT ACG GTC GAC GCT AGC TAA CGG TGT GGC CCG GGG GTA CAG CGC ACT CAC GTG GAG CTC GGA TCC-3′ (IF10) | Detection of virus using SPR aptasensor | [123] | |||
| n.d. | DNA | n.d. | 5′-GTG TGC ATG GAT AGC ACG TAA CGG TGT AGT AGA TAC GTG CGG GTA GGA AGA AAG GGA AAT AGT TGT CCT GTT GTT GCC ATG TGT ATG TGG G-3′. | Detection of virus using FET | [124] | |||
| HAS15-5 | RNA | n.d. | 5′-GGG TTC ACT GCA GAC TTG ACG AAG CTT ACA AAC AAG AGC AAA AAG GGA GUU GAC GUA GAC UGU GCG GAA TGG ATC CAC ATC TAC GAA TTC-3′ | Blocking HA activity | [132] | |||
| BV02 | DNA | n.d. | 5′-AAT TAA CCC TCA CTA AAG GGC TGA GTC TCA AAA CCG CAA TAC ACT GGT TGT ATG GTC GAA TAA GTT AA-3′ | Inhibition of viral replication in animal model | [133] | |||
| HA12-16 | RNA | n.d. | 5′-GGG TTC ACT GCA GAC TTG ACG AAG CTT GCU UGA CGG AGA UCA AGG GCG AGU CUC AUA CCA AGU UGA UGG GGA ATG GAT CCA CAT CTA CGA ATT C-3′ | Inhibition of viral replication in cells | [134] | |||
| 8-3S | RNA* | 170 pM | 5′-GGG CAA CCG CUG GAA CUU GAA GUC GGU AAU GCG AGC GGA AAG CCC-3′ | Blocking HA–Glycan interaction | [135] | |||
| H5N1 pA | PAN-1 ~ PAN-6 | DNA | 130–2000 nM | 5′-CCG TAA TAC GAC TCA CTA TAG GGG AGC TCG GTA CCG AAT TCC TTG GAC CAT TAA AAC ACG TGT CTG CAT CCA AGC TTT GCA GAG AGG ATC CTT-3′ (PAN-1) | Inhibition of endonuclease activity | [140] | ||
| H9N2 HA | C7 | DNA | n.d. | 5′-GGT AGT TAT AGT ATA TGG AAG GGG GTG TCG TAT GG-3′ | Inhibition of viral infection in cells | [131] | ||
| A9 and B4 | DNA | 46.23 ± 5.46 nM | 5′-GCT GCA ATA CTC ATG GAC AGC CTC CTG GGG TCA GGC TCA GAC ATT GAT AAA GCG ACA TCG GTC TGG AGT ACG ACC CTG AA-3′ (A9) | Inhibition of viral infection in cells | [136] | |||
| Influenza A NS1 | n.d. | DNA | 18.91 ± 3.95 nM | 5′-GCA ATG GTA CGG TAC TTC CCG CGG TCC GGG GTG GGT GGG TGG TGG GGG GTG CGG GGG GGC GGC CGC AAA AGT GCA CGC TAC TTT GCT AA-3′ | Inhibition of interferon antagonism | [138] | ||
| Influenza B HA | A-20 | RNA | 45 nM | 5′-GGG UGG ACG CGG UAC GAG CAA UUU GUA CCG GAU GGA UGU UCG CC-3′ | Inhibition of viral entry | [127] | ||
| RVFV | NP | n.d. | RNA | n.d. | 5′-GGU AGC CAU AUU AGC GCA UAA CCA UCA CAA CCG UGG GCU CAU UGG UGG CCA CUG CCA U-3′ | Inhibition of viral replication | [147] | |
| SFTSV | NP | SFTS-apt 1, 2 and 3 | DNA | 1–4 nM | 5′-ATC CAG AGT GAC GCA GCA CGA CCA CAG ATT GGA GAC TGA TAG TGC ACG AGC AAG GAC ATG GAC ACG GTG GCT TAG T-3′ (SFTS-apt 1) | Detection of virus using aptasensor | [151] | |
| EBOV | VP35 | 1G8-14 and 2F11-14 | RNA | 3.7 ± 0.2 nM | 5′-GGG AGA CAA GAA UAA ACG CUC AAG GCA UUU CUG CUA GUC UGG UUG UAA GAU AUU CAA CAC GUG AGU UUC GAC AGG AGG CUC ACA ACA GGC-3′ (1G8-14) | Inhibition of VP35–NP interaction | [153] | |
| VP24 | VPKS-2 and VPKS-5 | DNA | 0.5–20 nM | n.d. | Inhibition of VP24–KPNA1 interaction | [154] | ||
| GP | 39SGP1A | RNA * | 30 nM | 5′-GGG CGC UCA AUU UUU UAU UGC AUU UUU CUU UGA GCG CCC-3′ | Detection of viral infection | [155] | ||
| GP and NP | n.d. | DNA | 4.1–76.1 nM | 5′-GGG CGC UCA AUU UUU UAU UGA GCG CCC-3′ | Detection of viral infection | [156] | ||
| Group VI | HIV | Genome | AL4 and AS8 | RNA | n.d. | 5′-GGG AGU CGA CCG ACC AGA AAG CUA GGG AAC AGG GGA GGA GCA GGC AGU AGG UGC GAU GGU AUG UGC GUC UAC AUC UAG ACU CAU-3′ (AL4) | Inhibition of viral transcription | [167] |
| RNApt16 | RNA | 82 ± 13 nM | 5′-CCC CGG CAA GGA GGG G-3′ | Inhibition of viral production in cells | [168] | |||
| RT | RT 26 | DNA | n.d. | n.d. | Detection of virus | [161] | ||
| n.d. | DNA | 25 pM | 5′-GGG AGA UUC CGU UUU CAG UCG GGA AAA ACU GAA-3′ | Inhibition of viral production in cells | [170] | |||
| Class1 and 2 | RNA | n.d. | 5′-CAC AAG AUC CGA GGC AGA ACG GGA AAA UCU GCG AAG UAA-3′ (Class 1) | Inhibition of DNA polymerase activity | [171,172] | |||
| 70.8 and 70.15 | RNA | 27–63 nM | n.d. | Inhibition of viral replication in cells | [173,174] | |||
| RT1t49 | DNA | 4nM | 5′-ATC CCT GAT TAG CGA TAC TCA GAA GGA TAA ACT GTC CAG AAT TTG GA-3′ | Inhibition of polymerase and RNaseH activity | [175,176] | |||
| Inhibition of viral infection in cells | [180] | |||||||
| n.d. | DNA | 5nM | 5′-GCA TGA ATT CCC CGA AGA CGC AAA CTG AAG AGG CAC CGA AGG GGG GG-3′. | Inhibition of viral replication | [177] | |||
| RT6 | DNA | n.d. | 5′-CAG GCG TTA GGG AAG GGC GTC GAA AGC AGG GTG GG-3′ | Inhibition of RT activity | [178] | |||
| 70.15 | RNA | n.d. | 5′-ACC CAG GAG AUA AAG GGG AAA ACA CUG GAA AAC-3′ | Inhibition of viral infection in cells | [179] | |||
| F1Pk and F2Pk | RNA | n.d. | 5′-CCU AGG ACG AAA GCG AUA AUC GGG CCU GGA GGA UCA AAU UAA UGC U-3′ (F1Pk) | Inhibition of viral replication | [181] | |||
| 148.1-38m | RNA* | n.d. | 5′-GGG CGU UGC CUA CUC UCA AUC UGA GGU UCA AGG GCA CG-3′ | Inhibition of viral infection | [183] | |||
| ODN 93 and ODN 112 | DNA | n.d. | 5′-GGG GGT GGG AGG AGG GTA GGC CTT AGG TTT GTG A-3′ (ODN 93) | Inhibition of RNaseH activity | [184,185] | |||
| ODN | DNA | n.d. | 5′-GGG GGG GCC AGG CCA TGG CGT GAC TTG CTG GC-3′ | Inhibition of integrase activity | [187] | |||
| IN | T30177 | DNA | n.d. | 5′-GTG GTG GGT GGG TGG GT-3′ | Inhibition of integrase activity | [188] | ||
| 93del | DNA | n.d. | 5′-GGG GTG GGA GGA GGG T-3′ | Inhibition of viral replication ex vivo | [190] | |||
| T30175 | DNA | n.d. | 5′-GHG GTG GGT GGG TGG GT-3′ | Inhibition of integrase activity | [191] | |||
| PR | PR10.1 | RNA | 115 ± 22 nM | 5′-CUU CAU UGU AAC UUC UCA UAA UUU CCC GAG GCU UUU ACU UUC GGG GUC CU-3′ | Inhibition of viral production in cells | [193] | ||
| NC | N70-13 | RNA | 0.6 nM | 5′-GAC UGG GUA CGU UUC CGG UAG CCG GUA GGA-3′ | Inhibition of viral packaging | [195,196] | ||
| Gp120 | B40 | RNA * | 20.9 nM | 5′-GGG AGA CAA GAC UAG ACG CUC AAU GUG GGC CAC GCC CGA UUU UAC GCU UUU ACC CGC ACG CG-3′ | Inhibition of viral infection in cells | [198,199,200,201,202] | ||
| Gag | DP6-12 | RNA | 130 ± 9.3 nM | n.d. | Inhibition of viral infection in cells | [204] | ||
| Rev | n.d. | n.d. | n.d. | n.d. | Inhibition of viral infection in cells | [207] | ||
| RBA-14 | RNA | 5.9 nM | 5′-GGC UGG ACU CGU ACU UCG GUA CUG GAG AAA CAG CC−3′ | Inhibition of viral infection in cells | [205] | |||
| Tat | n.d. | RNA | n.d. | 5′-ACG AAG CUU GAU CCC GUU UGC CGG UCG AUC GCU UCG A-3′ | Detection of virus using aptasensor | [162,163] | ||
| n.d. | RNA | n.d. | 5′-UCG GUC GAU CGC UUC AUA A-3′ | Detection of virus using FET-based sensor | [164] | |||
| AntiTat5 | RNA | n.d. | 5′-ACG AAG CUU GAU CCC GUU UGC CGG UCG AUC GCU UCG AAA AAA AAA AAA CGA AGC UUG AUC CCG UUU GCC GGU CGA UCG CUU CG-3′ | Detection of virus using SE-based sensor | [166] | |||
| CCR5 | G3 | RNA | 110 nM | 5′-GCC UUC GUU UGU UUC GUC CA-3′ | Inhibition of viral infection in cells | [208] | ||
| NCL | AS1411 | DNA | 34.2 nM | 5′-GGT GGT GGT GGT TGT GGT GGT GGT GG-3′ | Inhibition of viral entry to cells | [209] | ||
| CycT1 | Apt1 and Apt4 | RNA | 1–2 nM | 5′-UCC CCC UAU GCG AAA AGC GAA UCA CUU CCA GCC UAC CCU-3′ (Apt1) | Inhibition of viral transcription in cells | [211] | ||
| Group VII | HBV | HBsAg | anti-HBsAg RNA aptamer | RNA | n.d. | 5′-GUA UGU GGG CUG AAC UCA AUC AGG UCC CAA UCC CCA ACA UAC ACA UGA CCC GUC GUU UAC GAU CAU UAU AGA CGG CCA UGA UUG ACA CGC AAU CAA CCC CCU AUA GUG AGU CGU AUU A-3′ | Detection of virus using FRET | [212] |
| HO1, HO2 and HO3 | DNA | n.d. | 5′-GGG AAT TCG AGC TCG GTA CCC ACA GCG AAC AGC GGC GGA CAT AAT AGT GCT TAC TAC GAC CTG CAG GCA TGC AAG CTT GG-3′ (HO1) | Detection of virus using MNPs | [213] | |||
| H01 | DNA | n.d. | 5′-ACC CAC AGC GAA CAG CGG CGG ACA TAA TAG TGC TTA CTA CGA CGC-3′ | Remove HBsAg in cell with no cytotoxicity | [218] | |||
| HBeAg | Aptamer 2-19 | DNA | n.d. | 5′ -GGG CGA AGA CCG GGA CGG GAG GAT TCT GTA GAT TGG TTT T-3′ | Detection of virus | [214] | ||
| Polymerase | Class I and Class II | RNA | n.d. | 5′-UGU UCA UGU CCU ACU GUU CCG AAC AAA AAU AAG AAG AAA AAU AAU AUU UGG GGC AUG GAC A-3′ (Class 1) | Inhibition of viral replication in cells | [215] | ||
| Core | Apt No 28 | DNA | n.d. | 5’-ACG CTC GGA TGC CAC TAC AGC TTC CCC TAA TCT GGC GCT CTC ATC TAA TTT CCC TTC CTG CTC ATG GAC GTG CTG GTG AC-3’ | Inhibition of viral infection in cells | [216] | ||
| capsid | AO-01 | DNA | 180 ± 82 nM | 5′-GCG GGT CGA CGT TTG CAC ACG CGA GCC GCC ATG TCT GGG CCA CAT CCA TGG GGC GG-3′ | Inhibition of viral production in cells | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Lee, S.-W. Aptamers for Anti-Viral Therapeutics and Diagnostics. Int. J. Mol. Sci. 2021, 22, 4168. https://doi.org/10.3390/ijms22084168
Kim T-H, Lee S-W. Aptamers for Anti-Viral Therapeutics and Diagnostics. International Journal of Molecular Sciences. 2021; 22(8):4168. https://doi.org/10.3390/ijms22084168
Chicago/Turabian StyleKim, Tae-Hyeong, and Seong-Wook Lee. 2021. "Aptamers for Anti-Viral Therapeutics and Diagnostics" International Journal of Molecular Sciences 22, no. 8: 4168. https://doi.org/10.3390/ijms22084168
APA StyleKim, T.-H., & Lee, S.-W. (2021). Aptamers for Anti-Viral Therapeutics and Diagnostics. International Journal of Molecular Sciences, 22(8), 4168. https://doi.org/10.3390/ijms22084168






